Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.1 Vicente López jun. 2014
ARTÍCULOS ORIGINALES
Azospirillum brasilense and Glomus intraradices co-inoculation stimulates growth and yield of cherry tomato under shadehouse conditions
La co-inoculación con Azospirillum brasilense y Glomus intraradices estimula el crecimiento y rendimiento de tomate cherry
Lira-Saldivar RH1, A Hernández1, LA Valdez2, A Cárdenas1, L Ibarra1, M Hernández3, N Ruiz4
1 Departamento de Agroplasticultura. Centro de Investigación en Química Aplicada. Saltillo, Coah., México. C.P. 25294.
2 Departamento de Horticultura, Universidad Autónoma Agraria Antonio Narro, Buenavista, Saltillo, Coah., México. C.P. 25315.
3 Departamento de Parasitología, Universidad Autónoma Agraria Antonio Narro, Buenavista, Saltillo, Coah., México. C.P. 25315.
4 Departamento de Tecnología de Semillas, Universidad Autónoma Agraria Antonio Narro, Buenavista, Saltillo, Coah., México. C.P. 25315.
Address Correspondence to: Dr. R. Hugo Lira-Saldivar, e-mail: hugo.lira@ciqa.edu.mx
Recibido / Received 22.IV.2013.
Aceptado /Accepted 11.VI.2013.
Abstract. The response of cherry tomato to biofertilization with beneficial microorganisms was evaluated under shadehouse conditions. Seeds were inoculated and/or co-inoculated with Azospirillum brasilense (Az) and/or Glomus intraradices (Gi). Thereafter, seedlings of six treatments received two applications of a suspension containing Az + Gi at 15 and 30 days after the transplant, and were compared against a non-inoculated treatment which only received conventional inorganic fertilization. Seed co-inoculation with A. brasilense and G. intraradices plus two applications of Az + Gi at 15 and 30 days after transplant increased on average 6% plant height, 11% leaf area, 10.5% dry biomass and 16% yield of cherry tomato in comparison to traditional fertilization.
Keywords: Arbuscular mycorrhiza; PGPR; Biofertilizers; Solanum lycopersicon.
Resumen. Se evaluó la respuesta del cultivo de tomate cherry a la biofertilización con microorganismos benéficos bajo condiciones de casasombra. Las semillas fueron inoculadas y co-inoculadas con Azospirillum brasilense (Az) y/o Glomus intraradices (Gi); después del trasplante, las plántulas de seis tratamientos recibieron dos aplicaciones de una solución conteniendo Az + Gi, y fueron comparadas contra el tratamiento testigo que solo recibió fertilización tradicional (NPK). La co-inoculación de las semillas con A. brasilense y G. intraradices más dos aplicaciones de Az + Gi a los 15 y 30 días después del trasplante, incrementaron en promedio 6% la altura de plantas, 11% el área foliar, 10,5% la biomasa seca y 16% el rendimiento de tomate cherry en comparación con la fertilización tradicional.
Palabras clave: Micorrizas arbusculares; PGPR; Biofertilizantes; Solanum lycopersicon.
INTRODUCTION
Cherry tomato (Solanum lycopersicon Mill.) is a novel crop for Mexican farmers that is becoming popular for cultivation under protected agriculture, including greenhouse (Hita et al., 2006) and shadehouse (Mantur & Sateesh, 2008) conditions. In recent years, shadehouses with insect-exclusion have become popular among tomato growers because of the lower need for pesticide application and decreased solar radiation intensity, which afects plants and fruits (Giordano et al., 2003). In México, shadehouses are economically convenient since its price is roughly one tenth of high-tech greenhouses. However, these highly productive controlled environments are also highly demanding of agricultural inputs such as chemical fertilizers.
