Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.1 Vicente López jun. 2014
ARTÍCULOS ORIGINALES
Endophytic fungi from Camellia sinensis show an antimicrobial activity against the rice blast pathogen Magnaporthe grisea
Hongos endofíticos de Camellia sinensis muestran una actividad antimicrobiana contra el tizón del arroz Magnaporthe grisea
Zhu XJ, YF Hu , X Chen, YH Wang, WP Fang, XH Li
Tea Science Research Institute, Nanjing Agricultural University, Nanjing 210095, P.R. China.
These authors contributed equally to this work.
Address Correspondence to: Xinghui Li, e-mail: lxh@njau.edu.cn
Recibido / Received 2.IX.2013.
Aceptado / Accepted 3.I.2014.
Abstract. The purpose of this study was to evaluate the antagonistic activity of two endophytic fungal strains, Pseudocercospora kaki and Penicillium sclerotiorum, isolated from the leaves of Camellia sinensis, against the rice blast pathogen Magnaporthe grisea. The inhibitory activity of the two endophytes against M. grisea in dual-culture was compared with that in monoculture. It was confirmed that the broth and its ethyl acetate extract of the dual-culture had a much stronger inhibition activity against M. grisea than the monocultures of P. kaki and P. sclerotiorum. The antagonism index of the broth and ethyl acetate extract from dual-culture to the mycelial growth of M. grisea was 78.02% ± 2.19% and 62.81% ± 2.29%, respectively, in different incubation periods. Qualitative analysis of ethyl acetate extract by GC-MS revealed that the number of bioactive compounds was greater in dual-culture than in monoculture. Compared to the ethyl acetate extract from monoculture, there were 10 constituents of bioactive compounds from dual-culture; however, there were only 6 types and 5 types from P. kaki monoculture and P. sclerotiorum monoculture, respectively. Glycerol; 4-Hydroxyphenyl ethanol; 1,2,3,4-Tetrahydroxy valeraldehyde and 1,2-Benzene-dicarboxylic acid dicyclohexyl ester were found in the ethyl acetate extracts of P. kaki, P. sclerotiorum and the dual culture. Based on the composition of the ethyl acetate extract of the dual culture of the endophytic fungi it might be possible to make a bioformulation for the biocontrol of the plant pathogen.
Keywords: Camellia sinensis; Endophytic fungi; Dual-culture; Antimicrobial activity; GC-MS.
Resumen. El propósito de este estudio fue evaluar la actividad antagonista de dos cepas de hongos endofíticos, Pseudocercospora kaki y Penicillium sclerotiorum, aislados de las hojas de Camellia sinensis, en relación al patógeno del tizón del arroz Magnaporthe grisea. La actividad inhibitoria de las dos cepas endófitas sobre M. grisea en cultivo dual se comparó con aquella en monocultivo. Se confirmó que el caldo y su extracto de etil acetato del cultivo dual tuvieron una actividad mucho más inhibitoria sobre M. grisea que los monocultivos de P. kaki y P. sclerotiorum. Los índices antagonistas del caldo y extracto de etil acetato del cultivo dual sobre el crecimiento del micelio de M. grisea fueron 78,02% ± 2,19% y 62,81% ± 2,29%, respectivamente, en diferentes períodos de incubación. El análisis cualitativo del extracto de etil acetato por GC-MS reveló que el número de compuestos bioactivos fue mayor en el cultivo dual que en el monocultivo. Comparado al extracto de etil acetato del monocultivo, hubo 10 compuestos bioactivos en el cultivo dual; sin embargo, hubo solo 6 tipos en el monocultivo de P. kaki, y 5 tipos en el monocultivo de P. sclerotiorum. Glicerol; 4-Hidroxifenil etanol; 1,2,3,4-tetrahidroxi valeraldehido y ester biciclohexil 1,2-benceno-ácido bicarboxílico se hallaron en los extractos de etil acetato de P. kaki, P. sclerotiorum y el cultivo dual. Basado en la composición del extracto de etil acetato del cultivo dual del hongo endofítico se podría hacer una formulación biológica para el control biológico del patógeno vegetal.
Palabras clave: Camellia sinensis; Hongos endofíticos; Cultivo dual; Actividad antimicrobiana; GC-MS.
