Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.1 Vicente López jun. 2014
ARTÍCULOS ORIGINALES
Volatile compounds of unifloral honey and floral nectar from Quillaja saponaria
Compuestos volátiles de miel monofloral y néctar floral de Quillaja saponaria
Santander F1, C Fredes1, G Nuñez1, G Casaubon2, MI Espinoza2, G Montenegro1
1 Department of Plant Science, School of Agronomy and Forestry.Pontificia Universidad Católica of Chile, Casilla 306-22, Santiago, Chile.
2 DICTUC Taste and Smell Center and ASIS‐UC Interdisciplinary Research Program on Tasty and Healthy Foods, Pontificia Universidad Católica of Chile, Casilla 306-22, Santiago, Chile.
Address Correspondence to: Francisca Santander, e-mail: f.santander.cadegan@gmail.com
Recibido / Received 20.XI.2012.
Aceptado / Accepted 15.VII.2013.
Abstract. Currently, the search for chemical markers related to the botanical origin of honey is an important issue because of its potential use as a complementary tool for melisopalinological analysis. The objective of this research was to compare the (1) volatile compounds of Quillaja saponaria Mol. (Fam. Quillajaceae) floral nectar with those of unifloral honey of this same species, and (2) volatile compounds in Q. saponaria honeys from the same geographical origin. For the identification and semiquantification of volatile compounds, Gas Chromatography with Mass Spectrometry (GC-MS) was performed. The nectar of Q. saponaria presented volatile compounds different from the compounds identified in its uniforal honey, which may be precursors of the compounds present in Q. saponaria honey. Ten volatile compounds were found in all the samples of Q. saponaria honey: 2-methyl butyric acid (2 – 21.6 µg/L), benzyl alcohol (1 – 6 µg/L), 2-phenylethanol (16 – 125.3 µg/L), ketoisophorone (2.6 – 15.9 µg/L), linalool (2.4 – 13.8 µg/L) and its oxides 1 and 2 (6 – 13.3 µg/L and 3 – 7 µg/L, respectively), ß-damascenone (4 - 12µg/L), pantolactone (2 – 7.5 µg/L) and furfural (7 – 44,2 µg/L). These compounds were common in unifloral honey with diferent floral sources from other countries. These results would indicate that Q. saponariahoney does not present specific volatile compounds that allow its clear diferentiation from other unifloral honey.
Keywords: Unifloral honey; Volatile compounds; SPME-GC-MS; Quillaja saponaria; Chile.
Resumen. Actualmente, la búsqueda de marcadores químicos relacionados con el origen botánico de las mieles es de gran interés debido a su uso potencial como una herramienta complementaria al análisis melisopalinológico. El objetivo de este estudio fue comparar (1) los contenidos de compuestos volátiles del néctar de Quillaja saponaria Mol. (Fam. Quillajaceae) con mieles monoforales de esta misma especie, y (2) los compuestos volátiles de mieles de Q. saponaria recolectadas provenientes del mismo origen geográfico. Para la identificación y semicuantificación de compuestos volátiles se utilizó Cromatografía de Gases con Espectrometría de Masa (GC-MS). El néctar de Q. saponaria presentó compuestos volátiles diferentes a los identificados en las mieles, los que podrían ser precursores de los compuestos presentes en dichas mieles. Diez compuestos volátiles fueron encontrados en las cinco mieles de Q. saponaria analizadas: ácido 2-metil butírico (2 – 21,6 µg/L), benzil alcohol (1 – 6 µg/L), 2-feniletanol (16 – 125,3µg/L), ketoisoforona (2,6 – 15,9 µg/L), linalool (2,4 – 13,8 µg/L ) y sus óxidos 1 y 2 (6 – 13,3 µg/L y 3 – 7 µg/L respectivamente), ß- damascenona (4 -12 µg/L), pantolactona (2 – 7,5 µg/L) y furfural (7 – 44,2µg/L), siendo estos compuestos comunes en mieles monoflorales de distintas fuentes florales descritas en mieles de otros países. Estos resultados indicarían que las mieles de Q. saponaria no presentan un tipo particular de compuestos volátiles que permitan diferenciarlas claramente respecto de otras mieles monoflorales.
Palabras clave: Miel monofloral; Compuestos volátiles; SPME-GC-MS; Quillaja saponaria; Chile.
INTRODUCTION
Honey aroma has been studied for several years due to its relation to organoleptic quality and authenticity (Pérez et al., 2002; Montenegro et al., 2008). Each unifloral honey has distinct aroma due to specific volatile compounds that may be derived from floral nectar; therefore, its organoleptic quality depends mainly on the origin foral source (Kaskoniene & Venskutonis, 2010). The differences in sensory properties of unifloral honey make possible to establish a relation between the main species present in honeys, and one or more compounds responsible for honey aroma and other volatile compounds identified by chemical analysis (Piasenzotto et al., 2003; Bogdanov et al., 2004; Manyi-Loh et al., 2011).