Biofertilizers, on the other hand, are a natural product carrying living microorganisms derived from roots or cultivated soil; as a result, they do not have any ill effect on soil health and the environment. Besides their role in atmospheric N fixation and P solubilization, biofertilizers also stimulate the synthesis of plant hormones, which renders a better nutrient uptake and increased tolerance to drought and moisture stress. A small dose of biofertilizer is sufficient to produce desirable results because each gram of biofertilizer carrier contains at least 10 million viable cells of a specifc strain of beneficial organisms (Anandaraj & Delapierre, 2010).
Plant growth promoting rhizobacteria (PGPR) are utilized as inoculants for biofertilization and phytostimulation (Choudhary et al., 2009; Datta et al., 2011) and can play an important role in the prevention of plant infectious diseases (Dashti et al., 2009). Likewise, arbuscular mycorrhizal (AM) associations between fungi and higher plants may provide adequate P nutrition, increasing P assimilation and positively affecting nitrogenase activity, which in turn promotes root and mycorrhizal growth (Perner et al., 2007). The use of biofertilizers can improve the efficiency of greenhouse tomato production (Mihov & Tringovska, 2009), positively affect the yield and quality of other horticultural crops (Meir et al, 2010), prevent soil organic matter depletion and improve N and K assimilation (Cavalcante et al., 2012). Some of the microorganisms most commonly recognized as plant productivity enhancers include the free-living rhizobacterium Azospirillum brasilense (Correa et al., 2007) and the AM fungus Glomus intraradices (Rouphael et al., 2010).
In order to promote an enhancing effect of biofertilizers, reports (Yadav, 2002) indicate that, compared to single applications, multiple applications of beneficial microorganisms on some vegetables result in increased plant responses. Kifle and Laing (2011) found that a dose of 106 cfu mL/L of Bacillus cereus and B. subtilis applied weekly or every two weeks, had the best effect on growth of lettuce seedlings and plant N content, suggesting that the dose response depended on the frequency of application. In a similar way, Javaid (2011) evaluated beneficial microorganisms applied every 2 weeks throughout the experimental period on potted rice plants. Therefore, we considered that seed biofertilizer inoculation, and subsequent dual applications of PGPR and AM to seedlings, could improve the colonization of cherry tomato roots. Thus, the aim of this work was to compare the effect of conventional inorganic fertilization with biofertilization with A. brasilense and G. intraradices on plant growth, biomass production and total yield of cherry tomato plants grown under shadehouse conditions.
MATERIALS AND METHODS
The experiment was carried out in a shadehouse (12.5 m× 25 m × 5.2 m) during the spring and summer 2011 in Saltillo, México (101° 02´ N, 25° 27´ W, 1520 m.a.s.l.). The insectproof polyethylene net was made of high strength round monofilaments characterized by its lightweight, transparency, and UV stabilizers incorporated during the manufacturing process. The average annual temperature of the study area is 19.1 °C and the average annual rainfall is 437 mm. Soil texture of the experimental site is clay 47%, silt 32%, and sand 21%, with a pH of 8.2 and organic matter content of 2.08%. A soil sample taken from the 0-60 cm profile was analyzed before the experiment was initiated; in general, the concentration of mineral nutrients resulted very low on N, P, K, and S, low in Fe, moderately low in Mn, B, and Cu, medium in Ca and Zn, and very high in Mg. White-on-black plastic mulch with 7 cm wide holes was laid mechanically before two rows (0.30 m space between rows) of cherry tomato seedlings (cv. Camelia) transplanted on April 15. After transplanting, plants were drip irrigated as necessary with a tape (T-tape, John Deere, USA) having 30.5 cm spaced emitters and a 0.98 L/h fow, placed along the top center of the beds.
