Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.1 Vicente López jun. 2014
ARTÍCULOS ORIGINALES
Morphological and immunochemical characterization of the pollen grains of Chenopodium album L. (Chenopodiaceae) in a temperate urban area in Argentina
Caracterización morfológica e inmunoquímica de granos de polen de Chenopodium album L. (Chenopodiaceae) en un área urbana templada de Argentina
Bianchimano AS1, MG Murray2,3, ME Aztiria1, B Montes2,3, ML Calfuán2, MI Prat1
1 Laboratorio de Inmunología. Departamento de Biología, Bioquímica y Farmacia. Universidad Nacional del Sur.
2 Laboratorio de Diversidad de Plantas Vasculares. Departamento de Biología, Bioquímica y Farmacia. Universidad Nacional del Sur.
3 Consejo Nacional de Investigaciones Científicas y Técnicas. INBIOSUR (CCT- Bahía Blanca).
Address Correspondence to: María Gabriela Murray. Universidad Nacional del Sur. San Juan 670, Bahía Blanca. C.P. 8000. Argentina. e-mail: mgmurray@criba.edu.ar
Recibido / Received 5.III.2013.
Aceptado / Accepted 16.V.2013.
Abstract. Chenopodium album is a very polymorphic, cosmopolitan, annual herb that grows spontaneously in modified soils in wasteland in the outlying urban zones of Bahía Blanca. In this city, the flowering period is mainly during February and March, which coincides with the highest concentrations of this pollen type in the atmosphere of the city. The objective of this study was to characterize the pollen grains of Chenopodium album, both morphologically and immunochemically, that were obtained from three different zones in the urban area of Bahía Blanca. Samples were collected from the three separate zones in the city that were far apart. The structure and morphology of the grains were analyzed using light and electron microscopy. The protein and antigenic profiles were studied with Tricine-SDS-PAGE and western blot with a polyclonal rabbit serum, respectively. The morphological analysis showed significant differences in relation to the diameters of the pollen grains in one of the studied areas. Differences in the protein expression were seen for the same area although the antigenic profile was conserved. The variations in the morphology and the protein profile may be caused by the effect of environmental conditions on the pollen, and the presence of urban contaminants from vehicular traffic.
Keywords: Chenopodium album; Immunobloting; Pollen; Proteins; Antigenic profile.
Resumen. Chenopodium album es una hierba cosmopolita, anual, muy polimórfica, que crece en forma espontánea en baldíos con suelos modificados de los barrios periféricos de Bahía Blanca. En esta ciudad, el periodo de floración es principalmente entre febrero y marzo, que coincide con la mayor concentración de este tipo de polen en la atmósfera. El objetivo de este estudio fue caracterizar morfológica e inmunoquímicamente a los granos de polen de Chenopodium album obtenidos en diferentes zonas del área urbana de la Bahía Blanca. Las muestras fueron colectadas en tres zonas de la ciudad. La estructura y la morfología de los granos fueron analizados con microscopía óptica y electrónica. Los perfiles proteicos y antigénicos fueron estudiados mediante Tricine-SDS-PAGE y western blot, empleando un suero policlonal obtenido en conejo. El análisis morfológico mostró diferencias significativas en relación al diámetro de los granos de polen en una de las áreas estudiadas. En esa misma área se encontraron diferencias en la expresión de proteínas, aunque el perfil antigénico fue conservado. Estas variaciones en la morfología y en el perfil proteico podrían ser causadas por los efectos de las condiciones ambientales sobre el polen y la presencia de contaminantes urbanos provenientes del tráfico vehicular.
Palabras clave: Chenopodium album; Immunobloting; Polen; Proteínas; Perfil antigénico.