The volatile compounds could be used to determine the main floral source in unifloral honey, providing a tool to detect fraud by addition of pollen and misdescription of pollen grains (Radovic et al., 2001; Bogdanov & Martin, 2002; Arvanitoyannis et al., 2005). Furthermore, it has been reported that pollen grains of kanuka (Kunzea ericoides) and manuka (Leptospermum scoparium) are indistinguishable by melisopalinological analysis (Stephens et al., 2010); therefore, alternative tools have to be developed to identify the real botanical origin of this kind of unifloral honeys to guarantee honey authenticity.
This issue has been addressed in various studies, and several aromatic markers have been identified in different types of honey all over the world, like citrus honey from Greece, Spain and Italy; orange, strawberry-tree, eucalyptus and dandelion honey from Italy; salvia, false acacia and chestnut honey from Croatia; cambará honey from Brazil; fir, acacia and lavender honey from France and cotton honey from Greece. These analyses are commonly performed by Gas Chromatography/Mass Spectrophotometry (GC-MS) (Alissandrakis et al., 2003, 2005a, 2005b, 2007a, 2007b; Piasenzotto et al., 2003; Bentivenga et al., 2004; Bianchi et al., 2005; Moreira & De Maria, 2005; Jerkovic et al., 2006, 2007, Castro-Vázquez et al., 2007; Daher & Gülaçar, 2008).
In previous research, volatile composition analysis and sensory evaluation were performed on three types of native unifloral honey from Chile. This allowed the researchers to relate distinctive aroma descriptors in unifloral honey to the presence of terpenes, norisoprenoids, and phenolic derivatives. Nevertheless, researchers had difficulty recognizing aroma in Quillaja saponaria Mol. (Fam. Quillajaceae) honey, possibly due to the use of different types of unifloral Q. saponaria honey. However, it may also be due to the fact that the honey contains other floral influences (Montenegro et al., 2009).
Plant species have a particular chemical composition. Because honey comes from the flower nectar of certain species, finding a correlation between the chemical composition and the species that originated each particular honey would be expected.
Chile has a great diversity of native flora that can be used by bees (Apis mellifera L.) for honey production (Montenegro, 2002; Montenegro et al., 2010). Nevertheless, little information exists about the volatile compounds present in Chilean native honey, and information about the chemical composition of native floral nectar has not yet been described.
The objective of this research was to compare the (1) volatile compounds of Quillaja saponaria Mol. (Fam. Quillajaceae) floral nectar with those of unifloral honey of this same species, and (2) the volatile compounds of Q. saponaria honeys from the same geographical origin to establish possible volatile compounds as chemical markers for Q. saponaria honey.
MATERIALS AND METHODS
Nectar and honey samples. Floral nectar and honey samples were collected from four sampling sites (Petorca, Limache, Huayacán, and Alicahue) in the Valparaiso Region (32° 02' - 33° 57', V Region) in Central Chile (Fig. 1). Native vegetation was predominant in all of the sampling sites.
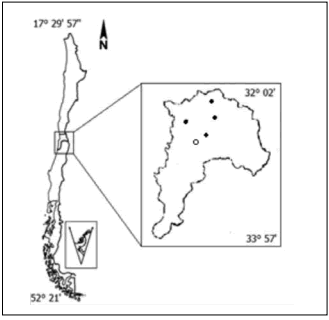
Fig. 1. Map of Valparaiso Region, Chile. Black circles indicate the origin of the Quillaja saponaria honey samples, the white circle indicates the origin of Quillaja saponaria nectar.
Fig. 1. Mapa de la Región de Valparaíso, Chile. Los puntos negros indican las zonas de origen de las muestras de miel de Quillaja saponaria, el círculo indica el origen del néctar de Quillaja saponaria.
During Q. saponaria flowering (November and December), a representative number of trees were identified and labeled. Plants with leaf damage were discarded. Floral nectar from Q. saponaria flowers (250 fowers) was taken randomly from 10 trees. The foral nectar was collected with glass capillary tubes as described by Southwick (1983) and Vidal et al. (2006).
During the corresponding harvest season, fifty-seven honey samples from the same geographical area were collected and studied using melisopalinological analysis to certify the Q. saponaria botanical origin as described in norm NCH 2981.OF2005 (Montenegro et al., 2008). The pollen grains were compared with a Pollen Grain Library for their identification. The data analysis was conducted using Statistical Analysis of Proportions, using maximum likelihood estimation to construct confidence intervals (Mead et al., 1993). Species that presented proportions lower than 0.03 (considering confidence interval) were discarded (Loveaux et al., 1978; Moar, 1985). Results and corresponding statistics are shown in Appendix 1 (Tables 6 to 62) (www.revistaphyton.fundromuloraggio.org.ar/vol83.html).
