Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.1 Vicente López jun. 2014
ARTÍCULOS ORIGINALES
Polyphenolic profile of Origanum vulgare L. ssp. viridulum from Argentina
Perfil de polifenoles de Origanum vulgare L. ssp. viridulum de Argentina
González MD, CM Luis, PL Lanzelotti
Departamento de Ciencias Básicas, Universidad Nacional de Luján. Cruce Rutas 5 y 7, Luján. C.P. 6700. Provincia de Buenos Aires, Argentina.
Address Correspondence to: María Dolores González, e-mail: doloresg@unlu.edu.ar
Recibido / Received 22.III.2013.
Aceptado / Accepted 7.XII.2013.
Abstract. Characterization of oregano in Argentina is limited. They are normally known with fancy names, but it is not possible to recognize whether they are genetically equivalent or not. This paper presents a non-volatile polyphenol study of one of the subspecies of Origanum vulgare, O. vulgare L. ssp. viridulum (Martrin-Donos) Nyman [= O. vulgare L. ssp. virens (Hoffmannsegg et Link) Ietswart]. The polyphenols 3-(3,4-dihydroxyphenyl)lactic acid, 3,4-dihydroxybenzoic acid, caffeic acid, 4-(3.4-dihydroxybenzoyloxymethyl)phenyl ß-glucoside and rosmarinic acid were isolated and identified by spectroscopic analysis from leaves and inflorescences of classified material. In addition, the polyphenol profile of the hydroalcoholic extract of this oregano was developed applying high performance liquid chromatography (HPLC) with diode array detector (DAD), and the identified compounds were located in the profile. The observation of the instrumental UV spectra revealed that this subspecies is also rich in flavone derivatives such as apigenin and luteolin glycosides. The profile found appears repeatedly in other samples of the same variety with diferent geographic origins, while differences were observed in profiles of other taxonomical groups of oreganos. This chemical profile is expected to be an additional support for botanists who diferentiate origanum materials, especially when most of the plant features have been lost in the culinary herb.
Keywords: Origanum vulgare L. ssp. viridulum; Polyphenols; Phenyl glucosides; Flavone glycosides; HPLC-DAD profile.
Resumen. La escasa caracterización de los oréganos argentinos hace que se los denomine en general con nombres de fantasía, sin saber si son o no genéticamente equivalentes. En este trabajo se presenta el estudio de los polifenoles no volátiles de una de las subespecies de Origanum vulgare L., O. vulgare L. ssp. viridulum (Martrin-Donos) Nyman [= O. vulgare L. ssp. virens (Hofmannsegg et Link) Ietswart]. De hojas e inflorescencias de material clasificado, se aislaron y se identificaron por análisis espectroscópico los polifenoles ácido 3-(3,4-dihidroxifenil)láctico, ácido 3,4-dihidroxibenzoico,ácido cafeico, 4 -(3,4-dihidroxibenzoiloximetil)fenil ß-glucósido y ácido rosmarínico. Además, se desarrolló el perfil analítico de los polifenoles del extracto hidroalcohólico de este orégano, aplicando cromatografía líquida de alta resolución (CLAR) y detector con arreglo de diodos (DAD) y los compuestos identificados se localizaron en el perfil. La observación de los espectros UV generados por el instrumento, reveló que esta subespecie también es rica en derivados de flavonas tales como glicósidos de apigenina y luteolina. El perfil encontrado apareció repetidamente en muestras de la misma variedad con diferentes orígenes geográficos, y a la vez presenta diferencias con el de otros grupos taxonómicos de oréganos. Se espera que este perfil químico sea un aporte adicional a los botánicos que diferencian materiales de orégano, especialmente cuando la mayoría de las características de la planta se han perdido en la especia comercial.
Palabras clave: Origanum vulgare L. ssp. viridulum; Polifenoles; Fenil glucósidos; Glicósidos de flavonas; Perfil HPLC-DAD.