INTRODUCTION
In recent years, there has been an increase in the allergic disease morbidity associated with pollen grains, especially in urban areas of industrialized countries (DAmato & Liccardi, 2002a). The pollen grain is the vehicle to transport the masculine gametophyte to the female reproductive structures for fertilization in seed plants or Spermatophytes (Raven & Johnson, 2001). The plants adopt reproductive strategies that enable the pollen grains to reach the gynoecium in the female flowers for fertilization. Currently, no more than 10% of the flowering plants are wind-pollinated (anemophilous pollination). However, although this strategy is very effective for plants, it often represents a health risk for people (Solomon, 2002). The presence of pollen grains in the atmosphere is determined by fundamental aspects such as the flowering of wind-pollinated plants and meteorological variables, especially temperature and precipitation. These variables have the greatest influence over the diferent stages of pollen grain dispersal in the atmosphere (Nilsson & Praglowski, 1974; Norris-Hill, 1997; Ahas et al., 2000; Silva Palacios et al., 2000; Barnes et al., 2001). The effects on the health of the population not only depend on the levels of pollen in the air, but also on individual susceptibility and environmental factors. Various authors have shown that some species of Chenopodiaceae (e.g. Beta vulgaris, Chenopodium album and Salsola kali) produce large quantities of pollen grains (Luoto et al., 2008) which cause allergic rhinitis in Iran (Tehrani et al., 2010), western USA (Newmark, 1979) and the south of Europe (DAmato & Liccardi, 2002b).
Although 92 species of this family occur in Argentina (Zuloaga & Morrone, 1999), there are approximately 33 species in Bahía Blanca. According to some authors, Salsola kali, Bassia scoparia (= Kochia scoparia) and Chenopodium album are responsible for the frequency and intensity of hay fever in this city (Herraiz Ballestero & Monticelli, 1943; Carignano et al., 1998).
The presence of environmental contaminants has been reported in different areas of the city (e.g. excessive levels of carbon monoxide, nitrous oxide and sulphur oxide: Carignano et al., 2003). These pollutants may affect pollen indirectly by inducing stress to plants, or directly by contaminating the anthers or pollen grains during dispersal (Guedes et al., 2009). On the other hand, they may also alter the physiology and ontogeny of the grain, leading to a lower level of production, a decrease in size and/or an increase in the number of deformed grains (Guedes et al., 2009; Rezanejad, 2009). Studies using nitrous oxide and sulphur oxide as treatments have shown that these toxic gases can produce changes in the soluble proteins found in pollen grains (Santra et al., 1991). In spite of numerous studies, the analysis of allergens from pollen grains of different environments, whether contaminated or not, have shown conflicting results, and it is not yet clear which factors cause variation in the quantity of allergens (Helander et al., 1997).
Chenopodium album (fat hen; lambs quarters) is a very polymorphic, cosmopolitan, annual herb (Kruckeberg & Rabinowitz, 1985) that grows spontaneously in modified soils in wasteland in the outlying zones of Bahía Blanca (Cabrera, 1963-1970; Lamberto et al., 1997). In this city, the flowering period of Chenopodium album is mainly during February and March, which coincides with the highest concentrations of this pollen type in the atmosphere of Bahía Blanca (Murray et al., 2010).
The objective of this study was to characterize the pollen grains of Chenopodium album, both morphologically and immunochemically, that were obtained from three different zones in the urban area of Bahía Blanca during February and March 2011. An integrated study of both aspects could contribute to the possible utilization of pollen grains as bioindicators of environmental contamination.
MATERIALS AND METHODS
Study area. Bahía Blanca is located at 20 m.a.s.l. with predominantly NNW winds. The typical climate pattern of the region is dominated by winds from the west, and the influence of a high pressure centre from the South Atlantic. The resulting circulation induces strong winds from the NW and N for more than 40% of the time (during any given year) with an average speed of 24 km/h, an hourly mean maximum of 58 km/h, and gusts that may exceed 100 km/h (Capelli et al., 2005). The climate is temperate/mesothermal, with constant precipitation throughout the year and hot summers (Kottek et al., 2006) (Fig. 1).
Fig. 1. Köppen-Geiger Climate Classification. C: warm temperate; f: fully humid; a: hot summer.
Fig. 1. Clasificación climática de Köppen-Geiger: C: templado; f: completamente húmedo; a: verano caluroso.
Collection and study of the pollen grains. Pollen grains of Chenopodium album were collected in three places with spontaneous vegetation in Bahía Blanca (zones A, B, C). Zone A (38° 42 S - 62° 14 W) and zone B (38° 41 S - 62° 15 W) are residential areas with a low level of vehicular traffic, whereas zone C (38° 41 S - 62° 18 W) is between thoroughfares with a high level of public transport, very close to a parking lot of the citys transport units. All three study areas are within the urban area of Bahía Blanca (Fig. 2).