The experimental plot consisted of a 7-m-long, 0.9-m-wide bed formed on 1.8-m centers. Plant density was 3.7 plants per square meter, having a double row with 18 plants per bed, and a distance of 0.3m between plants. The experimental design was a randomized complete block with seven treatments and four replications: (1) 200-150-100 (NPK) kg/ha dose of fertilization; (2) independent inoculations to tomato seeds with commercial A. brasilense (AzoFer, Biofabrica, México), with an inoculum concentration of 1 x 107 ufc/mL; or (3) G. intraradices (MicorrizaFer Biofabrica, México) containing at least 2000 propagules/g; and four different co-inoculations with both, Azospirillum and Glomus, consisting in: (4) A. brasilense and G. intraradices inoculated to seeds before sowing (AsGs); (5) seed inoculation of A. brasilense and G. intraradices plus two applications of A. brasilense after transplanting (AsGs + Ap); (6) seed inoculation of A. brasilense and G. intraradices plus two applications of G. intraradices after transplanting (AsGs + Gp); and (7) seed inoculation of A. brasilense and G. intraradices plus two applications of A. brasilense and G. intraradices (AsGs + ApGp) after transplanting.
One of the goals of this experiment was to verify whether biofertilization could reach the benefits of conventional fertilization of cherry tomato. Consequently, in order to guarantee colonization of plant roots the seeds were coated with G. intraradices and A. brasilense, either alone or in dual inoculation. Plant inoculations were performed 15 and 30 days after transplanting (DAT) by mixing both microorganisms in an aqueous suspension and adding 5 mL at the base of each plant following the manufacturer's instructions (1.5 g of AzoFer and 3.0 g of MicorrizaFer per plant). No inorganic fertilizers were supplied to the inoculated plants. Since the purpose of this study was to evaluate the performance of biofertilizers compared against conventional fertilizers under a high-input culture system, an absolute control (i.e., without either bioor conventional fertilization) was not established. Growth parameters measured at 30, 60 and 90 DAT included plant height, leaf area (LI-COR LI-300, Lincoln, Nebraska, USA) and shoot biomass. Fruit yield was recorded throughout the experiment. Data were statistically analyzed using the general linear model procedure with SAS (SAS Institute Inc., Cary, NC) and means were separated using the Tukey´s multiple range test (p<0.05).
RESULTS
The different fertilization treatments applied to cherry tomato significantly affected growth and physiology of plants. Among the seven treatments, significant differences (p<0.05) were detected in plant height (Fig. 1) and leaf area (Fig. 2) on each of the sampling dates. Thirty days after transplanting, the NPK fertilization treatment and the double AsGs + ApGp biofertilization produced the tallest plants, while the G. intraradices seed inoculation produced the shortest plants. However, the highest leaf area (Fig. 2) was associated with the AsGs + Ap biofertilization, while the conventional fertilization produced the plants with the lowest leaf area. Sixty days after transplanting, the plants showing the maximum leaf area were those treated with Glomus and the AsGs + ApGp biofertilization, whereas the plants with the minimum foliar surface were those with the AsGs + Ap treatment (Fig. 2). At the same time, however, the treatment that produced the tallest plants (Fig. 1) was the seed single inoculation with A. brasilense, and the treatment showing the shortest plants was the NPK fertilization.
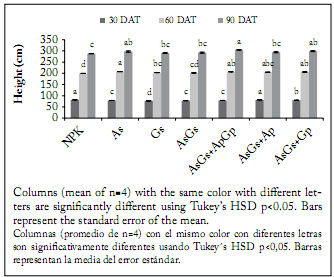
Fig. 1. Plant height of cherry tomato plants at 30, 60 and 90 days after transplanting (DAT) grown under different fertilization treatments. NPK = synthetic fertilization, As = seed inoculation with Azospirillum brasilense, Gs = seed inoculation with Glomus intraradices, Ap and Gp = plants inoculated twice (15 and 30 DAT) with A. brasilense and G. intraradices, respectively.
Fig. 1. Altura de plantas de tomate cherry a los 30, 60 y 90 días después del trasplante expuestas a diferentes tratamientos de fertilización. NPK = fertilización sintética, As = inoculación a la semilla con Azospirillum brasilense, Gs = inoculación a la semilla con Glomusintraradices, Ap y Gp = plantas inoculadas dos veces (15 y 30 DAT) con A. brasilense and G. intraradices, respectivamente.