INTRODUCTION
Several taxonomic groups belonging to Origanum genus (among species, subspecies and hybrids) have been reported as cultivated in Argentine (Xifreda, 1983; Rouquaud & Videla, 2000). However, different reports have assigned different botanical names to the same type of "oregano". That reveals not only the complexity of the genus but also the lack of knowledge we have on taxonomic aspects of the production (Argüello et al., 2012). In turn, fantasy names are used for cultivated varieties without taking into account whether they are genetically equivalent or not (Farías et al., 2010).
In Argentina, many efforts are being done to rationalize and improve "oregano" spice production. Growing internal and external commercial demands require the improvement of yields and herb quality. The Agricultural and Technology National Institute (INTA) proposed an Oregano National Web for agricultural studies. Cordoba University groups are also working in the same direction (Torres et al., 2010; Argüello et al., 2012). Other valuable efforts are Germoplasm banks which have established morphological and quality descriptors of Origanum spp. (Farías et al., 2010). Essential oil yield and composition have also been studied (Dambolena et al., 2010; Farías et al., 2010), but there are few studies about non volatile fractions of Argentine oreganos.
Many works in Origanum spp. extracts have reported antioxidant, antimicrobial, anti-inflammatory and cell damaging preventive activities (Shan et al., 2005; Yoshino et al., 2006; Ibrahim et al., 2010). Polyphenolic compounds have been found to be the active principles: rosmarinic acid (Petersend & Simmons, 2003) and other caffeic acid esters (Hu et al., 2005), other phenolic acids, (Kikuzaki & Nakatani, 1989; Hossain et al., 2010) and phenyl glycosides (Nakatani & Kikuzaki, 1987; Koukoulitsa et al., 2006). Flavonoids and their glycosides (Hussein et al., 1982; Fecka & Turek, 2008; Chatzopoulou et al., 2010)) have been identified in different oreganos.
The main purpose of this work was to report the identity of major polyphenolics in leaf and inflorescence extracts of O. vulgare L. ssp. viridulum (= O. vulgare L. ssp. virens) derived from a clone preserved in Argentine Littoral Germplasm Bank. Botanical descriptors like leaf shape, contour and base, calix shape and surface, floral bract size, shape and surface, inflorescence type and location have been established as characteristic descriptors on this clone (Farías et al., 2010).
Another aim was to develop a method to recognize those compounds in different plant materials. High performance liquid chromatography linked with diode array detection (HPLC-DAD) has proved to be a useful technique for herbal identification (Cai et al., 2012). In this study each one of the identified compounds has been located as the corresponding peak on an optimized HPLC - DAD chromatogram of a polar extract of this oregano.
Knowing the chemical composition of Argentine oreganos is important to (1) make decisions about production and (2) get quality standards in our country and worldwide. A chromatographic method was applied to find a chemical profile that may be used both (1) as a descriptor (with complementary information for oregano variety discrimination) and (2) to control results in managing studies (Treutter, 2010). Polyphenols have been pointed out as chemical markers in some species and their profiles seem to be less sensitive to environmental influences than to essential oil composition (Sztefanov et al., 2003).
MATERIALS AND METHODS
Plant material. Origanum vulgare L. ssp. viridulum (Martrin-Donos) Nyman [= O. vulgare L. ssp. virens (Hoffmannsegg et Link) Ietswaart] was kindly provided by Ing. Agr. Otto Brutti. It belongs to the Argentine Littoral Germplasm Bank collection (Escuela Agrotécnica "Las Delicias", Secretaría de Producción de Entre Ríos y Facultad de Ciencias Agropecuarias, UNER). This plant material is preserved there. It was identified as O. vulgare L. ssp. virens (Hofmannsegg et Link) Ietswart, according to calix shape and bract size, among other features (Ietswaart, 1980).
Other four tested samples were bought from plant growers in Buenos Aires province looking for descriptors established for O. vulgare L. ssp. virens. Afterwards, they were observed and confirmed according to calyx and bract shapes, sizes and surfaces (Ietswaart, 1980; Farías et al. 2010). A sample of O. vulgare L ssp. virens classified by Ing. Agr. Xifreda was provided by Dr. Beatriz Varela (Facultad de Farmacia y Bioquímica - Universidad de Buenos Aires).