Fig. 2. Map of Bahía Blanca to show the three study areas (A, B and C) and urban landmarks.
Fig. 2. Plano de la ciudad de Bahía Blanca donde se observan las tres zonas de estudio (A, B y C) y puntos de referencia urbanos.
The inflorescences were placed in jars of water inside a glass case so that the pollen released in vitro, both from the foor of the glass case and that attached to the flower parts, could be collected. Once all the pollen had been released from the anthers, it was filtered through a nylon mesh and stored at 4 °C. Several inflorescences from different plants were collected at each study site. For the morphological characterization, fresh pollen grains obtained from fresh plants were used.
The pollen structure was observed under both optical and scanning electron microscopes. For study under the optical microscope the pollen was mounted in water on a slide with a coverslip. A binocular microscope (Carl Zeiss) with a digital camera was used and observations were made at a magnification of 400x. The size of the pollen grains from the different study sites was compared by measuring the diameters of 30 pollen grains selected at random on each slide. For the optical analysis not acetolyzed pollen grains were used. Results were analyzed with the GraphPad Prism statistical programme and they were tested using ANOVA and Bonferronis Multiple Comparison Test.
For study under the scanning electron microscope (EVO 40 XVP Leo), dry pollen was used which was not acetolyzed. Pollen grains were metallized with gold and the structure of the exine was observed as well as any particles deposited on the surface.
Preparation of protein extract. The protocol of Varela et al. (2005) was used for the preparation of the protein extract. A 0.1 g sample of dry pollen was weighed, ground in a mortar, and it was then delipidified with cold concentrated acetone during 10 minutes under constant agitation. The resulting powder was left to dry for 24 hours at room temperature. Protein extraction from the dry powder was carried out with PBS 0.01 M (pH 7.4) with the addition of protease inhibitors (150 mM PMSF and trypsin inhibitor: 1 mg/mL), for 24 h at 4 °C, under constant agitation. After centrifuging at 12000 g for 20 minutes while still cold, the supernatant was quickly separated for protein determination with the Bradford test (1976), and the rest of the extract was stored at -20 °C.
Preparation of the polyclonal antibodies. The polyclonal antibody anti-Chenopodium album was obtained from immunized virgin rabbits using 500 µg of Chenopodium album pollen protein from zone A mixed with equal parts of Complete Freunds Adjuvant every two weeks during a period of 60 days (Baroni et al., 2002). The antibody titers were obtained by the indirect ELISA method (Voller et al., 1978).
Gel electrophoresis. A vertical electrophoresis in Tricine-SDS-PAGE gel at 12.5% was performed (Schägger & Gebhard, 1987) in a Hoefer SE 280 system (Amersham Pharmacia Biotech).
A sample of 18 mg of pollen protein extract from each zone was seeded (A, B, C) together with PM markers (Amersham, RPN 800E) in the 12000-225000 Da. range. The running conditions were: voltage 90V, 44mA, during 6 h. The gel was stained with Coomassie Brilliant Blue G-250.
Immunoblot. The electrophoretically separated proteins were transfered to a nitrocellulose membrane using a buffer consisting of 25 mM Tris, 192 mM Glycine, 20% methanol, pH 8.3 in a Hoefer TE 22 system of Amersham Pharmacia Biotech (Towbin et al., 1979). The membrane was blocked with TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.4) plus 5% skimmed milk overnight. The incubation with the antibody anti-Chenopodium album (1/300 dilution in 0.05% TBS-Tween, 1% skimmed milk) was carried out for two hours at room temperature under constant agitation. After six washings with TBS-Tween it was incubated for two hours with goat anti-rabbit IgG, marked with appropriately diluted peroxidase, and then, after further washing with TBS-Tween it was developed with 4-chloro-naphthol (SIGMA).
RESULTS
Morphological characterization of the pollen grains. Under the optical microscope the pollen grains were seen to be apolar, radially symmetrical, polipantoporate, with circular pores (Fig. 3). The size of the grains was very variable: 27.46 ± 1.58 µm diameter in zone A; 26.95 ± 1.87 µm in zone B and 24.21 ± 1.37 µm in zone C (Table 1). Signifhcant differences were seen between the diametres of grains from zones A and C, and B and C, but there were no differences between zones A and B (Table 2).