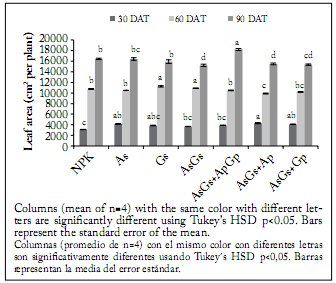
Fig. 2. Leaf area of cherry tomato plants at 30, 60 and 90 days after transplanting (DAT) and grown under different fertilization treatments. NPK = synthetic fertilization, As = seed inoculation with Azospirillum brasilense, Gs = seed inoculation with Glomus intraradices, Ap and Gp = plants inoculated twice (15 and 30 DAT) with A. brasilense and G. intraradices, respectively.
Fig. 2. Área foliar de plantas de tomate cherry a los 30, 60 y 90 días después del trasplante expuestas a diferentes tratamientos de fertilización. NPK = fertilización sintética, As = inoculación a la semilla con Azospirillum brasilense, Gs = inoculación a la semilla con Glomus intraradices, Ap y Gp = plantas inoculadas dos veces (15 y 30 DAT) con A. brasilense and G. intraradices, respectivamente.
At the final sampling date (90 DAT), the tallest plants were produced by the AsGs + ApGp treatment, while the shortest ones were generated by the NPK conventional fertilization. However, the plants with the largest leaf area belonged to the AsGs + ApGp treatment, while the plants with the smallest leaf area were those produced by the seed inoculation with A. brasilense plus G. intraradices and those obtained with the AsGs + Ap treatment. At the end of the experiment, leaf, stem, fruit, total shoot dry weight (Fig. 3) and yield (Fig. 4), were significantly affected by the treatments (p<0.05). The highest fruit production was observed in plants treated with the double AsGs + ApGp biofertilization. On the contrary, the treatments that induced the lowest dry biomass were those with seeds inoculated only with G. intraradices and A. brasilense plus G. intraradices; nevertheless, the treatment producing the lowest yield was the NPK fertilization. The plants also showed significant differences in whole-plant biomass accumulation when inoculation was made to seeds, and seeds and plants in comparison to plants treated with conventional fertilizers.
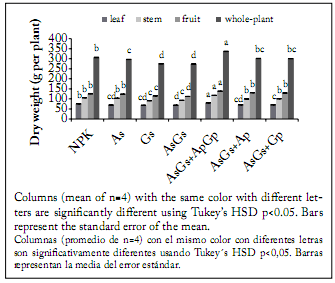
Fig. 3. Leaf, stem, fruit and whole-plant dry biomass of cherry tomato crop grown under different fertilization treatments. NPK = synthetic fertilization, As = seed inoculation with Azospirillum brasilense, Gs = seed inoculation with Glomus intraradices, Ap and Gp = plants inoculated twice (15 and 30 DAT) with A. brasilense and G. intraradices, respectively.
Fig. 3. Biomasa seca de hojas, tallo, frutos y de plantas de tomate cherry expuestas a diferentes tratamientos de fertilización. NPK = fertilización sintética, As = inoculación a la semilla con Azospirillum brasilense, Gs = inoculación a la semilla con Glomus intraradices, Ap y Gp = plantas inoculadas dos veces (15 y 30 DAT) con A. brasilense and G. intraradices, respectivamente.
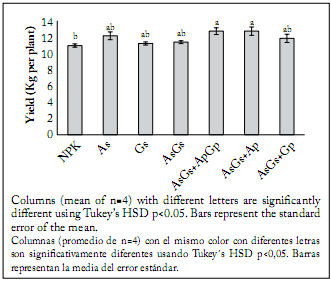
Fig. 4. Yield of cherry tomato plants grown under shadehouse with different fertilization treatments. NPK = synthetic fertilization, As = seed inoculation with Azospirillum brasilense, Gs= seed inoculation with Glomus intraradices, Ap and Gp = plants inoculated twice (15 and 30 DAT) with A. brasilense and G. intraradices, respectively.