INTRODUCTION
Rice blast, caused by Magnaporthe grisea B. Couch, is one of the most serious diseases that affect rice cultivation worldwide (Sesma, 2004; Park et al., 2008). This pathogen attacks both shoots and roots of the host plant. Chemical control methods of this disease can be expensive, ineffective and have a negative impact on both the environment and human health (Brent & Hollomon, 2007). A combination of high inoculum pressure, humid conditions that suit pathogen growth and frequent application of pesticides have resulted in the emergence of resistant pathogen strains (Takagaki et al., 2004; Yamada et al., 2004). Biological control, as a part of integrated pest management, has been suggested as the most suitable and sustained long-term solution.
Endophytic fungi are taxonomically and biologically diverse but all share a common characteristic of colonizing internal plant tissues without causing apparent harm to their host (Rodriguez et al., 2009). The definition of an endophyte has been broadened by many researchers, and can include now any organism that live in plant tissues whether the association is neutral, beneficial or detrimental (Sikora, 2007). Endophytic fungi may influence the plant metabolic state mainly through communication and transduction by contributingsharing genes and relevant bioactive products (Barrow et al., 2008). This pattern is thought to promote mutual relationships with the host plant. A parameter of mutual relationship, i.e. high colonization rates (62.00~100.00%) of endophytic fungi in Camellia sinensis (L.) O. Kuntze (tea plant), was obtained by Osono (2009). In recent studies, some endophytic fungi have received extensive attention. This is because they can produce resistant secondary metabolites with novel structure, and become a new and potential source of plant pest biocontrol factors (Backman & Sikora, 2008; Mejia et al., 2008). Though some endophytes are known to produce novel bioactive compounds with applicability in medicine and agriculture (Strobel, 2003), they are relatively understudied. During a screening of about 1000 endophytic fungal extracts for metabolites with activity against M. grisea, extracts of Alternaria spp., were found to inhibit appressorium formation of M. grisea without inhibition of spore germination and hyphal growth (Jeonl, 2010).
Many biological processes have to rely on two or more microorganisms, as they either cannot or can only be partially completed by a single species. Culturing different microbes together (dual-culture if two) forces direct interactions that may induce the production of compounds previously not observed when strains are cultured independently. The application of this dual-culture strategy represents a potentially important approach to the discovery of novel secondary metabolites (Holguin, 1996; Long, 2001; Oh, 2005).
Few studies on endophytic fungi from C. sinensis have been carried out compared with those made on other important economic crops. The aim of this study was to study the role of two endophytic fungi from C. sinensis against M. grisea, and to analyze the components of the secondary metabolites to characterize bioactive metabolites and understand their promising antifungal role.
MATERIALS AND METHODS
Fungal isolates. Two endophytic fungi, Pseudocercospora kaki and Penicillium sclerotiorum, isolated from leaves of tea plants, were obtained from the Tea Research Institute, Nanjing Agricultural University, China. The pathogenic M. grisea was collected from the Jiangsu Provincial Academy of Agricultural Sciences. Each fungal species was transferred from stored cultures to potato dextrose agar (PDA, 200 g of potato infusion, 20 g of dextrose, 20 g of agar in 1 L of distilled water at pH 5.6 ± 0.2) plates and cultured at 28 °C in the dark. Colonies 3-4 cm in diameter were obtained and used as inoculum for the experiments.
Antagonistic culture filtrate preparation and antagonism determination. Competitive interactions between P. kaki and P. sclerotiorum were evaluated in dual-culture experiments in Petri dishes (90 mm diameter) containing 20 mL of PDA under aseptic conditions. In each dish, two 5-mm diameter mycelial disks (one from P. kaki colonies, and one from P. sclerotiorum colonies) were placed on the agar surface 40 mm apart one from each other. Immediately after inoculation, the plates were sealed with plastic film and incubated in darkness at 28 °C for 12 days. With single fungal cultures as control, the experiment was repeated three times.
The inoculation proportion of P. kaki and P. sclerotiorum was kept in a ratio of 1:1, and then injected into the PD for next studies. Erlenmeyer flasks (150 mL) containing 100 mL of sterile PD were inoculated with two 12-mm disks of antagonistic fungus cut from the margin of preculture. The flasks were placed in a Gallenkamp orbital incubator (150 rpm) at 28 °C for 7 days. Single fungal culture was used as control. Te liquid cultures were filtered and sterilized through Stericup and Steritop (millipore) to remove hyphal fragments and conidia and to collect antagonist metabolites. To test the antagonistic activity, 10 mL of sterile PDA with a pH of 6.5 was poured into Petri dishes. The pH was adjusted with sodium hydroxide before autoclaving. Before its solidification, 5 mL of antagonist culture filtrate was added. After solidification, a 0.5 cm disk cut from the margin of preculture of M. grisea was placed at the centre of each plate. Control plates were added with 5 mL of distilled water. Each combination of culture filtrate and control was repeated three times. All plates were incubated at 28 °C in the dark and randomly distributed. Radial growth was recorded by measuring mean colony diameter at 4, 8, 11 and 14 days from study initiation, and required to reach the margin of the dish in controls. Fungal colony diameter was measured following the Criss-Cross Method. The antagonism Index (AI) was assessed according to the formula: AI= (RM-rm)/RM·100, where rm = ray of the colony towards the antagonist, and RM = average of the three rays of the colony in the other directions. The significance of the main efects and interaction effects of the antagonistic fungi was determined by ANOVA. Te antagonistic efects were compared using Duncan's multiple range test (p<0.05) (Campanile et al., 2007).