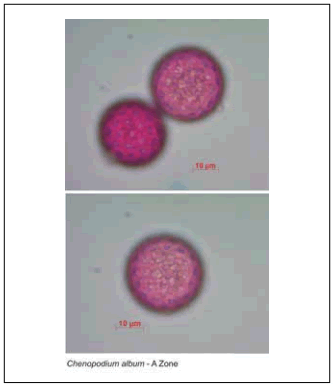
Fig. 3. Light Microscopy (LM) micrographs of Chenopodium album pollen from zone A (x400).
Fig. 3. Micrografías en el microscopio óptico de polen de Chenopodium album de la zona A (x400).
Table 1. Mean values and standard deviation of the diameters of grains of Chenopodium album pollen from each of the study areas.
Tabla 1. Valores medios y desviación estándar de los diámetros de los granos de polen de Chenopodium album, en cada una de lasáreas estudiadas.
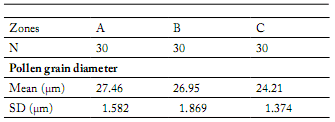
Table 2. Statistical analyses. Bonferronis Multiple Comparison Test of the mean diameters of the grains of Chenopodium album pollen from each of the study areas.
Tabla 2. Análisis estadísticos. Test de Bonferroni para la comparación múltiple de medias de los diámetros de los granos de polen de Chenopodium album, en cada una de las áreas estudiadas.

t = Student statistical; Ns = not significance; CI = confidence intervals.
Under the electron microscope numerous uniformally distributed spinules were seen on the exine, both on the surface and also on the opercula. Numerous foreign particles attached to the surface of the grains were also observed, from all the study areas (Fig. 4).

Fig. 4. SEM micrographs of Chenopodium album pollen from zones B (x7250) and C (x20000).
Fig. 4. Micrografías de microscopía electrónica de barrido de polen de Chenopodium album proveniente de las zonas B (x7250) y C (x20000).
Immunochemical characterization. The protein concentrations of the extracts of Chenopodium album determined using Bradfords method were as follows: zone A: 1.30 mg/mL, zone B: 0.65 mg/mL, and zone C: 1.40 mg/mL.
The protein profile of the extracts showed bands between 100 and 24 kDa in PM (Fig. 5). In all three extracts there were two notable bands in the 90 to 76 kDa zone, one band around 66 kDa, one at 43 kDa and another at 24 kDa. In samples A and B, PM bands also appeared between 52 and 43 kDa, between 31 and 24 kDa, and there were several PM bands lower than 24 kDa, but they were not found in zone C (Fig. 5). According to Amini et al. (2011), the allergens described for Chenopodium: Che a 1 (17 kDa glycoprotein), Che a 2 (14 kDa proflin) and Che a 3 (9.5 kDa polcalcine) were within the PM bands lower than 24 kDa.
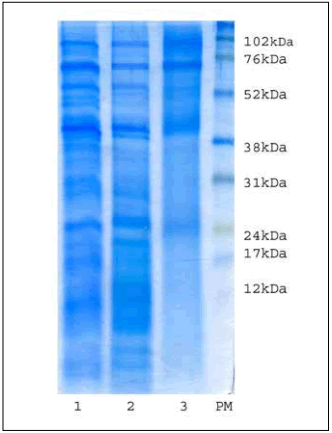
Fig. 5. Protein profiles. 1: Zone A, 2: Zone B, 3: Zone C, PM: weight markers.
Fig. 5. Perfles proteicos: 1: Zona A, 2: Zona B, 3: Zona C, PM: marcadores de peso molecular.
The antigenic profile of the extracts was conserved presenting PM bands between 76 and 35 kDa (Fig. 6).
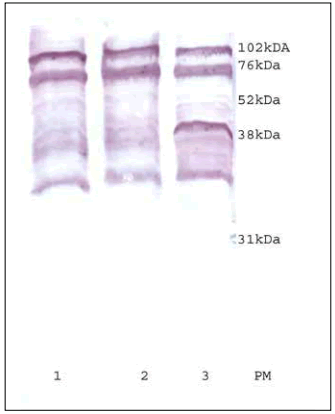
Fig. 6. Antigenic profiles. 1: Zone A, 2: Zone B, 3: Zone C, PM: weight markers.