Fig. 4. Rendimiento de plantas de tomate cherry desarrolladas bajo condiciones de casasombra con diferentes tratamientos de fertilización. NPK = fertilización sintética, As = inoculación a la semilla con Azospirillum brasilense, Gs = inoculación a la semilla con Glomus intraradices, Ap y Gp = plantas inoculadas dos veces (15 y 30 DAT) con A. brasilense and G. intraradices, respectivamente.
DISCUSSION
In the present study, the effectiveness of biologic fertilization, compared to mineral fertilization, on cherry tomato plants was assessed following two strategies: seed inoculation and/or seed-and-plant inoculation. Seed inoculation with either A. brasilense or G. intraradices, and seed co-inoculation produced shorter plants than conventional fertilization at 30 DAT. This response could be attributed to the effect of root colonization by the fungal partner. This is because it has been shown in some reports (Graham & Eissensat, 1998; Graham & Abbott, 2000) that early mycorrhizal establishment produces a slight growth delay of plant shoots. However, seed inoculated plants showed the same or higher height at 60 and 90 DAT than the control treatment (conventional fertilization). This result was also observed in the seed-and-plant inoculation treatments, except when A. brasilense and G. intraradices were inoculated twice in co-inoculation; this was because at 30 DAT the tomato plants showed no efect on height when compared to the conventional fertilization. These results suggest that soil nutrient availability was higher from synthetic fertilizers during the first 30 DAT than from nutrients provided through biofertilization. However, later (e.g., 60 and 90 DAT) the interaction of biofertilizers with roots allowed the plants to reach the efect of synthetic fertilization.
In this experiment, cherry tomato plants with the highest leaf area were found when the AsGs + ApGp treatment was applied. Plants with the smallest leaf area were those produced by only seed inoculation with A. brasilense plus G. intraradices and those obtained with the AsGs+Ap co-inoculation. It could be hypothesized that subsequent plant biofertilization adding G. intraradices will generate more leaf area than noninoculated plants. This further inoculation may have allowed a wider root colonization which in turn provided more nutrients (namely P) to the plant, resulting in an increased leaf area. This effect could be attributed to the fact that AM fungi (i.e., Glomus intraradices, Glomus mosseae and Glomus etunicatum) have the capacity to dissolve inorganic P and promote P nutrient availability by acidifying the soil and consequently facilitating the growth and development of the host plants (Shi et al., 2011). Thus, our results indicated that PGPR and AM applied to seed and plants significantly increased leaf area compared to the control treatment fertilized with NPK. Previous findings (Flores et al., 2010) pointed out that the PGPR A. brasilense has the potential to supply a considerable amount of N to pepper seedlings, as well as to stimulate plant growth and N uptake.
The increase in total biomass of cherry tomato plants could be attributed to the stimulating effect of A. brasilense and G. intraradices, since PGPR and AM fungi can positively impact plant growth as previously demonstrated (Hajiboland et al., 2010; Sabir et al., 2012). In a similar way, it was reported that tomato yield and its components were benefited with the inoculation of biofertilizers based on A. brasilense and A. chroococcum. This was because plants showed an increase from 12.6% to 51.9% dry matter compared to non-inoculated plants (Hernandez & Chailloux, 2004).