Ethyl acetate extract filtrate preparation and antagonism determination. To prepare the ethyl extract of P. kaki and P. sclerotiorum, fermentation broths were used after culturing them in separate and mixed media. Antifungal metabolites were extracted from 1.0 L of culture filtrates with ethyl acetate. Repeating the extraction once again with ethyl acetate, the elutes were dried in a rotational evaporator (<45 °C) and dissolved in 2 mL of ethyl acetate to the final concentrations of 1 mg/mL. Thereafter, they were sterilized through Stericup and Steritop (millipore). An agar plug of growing M. grisea (0.5 cm in diameter) was placed in the center of the PDA plate. Ethyl acetate extract filtrates of P. kaki and P. sclerotiorum, as well as dual-culture (100 µL) were placed into each of two wells surrounding the plug. The PDA plates were incubated at 28 °C for 5 days. Ethyl acetate was used as a negative control and the experiment was repeated three times. The antagonism index (AI) was assessed according to the formula mentioned above.
Gas Chromatography-Mass Spectrometry analysis. The ethyl acetate extract of the filtered fermentation broth was concentrated, and then the samples were transferred to GC vials. Then µL of N,O-bis(trimethylsilyl)-trifluoroacetamide (BSTFA) and 10 µL pyridine were added to them in each vial. The samples were then heated at 70 °C for 1 h. BSTFA and pyridine were then removed under the stream of nitrogen. With 100 µL of ethyl acetate added, the samples were loaded for gas chromatography-mass spectrometry (GC-MS) analysis. Wax components were separated using a 30 m, 0.32 mm HP-5 capillary column and helium as the carrying gas with constant flow of 2 mL/m in a GC-MS-AGILENT 6890/5975 (Plus) gas chromatography. GC was carried out with on-column injection temperature at 250 °C, oven temperature of 2 min at 40 °C, increasing at 40 °C /min to 220 °C, increasing at 10 °C/min to 290 °C, 20 min at 290 °C. The injection volume was 1.0 µL. Te composition was determined by comparing peak retention times with those of reference standards and by a GC-MS analysis of representative samples. The electron ionization system with ionization energy of 70eV was used. Compounds were identi?ed as trimethylsilyl (TMS) derivatives by comparing their mass spectra with the GC-MS spectral library (Willey 333.000) with data from the literature.
RESULTS
Interrelation of dual-culture experiments. At first, P. kaki and P. sclerotiorum were confronted on PDA for 12 days, using 5-mm diameter mycelial disks, in Petri dishes, and good compatibility with each other was observed. The identified volume in the ratio of 1:1 of P. kaki and P. sclerotiorum showed equal access to liquid medium by both. Then, dual-culture of P. kaki and P. sclerotiorum was cultured in the ratio of 1:1 for three days in PD. The culture medium's color turned light yellow (Fig. 1), and had a fragrance odor.

Fig. 1. Comparison of culture characteristics of dual-culture and monoculture of P. kaki and P. sclerotiorum incubated in potato dextrose liquid media. (A) Monoculture of P. kaki; (B) Dual-culture of P. kaki and P. sclerotiorum; (C) Monoculture of P. sclerotiorum.
Fig. 1. Comparación de las características del cultivo incubando P. kaki and P. sclerotiorum juntos o en monocultivo en medio líquido de papa dextrosa. (A) Monocultivo de P. kaki; (B) Cultivo mixto de P. kaki y P. sclerotiorum; (C) Monocultivo de P. sclerotiorum.
The inhibitory effect of different broths against M. grisea. The average colony diameter of M. grisea showed significant differences under the different culture broths used, and also varied with incubation time. Compared with the monoculture and control, the average colony diameter of M. grisea was smaller in dual-culture broth in the same incubation time (Table 1).