Fig. 6. Perfles antigénicos. 1: Zona A, 2: Zona B, 3: Zona C, PM: marcadores de peso molecular.
DISCUSSION
The allergenic species of Chenopodiaceae are among the most abundant in Bahía Blanca, being found on roadsides and in wastelands in the outskirts of the downtown, where many streets are unpaved. The pollen grains of this family have been widely studied in this city because of their notable representation in the area, their high concentrations in the atmosphere and their allergenic status. Several studies have been undertaken, focusing on different aspects, such as phenological (Rayer, 2012), aerobiological (Murray et al., 2008; 2010) and clinical (Carignano et al., 1998).
Environmental contamination from industry or from urban transport emissions may affect plant development and, in particular, the ontogeny and morphology of the pollen grains. This stress condition can also cause changes in the expression of the proteins present in them (Rezanejad, 2009).
The pollen grains are an important tool for study and analysis since they could be considered as bio-indicators of the degree of contamination at any particular place (Zobel& Nighswander, 1991; Guedes et al., 2009). Other authors (e.g., Helander et al., 1997), showed that environmental factors, such as the absence of light and soil conditions, are most important in causing changes in the allergenicity and expression of protein in Betula spp.
Rezanejad (2009) studied the pollen grains of Tuja orientalis and observed that samples from areas with high levels of vehicular traffic showed a larger number of irregular or fragile grains, and smaller diametres, than samples from uncontaminated areas. In our observations of Chenopodium album we did not found any diferences in the integrity of the grains. This may have been because the exine of the pollen grains of Chenopodium album is thicker than that of Tuja orientalis, and as a result it may be more resistant to the efects of deformation. In agreement with that observed by Rezanejad (2009) regarding the size of pollen grains, we found significant differences in pollen grain size (diameter) between zone C (high vehicular traffic) and the other two study areas.
It is reported in the literature that the protein profiles of Chenopodiaceae pollen grains show bands between 85, 66, 50, 45, 40, 25, 18, 15 and 10 kDa. Our results for zones A and B agree with those cited by different authors (Carnés et al., 2003; Barderas et al., 2004; Tehrani et al., 2010; Amini et al., 2011) even though the plants they analyzed came from varied habitats (Spain, Iran, Europe, North America, Saudi Arabia and Kuwait).
Comparison of the protein profiles of the three extracts analyzed showed components in the pollen from zone C that differ from a qualitative point of view. It is important to emphasize the effect of environmental pollutants, e.g. carbon monoxide, nitrous oxide and sulphur oxide, on the fertility of plants and on the expression of proteins (Helander et al., 1997; Guedes et al., 2009; Rezanejad, 2009). However, the results reported are contradictory. Guedes et al. (2009) report the loss of expression of some proteins from areas with greater environmental contamination. On the contrary, Helander et al. (1997) did not find any significant differences between pollen obtained from areas with different degrees of environmental contamination; they concluded that pollen grains from less contaminated areas show a lower protein content.
In our study, the antigenic profile studied by immunoblot with rabbit antiserum showed a conserved pattern of bands for the three zones, demonstrating two components in the region around 76 kDa in PM, several bands between 52 and 38 kDa and one band under 38 kDa. No bands were seen of molecular weight less than 35 kDa. These results differ from those recorded in the literature. However, it is worth noting that the characterization of allergenic extracts in the cited studies was carried out using antibodies of sensitized patients (Luoto et al., 2008; Barderas et al., 2004; Tehrani et al., 2010; Amini et al., 2011). The difference in relation to contact with the allergen between patients and experimental animals might be responsible for the discrepancies. Moreover, in the case of the patients, the antibody studied is of class IgE, whereas in the case of the rabbit, via intradermal inoculation, the antibodies generated are of the IgG isotype.
The variations observed in the morphology and in the protein profile would be caused by the effect of environmental conditions on the vegetation and the presence of urban contaminants from vehicular traffic.
This study is innovative for the region. The interpretation of the palinological, morphological and immunochemical data together will contribute to a better understanding of the real potential of the species as an allergen, and in the longterm it may lead to the possibility of using pollen grains as bio-indicators of contamination.
ACKNOWLEDGEMENTS
This work was supported by (1) Universidad Nacional del Sur (PGI-TIR), (2) Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET; (3) PIP Nº11420100100100), and (4) Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT; PICT Nº2697), Argentina.