According to this analysis, five unifloral Q. saponaria honey samples were identified and selected for the chemical research. The botanical origin (percentage of Q. saponaria), and companion species of these honey samples are described in Table 1.
Table 1. Percentage of Quillaja saponaria presence in honey, and companion species.
Tabla 1. Porcentaje de presencia de Quillaja saponaria en miel, y especies acompañantes.

*At least 3% presence of companion species was considered.
Chemical analysis. One sample of floral nectar and five certified Q. saponaria honey samples were kept in a cold chamber before the chemical analysis. A 2 cm 50/30 µm DVB/Carboxen/PDMS StableFlex fiber (Supelco, Inc., Bellefonte, PA) was used for aroma extraction.
The honey solution plus sodium chloride (0.2 g) and internal standard (1 µL of 4-nonalol solution 3.460 mg/mL) were placed in a 20 mL vial tightly capped with a Teflon/silicone septum (catalog number S126-0020, I-CHEM). The sample was equilibrated at 35 °C in a water bath for 5 min and extracted after stirring for 1 hour at the same temperature. After extraction, SPME fiber was inserted into the injection port of the GC/MS to desorb the analytes. GC/MS analyses were carried out on a HP 6890 Gas Chromatograph, coupled to a 5972A MSD Hewlett Packard mass spectrometer, and equipped with a 60 m x 0.25 mm x 0.25 µm DB-WAXETR capillary column (J&W Scientific). The injector was set at 260°C and column oven temperature was held for 5 min at 40 °C, then raised 3 °C/min until it reached 240 °C, at which point it was held for 25 min. Mass spectra was obtained by electron impact ionization (70 eV) scanning a mass range of 35-350 m/z. The MS quadrupole and MS source temperatures were 150 °C and 220 °C, respectively. The spectrometric data was compared with those from NIST-EPA-NIH libraries (http://www.nist.gov/srd/nist1a.htm) that have more than 130000 entries. Semiquantitative analysis was carried for the volatile compounds identified.
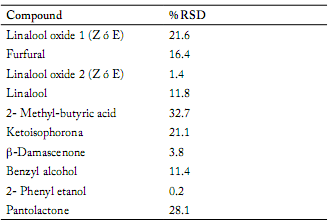
The repeatability of the extraction procedure was evaluated using nonalol as internal standard. The relative standard deviations (RSD) of the 10 common honey sample compounds are shown in Table 2.
Table 2. Relative standard deviation (% RSD) of 10 compounds obtained from a honey sample, estimated from 3 extractions.
Tabla 2. Desviación estándar relativa (% DER) de diez compuestos obtenidos de una muestra de miel, calculados a partir de 3 extracciones.
RESULTS AND DISCUSSION
The main factors that affect the composition of volatile compounds in honeys are: floral source, geographical origin, honey maturity, and harvest season (Gheldof & Engeseth, 2002; Kaskoniene & Venskutonis, 2010). Botanical origin seems to be the main factor (Kaskoniene & Venskutonis, 2010). The five Q. saponaria honey samples were selected from the same geographical area (V Region of Central Chile) during the same season (2011-2012). For these reasons, differences in the content of volatile compounds in Q. saponaria honey might be attributed mainly to the honey samples companion species and postharvest conditions.
Different methods of extraction for the determination of volatile compounds in honey by GC-MS have been described (Alissandrakis et al., 2003; Bianchi et al., 2005; Castro-Vázques et al., 2010). The use of CAR/PDMS columns has been described as an effective fiber for the isolation of aromatic compounds in honey (Plutowska et al., 2011), and was selected for this research. The RSD of the 10 common compounds in the five honey samples were found in a range of 0.2 - 32.7%. Similar values have been described by Alissandrakis et al. (2003) and Piasenzotto et al. (2003). Nevertheless, our results showed higher RSD for 2- methyl-butyric acid and pantolactone.
Volatile compounds in Q. saponaria nectar and comparison to its unifloral honey. An example of a Chromatogram obtained by GC-MS of floral nectar and the corresponding volatile compounds is shown in Figure 2.