Table 1. Growth of M. grisea in different culture broths at various times.
Tabla 1. Crecimiento de M. grisea en diferentes caldos de cultivo en varios momentos.
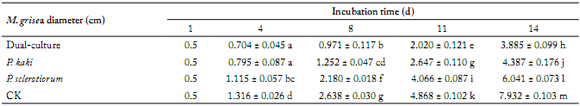
Note: Different letters indicate significant differences (p<0.05).
Nota: Letras diferentes indican diferencias significativas (p<0,05).
The colony diameter of M. grisea growing in different culture broths increased as the incubation time also increased (Fig. 2). The antagonism index was the largest against M. grisea when the dual-culture broth was used (78.02 ± 2.19%); the other two groups had different inhibitory effects, lower than those of the dual-culture. The antagonism index against M. grisea obtained by the dual-culture and P. kaki broths decreased as the incubation time increased (Fig. 2). However, when it was evaluated by the P. sclerotiorum broth, it was relatively stable and maintained at a lower level (Fig. 2). These results showed that the dual-culture exhibited a higher inhibitory effect against M. grisea in comparison to the monoculture broths, but the antagonism index decreased as the incubation time increased. It might be that the content of the active composition was reduced when the incubation time increased.

Fig. 2. Inhibitory effects of different broth on M. grisea growth. CK: Control (water); Monoculture of P. kaki; Monoculture of P. sclerotiorum; Dual-culture: P. kaki and P. sclerotiorum.
Fig. 2. Efectos inhibitorios de diferentes caldos de cultivo en el crecimiento de M. grisea. CK: Control (agua); Monocultivo de P. kaki; Monocultivo de P. sclerotiorum; Cultivo mixto: P. kaki y P. sclerotiorum.
The inhibitory effect of ethyl acetate extracts against M. grisea. The average colony diameter of M. grisea in the presence of ethyl acetate extract of dual-culture was only 1.319 ± 0.019 cm after 92 hours, almost half of that in the control. The antagonism index of the former was 62.81 ± 2.39%. However, the average colony diameters of M. grisea exposed to ethyl acetate extract of monoculture of P. kaki and P. sclerotiorum were 1.464 ± 0.050 cm and 2.056 ± 0.068 cm, respectively, and the antagonism indexes were 56.15 ± 3.40% and 29.29 ± 4.15%, respectively (Fig. 3). The ethyl acetate extracts of the monoculture of P. kaki and the dual-culture determined similar antagonism indexes. However, the antagonism index of P. sclerotiorum was significantly lower than that in P. kaki and dual-culture (Fig. 3). The dual-culture can increase the inhibition of M. grisea dramatically, compared with monocultures (Fig. 3).

Fig. 3. Inhibitory effect of ethyl acetate extracts from different broth on mycelia growth of M. grisea. CK: Control (ethyl acetate); Ethyl acetate extracts of P. kaki; Ethyl acetate extracts of P. sclerotiorum; Dual-culture: Ethyl acetate extracts of P. kaki and P. sclerotiorum.
Fig. 3. Efectos inhibitorios de extractos de etil acetato de varios caldos de cultivo en el crecimiento de micelio de M. grisea. CK: Control (etil acetato); Extractos de etil acetato de P. kaki; Extractos de etil acetato de P. sclerotiorum; Cultivo mixto: Extractos de etil acetato de P. kaki y P. sclerotiorum.
The compounds of ethyl acetate extract from culture filtrate by GC-MS analysis. The GC-MS running time for ethyl acetate extract of different culture filtrates was 38 min. The GC-MS Chromatogram of ethyl acetate extract of different culture filtrates is presented in Figure 4 in which waveforms are similar. These compounds were identified as a trimethylsilyl derivatives, by comparison with the equipment mass spectral library, and with literature data (Table 2).
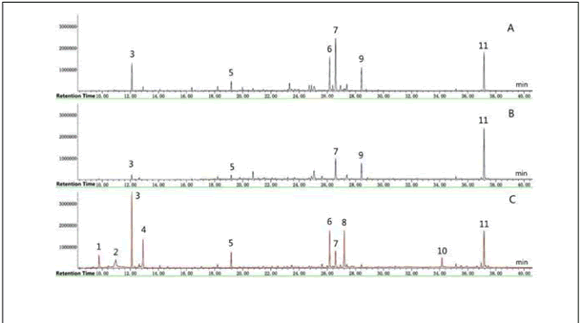
Fig. 4. GC-MS Chromatogram of the ethyl acetate fungal crude extract. (A) Ethyl acetate extracts of P. kaki. (B) Ethyl acetate extracts of P. sclerotiorum. (C) Ethyl acetate extracts of Dual-culture for P. kaki and P. sclerotiorum.