We would like to thank Flavia Barreiro for help in pollen collection.
REFERENCES
1. Ahas, R., J. Jaagus & A. Aasa (2000). The phenological calendar of Estonia and its correlation with mean air temperature. International Journal of Biometeorology 44: 159-166. [ Links ]
2. Amini, A., M. Sankian, M. Assarehzadegan, F. Vahedi y A. Varasteh (2011). Chenopodium album pollen profilin (Che a 2): homology modeling and evaluation of cross reactivity with allergenic profilins based on predicted potential IgE epitopes and IgE reactivity analysis. Molecular Biology Report 38: 2579-2587. [ Links ]
3. Barderas, R., M. Villalba, C. Pascual, E. Batanero & R. Rodríguez (2004). Profilin (Che a 2) and polcalcin (Che a 3) are relevant allergens of Chenopodium album pollen: Isolation, amino acid sequences, and immunologic properties. Journal of Allergy and Clinical Immunology 113: 1192-1198. [ Links ]
4. Barnes, C., F. Pacheco, J. Landuyt, F. Hu & J. Portnoy (2001). The effect of temperature, relative humidity and rainfall on airborne ragweed pollen concentrations. Aerobiologia 17: 61-68. [ Links ]
5. Baroni, M.V., G.A. Chiabrando, C. Costa, D.A. Wunderlin (2002). Assessment of the floral origin of honey by SDS-PAGE immunoblot techniques. Journal of Agricultural and Food Chemistry 50: 1362-1367. [ Links ]
6. Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analitycal Biochemistry 72: 248-254. [ Links ]
7. Cabrera, A.L. (1963-1970). Flora de la Provincia de Buenos Aires. Buenos Aires: Colección Científica INTA. Buenos Aires. [ Links ]
8. Capelli, A., C. Piccolo & A. Campos (2005). Clima urbano de Bahía Blanca. Buenos Aires: Ed. Dunke. [ Links ]
9. Carignano, C., L. Elosegui, M. Abrego, S. Spagnolo, M. Esandi, R. Frapiccini & O. Reissing (2003). Prevalencia de asma y síntomas indicadores en tres barrios de la ciudad en el marco de una encuesta de propósitos múltiples. Archivos de Alergia e Inmunología Clínica 34: 119-128. [ Links ]
10. Carignano, C., A. Iaquinandi, E. Aramayo, A. Valle, A. Andrada & S. Lamberto (1998). Polinosis en la región de Bahía Blanca. Archivos Argentinos de Alergia e Inmunología 29: 21-27. [ Links ]
11. Carnés, J., E. Fernández-Caldas, A. Marina, C. Alonso, C. Lahoz, C. Colás & A. Lezaun (2003). Immunochemical characterization of Russian thistle (Salsola kali) pollen extracts. Purification of the allergen Sal k 1. Allergy 58: 1152-1156. [ Links ]
12. DAmato, G. & G. Liccardi (2002a). The increasing trend of seasonal respiratory allergy in urban areas. Allergy 57 Suppl 71: 35-6. [ Links ]
13. DAmato, G. & G. Liccardi (2002b). Pollen related allergy in the European mediterranean area. Clinical & Experimental Allergy 24: 210-219. [ Links ]
14. Guedes, A., N. Ribeiro, F. Ribeiro, M. Oliveira, F. Noronha & I. Abreu (2009). Comparison between urban and rural pollen of Chenopodium alba and characterization of adhered pollutant aerosol particles. Aerosol Science 40: 81-86. [ Links ]
15. Helander, M.L., J. Savolainen & J. Ahlholm (1997). Effects of air pollution and other environmental factors on birch pollen allergens. Allergy 52: 1207-1214. [ Links ]
16. Herraiz Ballestero, L. & J. Monticelli (1943). Polinosis. Buenos Aires: Librería Hachette S.A. [ Links ]
17. Kottek, M., J. Grieser, C. Beck, B. Rudolf & F. Rubel (2006). World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15: 259-263. [ Links ]
18. Kruckeberg, A.R. & D. Rabinowitz (1985). Biological aspects of endemism in higher plants. Annual Review of Ecological and Systematics 16: 447-479. [ Links ]