Compound isolation and identification. All chemicals and solvents used were analytical grade. Silica gel 60 (0.040-0.063 mm) (for column chromatography) and TLC Silica gel 60 F254 Aluminium sheets were obtained from Merck (Darmstadt, Germany); Sephadex LH-20 was obtained from Pharmacia Biotech (Uppsala, Sweden).
Air dried, destemming and crushed flowering buds of O. vulgare L. ssp. viridulum were refluxed for 1 hour with H2O/MeOH 6:4. Methanol was evaporated in vacuo and the water residue was successively subjected to ethyl acetate and n-butanol extraction, frst at pH 8, thereafter at pH 3. Ethyl acetate extract at pH 3 was evaporated and fractionated by column chromatography over silica gel (eluted with chloroform-ethyl acetate - methanol continuous gradient). After comparison by thin layer chromatography (TLC) analysis with anisaldehide/sulfuric acid or 3% ferric chloride in methanol as chromogenic reactives, nine main fractions were obtained. Compounds (2) (0.075 g) and (3) (0.010 g) were purified from fraction 3 by rechromatography. Compound (11) (0.150 g) was purified from fraction 6. Compound (1) was isolated from more polar fractions (F 8) after repurification on Sephadex LH-20 column chromatography. Butanolic acid extract at pH 3 residue was fractioned on column chromatography on silica gel (ethyl acetate-methanol-water gradient). Compounds (11) and (1) were also found here in the early fractions. Compound (6a) eluted in more polar fractions. Isolation of 6a was obtained after repurification with Sephadex LH-20 column with methanol (0.180 g). Compound 6a (0.080 g) was acetylated with acetic anhydride in pyridine and then purified on column chromatography on silica gel (Hexane-chloroform gradient) to yield 0.040 g of Compound 6b (peracetate derivative of 6a) (Fig.1).

Fig. 1. Main polyphenolics of O. vulgare L. ssp. viridulum (= O. vulgare L. ssp. virens) hydroalcoholic extract.
Fig. 1. Principales polifenoles del extracto hidroalcóholico de O. vulgare L. ssp. viridulum (= O. vulgare L. ssp. virens).
UV spectra and absorptions were recorded on a Shimadzu PR-1 spectrophotometer. 1H-NMR and 13C-NMR spectra were recorded on a Bruker AC 300 NMR spectrometer (Facultad de Farmacia y Bioquímica-Universidad de Buenos Aires).
High Performance Liquid Cromatography analysis. A HPLC Konik 500-A system with UV detection was used for routine investigation and a HPLC WATERS ALLIANCE 2695 system (Waters Corp., MA, USA), equipped with autosampler and a Photodiode Array Detector (PDA 2996) was used to obtain online UV spectra of each peak. The separation was performed on a Phenomenex Luna 5 µ C18 (2) 100 A (250 mm × 4.6 mm, 5 µm) column for both systems. The mobile phase was: (A) formic acid 0.25% in water and (B) formic acid 0.25% in methanol. The optimized gradient elution was 95% A at 0 minute, to 75% A at 8 minutes, to 65% A at 45 minutes, to 95% A at 50 minutes, then 15 minutes to recover its initial condition. Flow rate was 1.0 mL/min and column was at room temperature. The detection wavelength was set at 280 nm.
All samples were dried and torn into pieces as the commercial materials. In the optimized method, 0.300 g plant material were extracted with 10 mL of H2O: methanol 6:4 in 60 °C water bath during 30 minutes, then at 90 °C during 15 minutes. Extracts were centrifugated and taken to 10 mL. An aliquot was filtered through 0.22 µm filter before HPLC analysis.