Fig. 4. Cromatograma de GC-MS del extracto crudo de etil acetate del hongo. (A) Extractos de etil acetato de P. kaki. (B) Extractos de etil acetato de P. sclerotiorum. (C) Extractos de etil acetato del cultivo mixto de P. kaki y P. sclerotiorum.
Table 2. Compounds identified in the ethyl acetate fungal crude extract.
Tabla 2. Compuestos identificados en el extracto crudo de etil acetate de hongos.

A total of 11 compounds were identified in the ethyl acetate extracts of different culture filtrates by GC-MS analysis. The active principles with their retention time (RT), molecular formula and concentration (%) are presented in Table 2. The prevailing compounds were Glycerol, 4-Hydroxyphenyl ethanol, 1,2,3,4-Tetrahydroxy valeraldehyde and 1,2-Benzenedicarboxylic acid dicyclohexyl, other major and minor constituents were also present. However, 5 kinds of compounds, which were 1,2,3-Butanetriol; Propionic acid; succinic acid; Dibutyl phthalate; Adipic acid, di(oct-4-yl ester) belonged to ethyl acetate from dual-culture filtrate.
DISCUSSION
Most previous studies using dual-culture techniques have provided evidence for the induction of antibiotic biosynthesis. Antibiotic activity has increased in extracts when microbial strains were cultured together; dual-culture has been applied to fnd new metabolites for drug development. In Cueto's (2001) study, the pestalone was produced by a cultured marine fungus only when a unicellular marine bacterium, strain CNJ-328, was dual-cultured in the fungal fermentation. It showed a potent antibiotic activity against methicillin-resistant Staphylococcus aureus and vancomycinresistant Enterococcus faecium. Strobel et al. (2008) reported that using endogenous fungal dual-culture or endogenous fungal metabolite mixture preparations produced a strong inhibitory efect, and the dual-culture of Muscodor vitis genus and Oidium sp. plus isobutyric acid produced an additive antibiosis efect against P. ultimum.
In previous studies, synergism because of dual-culture has increased when two fungi were initially inoculated in equal' numbers (Mellefont, 2008). In this research, compared with the monoculture, the characteristics of the dual-culture changed with incubation time. This indicates that the broth under the dual-culture was likely producing condition had possibly produced some new compounds. Daily examination under a stereomicroscope (Innocenti et al., 2002) would be the next step to confirm the colony growth and the type of interaction. Identi?cation and selection of effective antagonistic organisms are the?rst and foremost steps in biological control. Our?ndings showed that P. kaki and P. sclerotiorum demonstrated a signi?cant antagonistic activity towards M. grisea in dual culture (Fig. 2), when compared with their activities on monocultures. Moreover, growth of M. grisea was the lowest when the extract with ethyl acetate from the dualculture solution was applied (Table 1). This indicates that the dual culture might be an important source of active chemical components. Further research should focus on the isolation and purifcation of the bioactive compounds. Studies on the antagonistic effects of economically important crop species to other pathogenic fungi are needed. This might help in obtaining bioformulations to control them. Our research results demonstrated that it is also possible to look for new bioactive metabolites, obtained through mixed culture fermentation, by applying the improved techniques for screening and charactering these compounds.
ACKNOWLEDGEMENTS
This study was financially supported by the earmarked fund for Modern Agro-industry Technology Research System of China (CARS-23), Specialized Research Fund for the Doctoral Program of Higher Education (20130097120013), the Priority Academic Program Development of Jiangsu Higher Education Institutions, Science and Technology Plan of Jiangsu Province (BE2013426, BE2011319), Science and Technology Plan of Suzhou (SZGD201067), and Science and Technology Plan of Nanjing (201301076). Sunil Kumar Talor is acknowledged for his critical reading of our manuscript.