19. Lamberto, S.A., A.F. Valle, E.M. Aramayo & A.C. Andrada (1997). Manual ilustrado de las plantas silvestres de la región de Bahía Blanca. Bahía Blanca: Departamento de Agronomía. Universidad Nacional del Sur. [ Links ]
20. Luoto, S., W. Lambert, A. Blomqvist & C. Emanuelsson (2008). The identification of allergen proteins in sugar beet (Beta vulgaris) pollen causing occupational allergy in greenhouses. Clinical and Molecular Allergy 6: 1-10. [ Links ]
21. Murray, M.G., C. Galán & C.B. Villamil (2008). Aeropalynological research in Salitral de la Vidriera, Buenos Aires province, Argentina. Aerobiologia 24: 181-190. [ Links ]
22. Murray, M.G., C. Galán & C.B. Villamil (2010). Airborne pollen in Bahía Blanca, Argentina: seasonal distribution of pollen types. Aerobiologia 26: 195-207. [ Links ]
23. Newmark, F. (1979). The hay fever plants of Colorado. Ann Allergy 49: 18-24. [ Links ]
24. Nilsson, S. & J. Praglowski (1974). Pollen and spores incidence and phenology in the Stockholm area during 1972. Grana 14: 78-84. [ Links ]
25. Norris-Hill, J. (1997). The influence of ambient temperature on the abundance of Poaceae pollen. Aerobiologia 13: 91-97. [ Links ]
26. Raven, P.H. & G.B. Johnson (2001). Biology. McGraw Hill Higher Education: London. [ Links ]
27. Rayer, A. (2012). Producción polínica de Chenopodium album L. en la ciudad de Bahía Blanca. Tesis de Licenciatura en Ciencias Biológicas, Universidad Nacional del Sur, Argentina. [ Links ]
28. Rezanejad, F. (2009). Air pollution effects on structure proteins and flavonoids in pollen grains of Tuja orientalis L (Cupressaceae). Grana 48: 205-213. [ Links ]
29. Santra, S.C., S. Gupta & S. Chanda (1991). Air pollutants and aeroallergens interaction. Grana 30: 63-66. [ Links ]
30. Schägger, H. & V. Gebhard (1987). Tricine-Sodium Dodecyl Sulfate-Polyacrylamide gel Electrophoresis for the Separation of Proteins in the range from 1 to 100 kDa. Analitycal Biochemistry 166: 368-379. [ Links ]
31. Silva, Palacios I., R. Tormo Molina & A.F. Muñoz Rodríguez (2000). Influence of wind direction on pollen concentration in the atmosphere. International Journal of Biometeorology 44: 128-133. [ Links ]
32. Solomon, W.R. (2002). Airborne pollen: A brief life. Journal of Allergy Clinical Immunology 109: 895-900 [ Links ]
33. Tehrani, M., M. Sankian, M. Assarehzadegan, R. Falak, F. Jabbari & A. Varasteh (2010). Immunochemical Characterization of Amaranthus retroflexus Pollen Extract: Extensive Cross-reactive Allergenic Components among the Four Species of Amaranthaceae/Chenopodiaceae. Iran Journal of Allergy, Asthma and Immunology 9: 87-95. [ Links ]
34. Towbin, H., I. Staehlin & J. Gordon (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proceedings of the National Academy of Science 76: 4350-4354. [ Links ]
35. Varela, S., J. Subiza, J.L. Subiza, R. Rodríguez, B. García, M. Jerez, J.A. Jiménez & R. Panzani (2005). Platanus pollen as an important cause of pollinosis. Journal of Allergy and Clinical Immunology 100: 748-754. [ Links ]
36. Voller, A., A. Bartlett & D. Bidwell (1978). Enzyme immunoassays with special reference to ELISA techniques. Journal of Clinical Pathology 31: 507-520. [ Links ]
37. Zobel, A. & J.E. Nighswander (1991). Accumulation of phenolic compounds in the necrotic areas of Austrian and red pine needles after spraying with sulphuric acid: a possible bioindicator of air pollution. New Phytologist 117: 565-574. [ Links ]
38. Zuloaga, F.O. & O. Morrone (1999) Catálogo de las Plantas Vasculares de la República Argentina II. Vol. 74. St. Louis, Missouri: Monographs in Systematic Botany from the Missouri Botanical Garden. [ Links ]