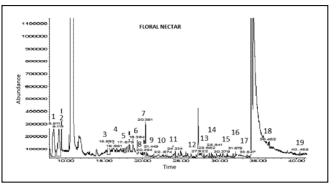
Fig. 2. GC-MS chromatogram of volatile compounds of Quillaja saponaria floral nectar. Peaks and corresponding retention time (RT): 1=ethyl acetate (RT: 8.511), 2=trimethyl acetate (RT: 9.114), 3= buthyl acetate (RT: 15.593), 4=ß-pinene (RT: 16.651), 5=methyl laurate (RT: 17.679), 6=hexanal dimethyl acetal (RT: 19.364), 7=limonene (RT: 20.381), 8=methyl caproate (RT: 20.494), 9= 1,8-cineole (RT: 21.449), 10=ethyl hexanoate (RT: 22.674), 11=cymene 1 (RT: 24.334), 12=6-methyl-5-hepten-2-one (RT: 27.922), 13=cymene 2 (RT:28.682), 14=octanal dimethyl acetal (RT: 28.941), 15=nonanal (RT: 30.379), 16=thujone (RT: 31.875), 17=nonanal dimethyl acetal (RT: 33.547), 18=benzaldehyde (RT: 36.462), 19=methyl benzoate (RT: 40.458).
Fig. 2. Cromatograma de GC-MS de los compuestos del nectar de flores. Peaks y tiempo de retención (TR): 1= etil acetato (TR: 8,511), 2=trimetil acetato (TR: 9,114), 3=butil acetato (TR: 15,593), 4=ß-pineno (TR: 16,651), 5=metil laurato (TR: 17,679), 6=hexanal dimetil acetal (TR: 19,364), 7=limoneno (TR: 20,381), 8=metil caproato (TR: 20,494), 9= 1,8-cineole (TR: 21,449), 10=etil hexanoato (TR: 22,674), 11=cymene 1 (TR: 24,334), 12=6-metil-5-hepten-2-one (TR: 27,922), 13=cymene 2 (TR: 28,682), 14=octanal dimetil acetal (TR: 28,941), 15=nonanal (TR: 30,379), 16=thujone (TR: 31,875), 17=nonanal dimetil acetal (TR: 33,547), 18=benzaldehído (TR: 36,462), 19=metil benzoate (TR: 40,458).
Floral nectar was mainly composed of esters in total concentrations of 189.0 µg/L; ethyl acetate, trimethyl acetate, buthyl acetate, methyl laurate, methyl caproate, ethyl hexanoate, methyl octanoate and methyl benzoate were identified. The group of second importance was terpenes, in concentrations of 80.5 µg/L; ß-pinene, limonene, 1,8-cineole, cymenene 1 and 2 and thujone were identified (Table 4, Fig. 2). These results are the first report of Q. saponaria nectar volatile composition.
The volatile compounds identified in the Q. saponaria nectar differ both in the type and relative concentration when compared to the five unifloral honey samples (Table 3, 4 and 5). Nevertheless, there were two compounds (nonanal and benzaldehyde) found in both nectar and honey of Q. saponaria. Nonanal was found in higher mean concentrations in honey (9.8 µg/L) than nectar (3.8 µg/L), whereas bezalde- hyde was found in higher concentration in nectar (9.6 µg/L) than honey (1.3 µg/L) (Table 5). These differences may be explained by the presence of compounds in nectar that could be precursors of other compounds present in honey, and agree with results obtained by other researchers (Rowland et al., 1995; Alissandrakis et al., 2003, Alissandrakis et al., 2007a, Alissandrakis et al., 2007b) where floral nectar and honey do not always share identical volatile compounds.
Table 3. Acid and alcohol composition (µg/L) of flower nectar and five Chilean unifloral honey samples of the same botanical origin (Quillaja saponaria) obtained via SPME-GC/MS.
Tabla 3. Composición de ácidos y alcoholes obtenidos mediante SPME-GC/MS de néctar de flores y cinco mieles monoflorales chilenas del mismo origen botánico (Quillaja saponaria).
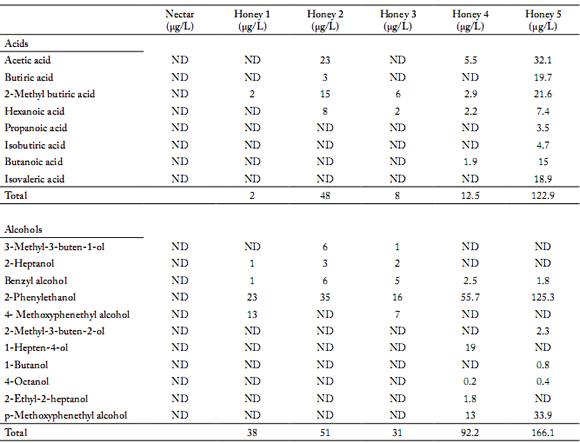
ND: Not detected
Table 4. Ketone, terpene, aldehyde and ester composition (µg/L) of flower nectar and five Chilean unifloral honey samples of the same botanical origin (Quillaja saponaria) obtained via SPME-GC/MS.
Tabla 4. Composición de cetonas, terpenos, aldehídos y ésteres obtenidos mediante SPME-GC/MS de néctar de flores y cinco mieles monoflorales chilenas del mismo origen botánico (Quillaja saponaria).