REFERENCES
1. Backman P.A. & R.A. Sikora (2008). Endophytes: an emerging tool for biological control. Biological Control 46: 1-3. [ Links ]
2. Barrow J.R., M.E. Lucero, I. Reyes-Vera & K.M. Havstad (2008). Do symbiotic microbes have a role in plant evolution, performance and response to stress? Communicative and Integrative Biology 1: 1-5. [ Links ]
3. Brent, K.J. & D.W. Hollomon (2007). Fungicide Resistance: The Assessment of Risk, 2nd eds. The Fungicide Resistance Committee (FRAC 2007), FRAC Monograph no.2. Essex, UK: Aimprint. [ Links ]
4. Campanile G., A. Ruscelli & N. Luisi (2007). Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. European Journal of Plant Pathology 117: 237-246. [ Links ]
5. Cueto, M., P.R. Jensen, C. Kauffman, W. Fenical, E. Lobkovsky & J. Clardy (2001). Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. Journal of Natural Products 64: 1444-1446. [ Links ]
6. Holguin, G. & Y. Bashan (1996). Nitrogen-fixation by Azospirillum brasilense Cd is promoted when co-cultured with a mangrove rhizosphere bacterium (Staphylococcus sp.). Soil Biology and Biochemistry 28: 1651-1660. [ Links ]
7. Innocenti, G., N.G. Garibyan & S.M. Badalyan (2002). Antagonistic activity of xylotrophic mushrooms against pathogenic fungi of cereals in dual culture. Phytopathologia Mediterranea 41: 200-225. [ Links ]
8. Jeonl, Y.T., K.H. Ryu, M.K. Kang et al. (2010). Alternariol Monomethyl Ether and a, ß-Dehydrocurvularin from Endophytic Fungi Alternaria spp. Inhibit Appressorium Formation of Magnaporthe grisea. Journal of Korean Society Applied Biological Chemistry 53: 39-42.
9. Long, R. & F. Azam (2001). Antagonistic Interactions among Marine Pelagic Bacteria. Applied and Environmental Microbiology 67: 4975-4983. [ Links ]
10. Mejia, L.C., E.I. Rojas, Z. Maynard, S. Van Bael, A.E. Arnold, P. Hebbar, G.J. Samuels, N. Robbins & E.A. Herre (2008). Endophytic fungi as biocontrol agents of Teobroma cacao pathogens. Biological Control 46: 4-14. [ Links ]
11. Mellefont, L., T. McMeekin & T. Ross (2008). Effect of relative inoculum concentration on Listeria monocytogenes growth in coculture. International Journal of Food Microbiology 121: 157-168. [ Links ]
12. Oh, D.C., P.R. Jensen, C.A. Kauffman & W. Fenical (2005). Libertellenones A-D: induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorganic and Medicinal Chemistry 13: 5267-5273. [ Links ]
13. Osono, T. & D. Hirose (2009). Effects of prior decomposition of Camellia japonica leaf litter by an endophytic fungus on the subsequent decomposition by fungal colonizers. Mycoscience 50: 52-55. [ Links ]
14. Park, D., M. Beckmann, D.P. Enot, D.P. Overy, Z.C. Rios, M. Gilbert, N. Talbot & J. Draper (2008). Rice blast infection of Brachypodium distachyon as a model system to study dynamic host ⁄pathogen interactions. Nature-Protocol 3: 435-445.
15. Rodriguez, R.J., J.F. White Jr, A.E. Arnold & R.S. Redman (2009). Fungal endophytes: diversity and functional roles. The New Phytologist 182: 314-330 [ Links ]
16. Sesma, A. & A.E. Osbourn (2004). The rice leaf blast pathogen undergoes developmental processes typical of root infecting fungi. Nature 431: 582-586. [ Links ]
17. Sikora, R.A., K. Schäfer & A.A. Dababat (2007). Modes of action associated with microbially induced in planta suppression of plant-parasitic nematodes. Australasian Plant Pathology 36: 124-134. [ Links ]
18. Strobel, G. (2003). Endophytes as sources of bioactive products. Microbes and Infection 5: 535-544. [ Links ]
19. Strobel, G.A., S. Spang, K. Kluck, W.H. Hess, J. Sears & T. Livinghouse (2008). Synergism among volatile organic compounds resulting in increased antibiosis in Oidium sp. FEMS Microbiological Letters 283: 140-145. [ Links ]
20. Takagaki, M., K. Kaku, S. Watanabe, K. Kawai, T. Shimizu et al. (2004). Mechanism of resistance to carpropamid in Magnaporthe grisea. Pest Management Science 60: 921-926. [ Links ]
21. Yamada, N., T. Motoyama, M. Nakasako, S. Kagabu, T. Kudo & I. Yamaguchi (2004). Enzymatic characterization of scytalone dehydratase Val75Met variant found in melanin biosynthesis dehydratase inhibitor (MBI-D) resistant strains of the rice blast fungus. Bioscience, Biotechnology and Biochemistry 68: 615-621. [ Links ]