Positive effects of biofertilizers on growth of other horticultural crops have also been documented (Singh & Singh 2009). These authors reported that nitrogen fixing bacteria and bioregulators exhibit significant efect on the growth characters in strawberry plants. The maximum growth in terms of plant height, number of leaves, leaf area, crowns/plant and total biomass of strawberry plants was observed in the treatment consisting of Azotobacter + Azospirillium + 60 kg N/ha + 100 ppm GA3. This treatment was also associated with the highest chlorophyll content in leaves, maximum fruit set, yield and optimum fruit quality. On the other hand, Mihov and Tringovska (2010) studied the energy efficiency improvement of greenhouse tomato production by applying biofertilizers in a similar experiment. These authors reported that a commercial bacterial fertilizer (BioLife) and a mycorrhizal inoculum (Media Mix) increased the energy output-input ratio of yield to 1.19 and 1.11, respectively.
Regarding the plant growth-promoting activity of two Azospirillum strains, it has been reported that Azospirillum induced greater plant growth promotion on cherry tomato than on fresh-market tomato; on the other hand, leaf and plantdeath of cherry tomato plants were delayed on Azospirillumtreated plants compared with non-treated controls (Romero et al., 2003).
CONCLUSIONS
Compared to conventional chemical fertilization, biofertilizers increased plant growth, biomass production and yield. Thus, the incorporation of such beneficial microorganisms was positive for plant development producing a yield as high as that obtained with the use of mineral fertilizers under shadehouse conditions.
Biofertilizers based on A. brasilense and G. intraradices affected growth and yield of tomato. Overall, plant growth and yield increased by biofertilizer co-inoculation in comparison to single inoculation with either beneficial microorganism, suggesting a synergic effect.
Seed inoculation plus one application of both A. brasilense and G. intraradices to seedlings at 15 days after transplanting may improve the beneficial effect on cherry tomato growth and yield.
The use of biofertilizers is a promissory practice among the organic or sustainable production systems of cherry tomato.
More research that integrates knowledge of single inoculation and co-inoculation of arbuscular mycorrhiza and rhizobacteria on cherry tomato crop is needed.
REFERENCES
1. Anandaraj, B & L.R.A. Delapierre (2010). Studies on influence of bioinoculants (Pseudomonas fluorescens, Rhizobium sp., Bacillus megaterium) in green gram. Journal of Bioscience and Technology 1: 95-99. [ Links ]
2. Cavalcante, L.F., I.H.L. Cavalcante, F. Rodolfo, M.Z. Beckmann-Cavalcante & G.P. dos Santos (2012). Leaf-macronutrient status and fruit yield of biofertilized yellow passion fruit plants. Journal of Plant Nutrition 35: 176-191. [ Links ]
3. Correa, O.S., A.M. Romero, M.S. Montecchia & M.A. Soria (2007). Tomato genotype and Azospirillum inoculation modulate the changes in bacterial communities associated with roots and leaves. Journal of Applied Microbiology 3: 781-786. [ Links ]
4. Choudhary, D.K., A. Prakash, V. Wray & N.J. Bhavdish (2009). Insights of the fluorescent pseudomonads in plant growth regulation. Current Science 97: 170-179. [ Links ]
5. Dashti, N.H., M.S. Montasser, N.Y. Ali, R.G. Bhardwaj & D.L. Smith (2007). Nitrogen biofixing bacteria compensate for the yield loss caused by viral satellite RNA associated with cucumber mosaic virus in tomato. Plant Pathology Journal 23: 90-96. [ Links ]
6. Datta, M., R. Palit, C. Sengupta, M.K. Pandit & S. Banerjee (2011). Plant growth promoting rhizobacteria enhance growth and yield of chilli (Capsicum annuum L.) under field conditions. Australian Journal of Crop Science 5: 531-536. [ Links ]