Table 5. Norisoprenoid, lactone, sulphide, furan and other composition (µg/L) of flower nectar and five Chilean unifloral honey samples of the same botanical origin (Quillaja saponaria) obtained via SPEME-GC/MS.
Tabla 5. Composición de norisoprenoides, lactonas, sulfidos, furanos y otros obtenidos mediante SPME-GC/MS de néctar de flores y cinco mieles monoflorales chilenas del mismo origen botánico (Quillaja saponaria).
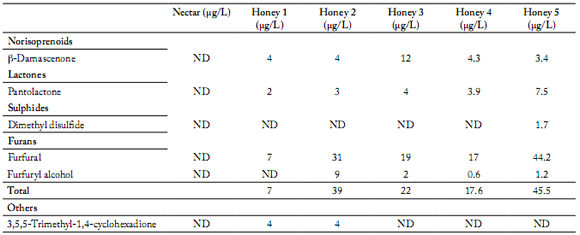
ND: Not detected.
Comparison of volatile compounds in Q. saponaria honey. An example of Chromatograms obtained by GC-MS of each Q. saponaria honey, and the corresponding volatile compounds, are shown in Figure 3.

Fig. 3. GC-MS chromatogram of volatile compounds of Quillaja saponaria honeys. Peaks and corresponding retention time (RT): 1= linalool oxide 1 (RT: 29.254 - 29.741), 2= furfural (RT: 30.344 - 30.818), 3= linalool oxide 2 (RT: 30.486 - 30.970), 4= linalool (RT: 30.486 - 30.970), 5= 2-methyl butyric acid (RT: 38.656 - 39.118), 6= ketoisophorone (RT: 39.535 - 40.099), 7= ß- damascenone (RT: 44.300 - 44.881), 8= benzyl alcohol (RT: 46.189 - 46.704), 9= 2-phenylethanol (RT: 47.405-47.943), 10= pantolactone (RT: 51.457-51.994).
Fig. 3. Cromatograma de GC-MS de los compuestos de miel de Quillaja saponaria. Peaks y tiempo de retención (TR): 1= óxido de linalool 1 (TR: 29,254-29,741), 2= furfural (TR: 30,344-30,818), 3= óxido de linalool 2 (TR: 30,486-30,970), 4= linalool (TR: 30,486-30,970), 5= ácido 2--metil butírico (TR: 38,656-39,118), 6= ketoisoforona (TR: 39,535-40,099), 7= ß- damascenona (TR: 44,300-44,881), 8= benzil alcohol (TR: 46,189-46,704), 9= 2-feniletanol (TR: 47,405-47,943), 10= pantolactona (TR: 51,457-51,994).
The volatile compounds identified in the five Q. saponaria honey samples differ both in type and relative concentration. In the five honey samples, 2-methyl butyric acid was found as the only type of acid in concentrations between 2.0 - 21.6 µg/L (Table 3). Among alcohols, benzyl alcohol and 2-phenylethanol were identified in concentrations between 1.0 - 6.0 µg/L and 16.0 - 125.5 µg/L, respectively (Table 3). Ketoisophorone was the only ketone identified in the five honey samples in concentrations between 2.6 – 15.9 µg/L (Table 4). Furans were identified in the form of furfural with concentrations that varied between 7.0 – 44.5 µg/L (Table 5). Linalool and oxide 1 and 2 were the only terpenes found in all fve samples with concentrations that oscillated between 6.0 – 13.3 µg/L, 3.0 – 7.0 µg/L and 3.0 – 13.8 µg/L, respectively (Table 4). One norisoprenoid was identified in the form of ß-damascenone in concentrations between 3.4 – 12.0 µg/L and one lactone was identified in the form of pantolactone in concentrations of 2.0 – 7.7 µg/L (Table 5).
These results could be explained by the different companion species in each honey. To be classified as a unifloral honey by melissopalinoligical analysis, at least 45% of the honey sample's pollen grain must come from a single species (Montenegro et al., 2008). The botanical origin results in this research indicate that the fve unifloral honey samples had a Q. saponaria percentage of between 55.3 – 87.2%, and the companion species oscillated between 22.8 - 44.7% (Table 1). An important variety of the companion species was identified in the five honey samples; native species (Schinus molle, Gentianella ottonis, Trevoa quinquinervia, Retanilla trinervia) predominated. According to our knowledge, the volatile compounds of these Chilean native species have not been described yet.
The nature of the honey used in most of this type of research makes it possible to easily correlate results of chemical composition among the different honey samples. This is because mostly cultivated species are used to obtain honey (citrus, lavender, eucalyptus, salvia, among others), which results in a very high percentage of the predominant species in each honey and a lower variability between honeys of the same botanical origin. This research, on the other hand, was focused on sampling sites where non-cultivated species (mostly native plants) predominated. This situation meant that there were a wider variety of species where honeybees collected nectar, resulting in a lower percentage of the predominant species.