RESULTS
Phytochemical Analysis. Compounds 1, 2, 3, 6a and 11 structures are shown in Figure 1. The results are:
3-(3,4-dihydroxyphenyl)lactic acid (1): amorphous solid, 1H-NMR (ppm, D2O): 2.85 (dd (J=8 y 14 Hz),1H, 7a-H) ; 3.02 (dd (J= 4 and 14 Hz), 1H, 7b-H); 4.20 (dd (J= 4 y 8 Hz), 1H, 8-H); 6.72 (dd,(J=1.5 and 8 Hz) 6-H), 6.75 (d, J=8 Hz, 5-H); ), 6.85 (d, J=1,5 Hz, 2-H), according to literature (Siebl et al., 1998).
3,4-dihydroxybenzoic acid (protocatechuic acid) (2), Caffeic acid (3), Rosmarinic acid (11): TLC and HPLC chromatographic mobility and 1H- and 13C- NMR (CD3OD)), according to literature (Siebl et al., 1998). Compound 3 was also compared with an authentic standard.
4-(3,4-dihydroxybenzoyloxymethyl)phenyl ß-glucoside (6a): white solid 1H-NMR (ppm, D2O): 2.7-3.5 (m, 6H, 2'',3'',4'' 5'',6''H-glucose); 4.70-4.77 (bs, 3H, 1''-H, methylene); 6.55-6.60 (bd, 3H,2-, 3'-, 5'-H); 6.88 (d,2H, 2'-,6'-H); 6.95-7.2 (m, 2H, 6-, 5-H), according to literature data (Nakatani & Kikuzaki, 1987; Siebl et al., 1998).
4-(3,4-dihydroxybenzoyloxymethyl)phenyl ß-glucoside (6b) hexaacetate derivative: amorphous colourless solid , 1H-NMR (ppm, CDCl3): 4.15 (dd J=2.2 and 12 Hz),1H, 6'-H); 4.30 (dd, J=5.4 and 12Hz),1H, 6'-H); 5.0 (d (J= 6.5 Hz) 1H, 1''-H); 5.18 (t, J=9.4 Hz, 1H, 2''-H); 5.25-5.35 (m, 2H, 3'-,4'-H); 5.30 (s, 2H, methylene); 7.02 (d (J=8.6 Hz), 2H, 3'- ,5'-H); 7.29 (d (J=8.5 Hz) 1H, 5-H); 7.38 (d (J= 8.6 Hz), 2H, 2'-,6'-H); 7.88 (d (J =1.2 Hz) 1H, 2-H); 7.97 (dd (J=8.5 and 1.2 Hz),1H, 6-H); 13C-NMR (ppm, CDCl3): 62.5, 71.3, 74.8, 77.9, 78.1, 102.1 (6C, glucose moiety); 67.0 (methylene); 130.7 (2 protonated aromatic C); 117.7 (2 protonated aromatic C); 168.1 (ester carbonyl) among other signals, according to literature data (Nakatani & Kikuzaki, 1987).
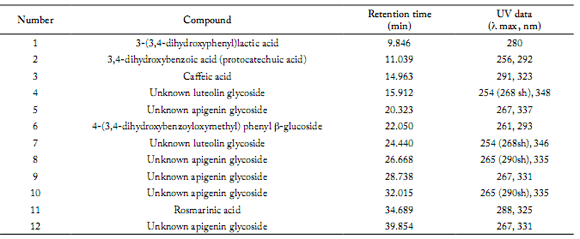
On-line UV spectra of flavone glycosides detected: Compounds (4) and (7) showed a typical UV spectra for luteolin derivates and compounds (5), (8), (9), (10) and (12) showed a typical UV spectra for apigenin derivates (Mabry et al., 1970).
Liquid chromatography analysis. A typical chromatogram of Origanum vulgare L ssp. viridulum extracted under the conditions described above is shown in Figure 2. Retention times and UV data of compounds 1 to 12 are shown in Table 1. The repeatability and reproducibility was assessed by analyzing extract solutions of the sample in different days. Relative standard deviation of retention times was always below 5.0%, showing that samples were stable and the HPLC-DAD system was reliable. Identity and purity were assessed by comparing UV spectra of the same peak in diferent chromatograms with correlation over 0.985.