7. Flores, P., J. Fenolla, P. Hellina & P. Aparicio-Tejo (2010). Isotopic evidence of significant assimilation of atmospheric-derived nitrogen fixed by Azospirillum brasilense co-inoculated with phosphate-solubilising Pantoea dispersa in pepper seedling. Applied Soil Ecology 46: 335-340 [ Links ]
8. Giordano, I., A. Pentagelo, G. Graziani & V. Fogliano (2003). Effects of plastic screens on virus infection yield and qualitative characteristics of small tomatoes. Acta Horticulturae 614: 735-740. [ Links ]
9. Graham, J.H. y D.M. Eissenstat (1998). Field evidence for the carbon cost of citrus mycorrhizas. New Phytologist 140: 103-110. [ Links ]
10. Graham, J.H. & L.K. Abbott (2000). Wheat responses to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant and Soil 220: 207-218. [ Links ]
11. Hajiboland, R., N. Aliasgharzadeh, S.F. Laiegh & C. Poschenrieder (2010). Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant and Soil 331: 313-327. [ Links ]
12. Hernández, I.M. & M. Chailloux (2004). Las micorrizas arbusculares y las bacterias rizosféricas como alternativa a la nutrición mineral del tomate. Cultivos Tropicales 25: 5-12. [ Links ]
13. Hita, O., I. Romacho, T. Soriano, M.I. Morales, I. Escobar y E.M. Suárez-Rey (2006). Comparison of two Mediterranean green- house technological packets with cherry tomato. ISHS I. S: Advances in Soil & soilless Cultivation under Protected Environment Agadir, Morocco. February 19-24. [ Links ]
14. Javaid, A. (2011). Efects of biofertilizers combined with different soil amendments on potted rice plants. Chilean Journal of Agricultural Research 71: 157-163. [ Links ]
15. Kifle, M.H. & M.D. Laing. (2011). Determination of optimum dose and frequency of application of free-living diazotrophs (FLD) on lettuce. African Journal of Agricultural Research 6: 671-675. [ Links ]
16. Mantur, S.M. & R.P. Sateesh (2008). Influence of spacing and pruning on yield of tomato grown under shadehouse. Karnataka Journal of Agricultural Science 21: 97-98. [ Links ]
17. Mihov, M. y I. Tringovska (2010). Energy efficiency improvement of greenhouse tomato production by applying new biofertilizers. Bulgarian Journal of Agricultural Science 16: 454-458. [ Links ]
18. Perner, H., D. Schwarz, C. Bruns, P. Mäder & E. George (2007). Effect of arbuscular mycorrhizal colonization and two levels of compost supply on nutrient uptake and fowering of pelargonium plants. Mycorrhiza 17: 469-474. [ Links ]
19. Romero, A.M., O.S. Correa, S. Moccia & J.G. Rivas (2003). Effect of Azospirillum-mediated plant growth promotion on the development of bacterial diseases on fresh-market and cherry tomato. Journal of Applied Microbiology 4: 832-838. [ Links ]
20. Rouphael, Y., M. Cardarelli, E. Di Mattia, M. Tullio, E. Rea & G. Colla (2010). Enhancement of alkalinity tolerance in two cucumber genotypes inoculated with an arbuscular mycorrhizal biofertilizer containing Glomus intraradices. Biology and Fertility of Soils 46: 499-509. [ Links ]
21. Sabir, A, M.A. Yazici, Z. Kara & F. Sahin (2012). Growth and mineral acquisition response of grapevine rootstocks (Vitis spp.) to inoculation with different strains of plant growth-promoting rhizobacteria (PGPR). Journal of the Science of Food and Agriculture 92: 2148-2153. [ Links ]
22. Shi, Z., F. Wang, C. Zhang & Z. Yang (2011). Exploitation of phosphorus patches with diferent phosphorus enrichment by three arbuscular mycorrhizal fungi. Journal of Plant Nutrition 34: 1096-1106. [ Links ]
23. Singh, A. & J.N. Singh (2009). Effect of biofertilizers and bioregulators on growth, yield and nutrient status of strawberry cv. Sweet Charlie. Indian Journal of Horticulture 66: 220-224. [ Links ]
24. Yadav, S.P. (2002). Performance of effective microorganisms (EM) on growth and yields of selected vegetables. Nature Farming & Environment 1: 35-38. [ Links ]