Honey samples N° 1, 2, 4 and 5 had similar concentrations of ß-damascenone (Table 5). This compound has been previously described in rhododendron, buckwheat, thyme and citrus honey (Castro-Vásquez et al., 2007; Alissandrakis et al., 2007 b; Kaskoniene & Venskutonis, 2010).
Honey samples N° 1, 2, 3 and 4 had similar concentrations of pantolactone (Table 5), linalool and its oxides (Table 4) and benzil alcohol (Table 3). Linalool and its oxides have been described in citrus, thyme, quillay, Christ thorn, cotton, chestnut, heather, rape, sunflower, lavender, false acacia and acacia honey (Radovic et al., 2001; Alissandrakis et al., 2005b; Castro-Vásquez et al., 2007; Jerkovic et al., 2007; Montenegro et al., 2009; Jerkovic et al., 2009). Linalool has cytotoxic activity over fungus and several bacteria, which could be related to the antibacterial activity of this honey (Bakkali et al., 2008; Montenegro et al., 2009). Benzyl alcohol has been described in citrus, cotton, Christ thorn and false acacia honey (Alissandrakis et al., 2005b; Castro-Vásquez et al., 2007; Jerkovic et al., 2007; Jerkovic et al., 2009). Pantolactone has been found in chestnut honey and eucalyptus extracts, and honey from Spain (Castro-Vázquez et al., 2003; Castro-Vázquez et al., 2010). In the work of Castro-Vázquez et al. (2010), this compound was described as having a woody and toasty caramel taste.
Honey sample N°5 had higher contents of furfural and 2-phenylethanol (Tables 3 and 5) than the rest of the samples. Furans were detected in a significantly higher concentration in two out of the five honey samples analyzed (Table 5). Furan derivatives have been described as indicators of heat treatments during extraction and storage conditions. Since all honey used was harvested in the same season, these results might indicate that honey samples N°2 and N°5 were exposed to high temperatures during the extraction process and/or storage, which is congruent with the HMF content in honey N°5 (Alissandrakis et al., 2003; Piasenzotto et al., 2003). Moreover, honey N°5 presented high concentrations of 2-phenylethanol. This compound in high concentrations has been described in citrus, false acacia, Christ thorn and cambará honey (Piasenzotto et al., 2003; Alissandrakis et al., 2005a; Moreira & De Maria, 2005; Castro-Vázquez et al., 2007; Jerkovic et al., 2007; Jerkovic et al., 2009). Wani et al. (2010) reported that phenylethanol has antiseptic activity and this activity may also be associated with the antibacterial activity of Q. saponaria honey as reported by Montenegro et al. (2009). Phenylethanol has also been described as a characteristic compound of the first stage of the fermentation process in honey. The presence of 2-phenylethanol has been associated with exposure to high temperatures during the process of extraction and/ or storage (Vidrih & Hribar, 2007). Because of this, the high concentration of these compounds in honey samples N°4 and N°5 may be attributed to the inadequate storage after their harvest.
CONCLUSION
The chemical composition of Q. saponaria nectar was different than the volatile compounds found in Q. saponaria honey, and a relationship between them could not be established. This indicates that the volatile compounds of the floral nectar should be analyzed as precursor aroma compounds present in honey, thereby establishing a relationship between both types of compounds in order to use the chemical composition of nectar to support the search for volatile compounds as chemical markers of botanical origin.
The impact of companion species on the chemical composition of Q. saponaria honey was demonstrated by the variable volatile compositions found in the fve honey samples analyzed. Even though five unifloral honey samples shared ten volatile compounds, the variable concentration of these compounds made it difficult to establish a clear relationship between the Q. saponaria honey samples. Moreover, these common chemical compounds were also present in unifloral honey of other botanical origins. For this reason, a relationship between botanical origin and volatile composition could not be established. Given this, the search for chemical compounds as indicators of botanical origin in Q. saponaria honey should be complemented with the analysis of other types of chemical compounds present in this kind of unifloral honey.
ACKNOWLEDGEMENTS
This research was supported by Fondecyt Grant 1110808 to Gloria Montenegro.
REFERENCES
1. Alissandrakis, E., D. Daferera, P.A. Tarantilis, M. Polissiou & P.C. Harizanis (2003). Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chemistry 82: 575-582. [ Links ]
2. Alissandrakis, E., P.A. Tarantilis, P.C. Harizanis & M. Polissiou (2005a). Evaluation of four isolation techniques for honey aroma compounds. Journal of the Science of Food and Agriculture 85: 91-97. [ Links ]
3. Alissandrakis, E., A.C. Kibaris, P.A. Tarantilis, P.C. Harizanis & M. Polissiou (2005b). Flavour compounds of Greek cotton honey. Journal of the Science of Food and Agriculture 85: 1444-1452. [ Links ]
4. Alissandrakis, E., P.A. Tarantilis, P.C. Harizanis & M. Polissiou (2007a). Aroma investigation of unifloral Greek citrus honey using solid-phase microextraction coupled to gas chromatographic–mass spectrometric analysis. Food Chemistry 100: 396-404. [ Links ]
5. Alissandrakis, E., P.A. Tarantilis, P.C. Harizanis & M. Polissiou (2007b). Comparison of the volatile composition in thyme honeys from several origins in Greece. Journal of Agricultural and Food Chemistry 55: 8152-8157. [ Links ]
6. Arvanitoyannis, I.S., C. Chalhoub, P. Gotsiu, N. Lydakis-Simantiris & P. Kefalas (2005). Novel Quality Control Methods in Conjunction with Chemometrics (Multivariate Analysis) for Detecting Honey Authenticity. Critical Reviews in Food Science and Nutrition 45: 193–203. [ Links ]
7. Bakkali, F., S. Averbeck, D. Averbeck & M. Idaomar (2008). Biological effects of essential oils- a review. Food and Chemical Toxicology 46: 446-475. [ Links ]
8. Bentivenga, G., M. D´Auria, P. Fedeli, G. Mauriello & R. Racioppi (2004). SPME-GC-MS analysis of volatile organic compounds in honey from Basilicata: Evidence for the presence of pollutants from anthropogenic activities. International Journal of Food Science& Technology 29: 1079-1086. [ Links ]
9. Bianchi, F., M. Careri & M. Musci (2005). Volatile norisoprenoids as markers of botanical origin of Sardinian strawberry-tree (Arbustus unedo L.) honey: Characterization of aroma compounds by dynamic headspace extraction and gas chromatography-mass spectrometry. Food Chemistry 89: 527-532. [ Links ]
10. Bogdanov, S. & P. Martin (2002). Honey Authenticity: A Review. Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene 93: 232-254. [ Links ]
11. Bogdanov, S., K. Ruoffa & L. Persano-Oddo (2004). Physicochemical methods for characterization of unifloral honeys: a review. Apidologie 35: S4-S17. [ Links ]
12. Castro-Vázquez, L., M.S. Pérez-Coello & M.D. Cabezudo (2003). Analysis of volatile compounds of rosemary honey. Comparison of different extractions. Chromatographia 57: 227-233. [ Links ]
13. Castro-Vázquez, L., M.C. Díaz- Maroto & M.S. Pérez-Coello (2007). Aroma composition and new chemical markers of Spanish citrus honey. Food Chemistry 103: 601-606. [ Links ]
14. Castro-Vázquez, L., M.C. Díaz-Maroto, C. de Torres & M.S. Pérez-Coello (2010). Effect of geographical origin on the chemical and sensory characteristics of chestnut honeys. Food Research International 43: 2335-2340. [ Links ]
15. Daher, S. & F.O. Gülaçar (2008). Analysis of phenolic and other aromatic compounds in honeys by Solid-Phase Microextraction followed by Gas Chromatography-Mass Spectrometry. Journal of Agricultural and Food Chemistry 56: 5776-5780. [ Links ]
16. Gheldof, N. & N.J. Engeseth (2002). Antioxidant capacity of honey from various floral source based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. Journal of Agricultural and Food Chemistry 50: 3050-3055. [ Links ]
17. Jerkovic, I., J. Mastelic, Z. Marijanovic, Z. Klein & M. Jelic (2007). Comparison of hydrodistillation and ultrasonic solvent extraction for the isolation of volatile compounds from two unifloral honeys of Robinia pseudoacacia L. and Castanea sativa L. Ultrasonics Sonochemistry 14: 750-756. [ Links ]
18. Jerkovic, I., J. Mastelic & Z. Marijanovic (2006). A variety of volatile compounds as markers in unifloral honey from Dalmatian sage (Salvia officinalis L.). Chemistry and Biodiversity 3: 1307-1316. [ Links ]
19. Jerkovic, I., C.I.G. Tuberoso, Z. Marijanovic, M. Jelic & A. Kasum (2009). Headspace, volatile and semivolatile patterns of Paliurus spina-christi unifloral honey has marker of botanical origin. Food Chemistry 112: 239-245. [ Links ]
20. Kaskoniene, K. & P.R. Venskutonis (2010). Floral markers in honey of various botanical and geographical irigins: a review. Comprehensive Reviews in Food Science 9: 620-634. [ Links ]
21. Loveaux, J., A. Maurizio & G. Vorwhol (1978) Methods of melissopalynology. Bee World 59: 139-157. [ Links ]
22. Manyi-Loh, C.E., R.N. Ndip & A.M. Clarke (2011). Volatile compounds in honey: a review on their involvement in aroma, botanical origin determination and potential biomedical activities. International Journal of Molecular Sciences 12: 9514-9532. [ Links ]
23. Mead, R., R.N. Curnow & A.M. Halstead (1993). Statistical Methods in Agriculture and Experimental Biology, Chapman & Hall, London, UK. 415 p. [ Links ]
24. Moar, N.T. (1985) Pollen analysis of New Zealand honey. New Zealand Journal of Agricultural Research 28: 39-70. [ Links ]
25. Montenegro, G., M. Gómez, R. Pizarro, G. Casaubon & R. Peña (2008). Implementación de un panel sensorial para mieles chilenas. Ciencia e Investigación Agraria 35: 51-58. [ Links ]
26. Montenegro, G. (2002). Chile, nuestra flora útil. Guía de plantas de uso apícola alimentario, medicinal folclórico artesanal y ornamental. Ed. Universidad Católica de Chile. Santiago, Chile. 267 p. [ Links ]
27. Montenegro, G., S. Rodriguez, S. Vío, M. Gómez, P. Pizarro, A.M. Mujica & X. Ortega (2010). Investigación científica y tecnológica en productos apícolas. Fundación COPEC-Universidad Católica. Santiago, Chile. 240 p. [ Links ]
28. Montenegro, G., M. Gómez, J. Díaz-Forestier & R. Pizarro (2008). Aplicación de la Norma Chilena Oficial de denominación de origen botánico de la miel para la caracterización de la producción apícola. Ciencia e Investigación Agraria 35: 181-190. [ Links ]
29. Montenegro, G., M. Gómez, G. Casaubon, A. Belancic, A.M. Mujica & R.C. Peña (2009). Analysis of volatile compounds in three unifloral native Chilean honeys. Phyton, International Journal of Experimental Botany 78: 61-65. [ Links ]
30. Moreira, R.F.A. & C.A.B. De Maria (2005). Investigation of the aroma compounds from headspace and aqueous solution from cambará (Gochnatia velutina). Flavour Fragrance Journal 20: 13-17. [ Links ]
31. Muñoz, O., S. Copaja, H. Speisky, R. Peña & G. Montenegro (2007). Contenido de flavonoides y compuestos fenólicos de mieles chilenas e índice antioxidante. Quimica Nova 30: 848-851. [ Links ]
32. Pérez, R., C. Sánchez-Brunete, R. Calvo & J. Tadeo (2002). Analysis of volatiles from Spanish honeys by Solid-Phase Microextraction and Gas Chromatography--Mass Spectrometry. Journal of Agricultural and Food Chemistry 50: 2633-2637. [ Links ]
33. Piasenzotto, L., L. Gracco & L. Conte (2003). Solid phase microextraction (SPME) applied to honey quality control. Journal of the Science of Food and Agriculture 83: 1037-1044. [ Links ]
34. Plutowska, B., T. Chmiel & W. Wardencki (2011). A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chemistry 126: 1288-1298. [ Links ]
35. Radovic, B.S., M. Careri, A. Mangia, M. Musci, M. Gerboles & E. Ankleam (2001). Contribution of dynamic headspace GC-MS analysis of aroma compounds to authenticity testing of honey. Food Chemistry 72: 511-520. [ Links ]
36. Rowland, C.Y., A.J. Blackmand, B.R. D´Arcy & G.B. Rintoul (1995). Comparison of organic extractives found in leatherwood (Eucryphia lucida) honey and leatherwood fowers and leaves. Journal of Agricultural and Food Chemistry 43: 753-763. [ Links ]
37. Stephens, J.M., R.C. Schlothauer, B.D. Morris, D. Yang, L. Fearnley, D.R. Greenwood & K.M. Loomes (2010). Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chemistry 120: 78-86. [ Links ]
38. Southwick, E. (1983). Nectar biology and nectar feeders of common milkweed, Asclepias syriaca L. Bulletin of the Torrey Botanical Club 110: 324-334. [ Links ]
39. Vidal, M.G., D. Jong, H.C. Wien & R. Morse (2006). Nectar and pollen production in pumpkin (Cucurbita pepo L.). Revista Brasileira de Botanica 29: 267-273. [ Links ]
40. Vidrih, R. & J. Hribar (2007). Studies on the sensory properties of mead and the formation of aroma compounds related to the type of honey. Acta Alimentaria 36: 151-162. [ Links ]
41. Wani, M.A., K. Sanjana, D.M. Kumar & D.K. Lal (2010). GC – MS analysis reveals production of 2 – Phenylethanol from Aspergillus niger endophytic in rose. Journal of Basic Microbiology 50: 110-114. [ Links ]