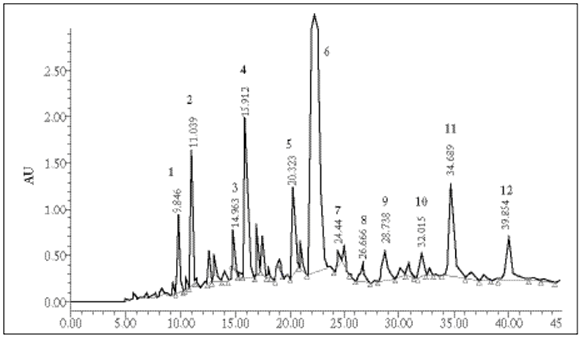
Fig. 2. Origanum vulgare L. ssp. viridulum HPLC chromatogram, under the conditions described in the text. Compounds 1, 2, 3, 6 and 11 were isolated and their structures identified spectroscopically. Compound 3 was also compared with authentic standard. Compounds 4, 5, 7, 8, 9, 10 and 12 were tentatively characterized according to DAD spectra.
Fig. 2. Cromatograma de CLAR del O. vulgare L. ssp. viridulum en las condiciones descriptas en el texto. Los compuestos 1, 2, 3, 6 y 11 fueron aislados e identificados espectroscópicamente. El compuesto 3 se comparó además con estándar. Los compuestos 4, 5, 7, 8, 9, 10 y 12 fueron caracterizados tentativamente de acuerdo a su espectro UV con arreglo de diodos (DAD).
Table 1. Main polyphenolics in O. vulgare L. ssp. viridulum hydroalcoholic extract: UV (λ max, nm) and Retention times (minutes) under the conditions described in the text.
Tabla 1. Principales polifenoles del extracto hidroalcóholico del O. vulgare L. ssp. viridulum: UV (λ máx, nm) y Tiempos de retención (minutos) en las condiciones descriptas en el texto.
Polar phenolic compounds were separated with a C18 reverse phase although a mobile phase gradient was necessary to achieve peak resolution of the complex mixture in the extract. The chosen UV wavelength (280nm) allowed the detection of most phenolic compounds.
In our preliminary studies, similar chromatographic profiles were obtained in extracts run in the same conditions from plants classified after Iestewart as O. vulgare spp. virens (Plant materials). Only slight quantitative differences could be observed on peak areas for assays of different O. vulgare L. ssp. viridulum (= O. vulgare L. ssp. virens) populations, but no qualitative ones, as expected to genetic expression (Treutter, 2010).
DISCUSSION
This phytochemical study describes major polar polyphenolics of O. vulgare L. ssp. viridulum (= O. vulgare L. ssp. virens). As a characteristic of the Lamiaceae family, rosmarinic acid (11) can be detected (Petersen & Simmonds, 2003) as well as protocatechuic acid (2) and caffeic acid (3) (Janicsak et al., 1999). The compound 3-(3,4-dihydroxyphenyl)lactic acid (1), also known as danshensu, was found in this oregano and has been identifed in salvia (Hu et al., 2005). Hydroquinone and its ß-glucoside, arbutin, could not be found in this subespecies although they had been isolated from "native" Origanum x applii (González & Rolando, 2005).
In UV detection, absolute concentration of compounds depends not only on the peak area but also on the particular absorption of each compound at a given wavelength (280 nm). However, compound 6a, identified as 4-(3,4-dihydroxybenzoyloxymethyl)phenyl ß-glucoside (Fig. 1, Fig. 2) was undoubtedly one of the major compounds of the hydroalcoholic extract of O. vulgare L. ssp. viridulum. Characteristic signals in the proton nuclear magnetic resonance (1H-NMR) spectra of the peracetate derivative 6b (singlet at 5.20 ppm for 2H-7', doublets at 7.02 ppm for H-3' and 5' and 7.38 ppm for H-2' and 6') support the structure assigned (Fig. 1), together with the remaining signals in 1H- and 13C-NMR. Some phenol glucosides have been reported in origanum (Nakatani & Kikuzaki, 1987; Kikuzaki & Nakatani, 1989; Koukoulista et al., 2010; Rao et al., 2011). Compound 6a was first isolated from O. vulgare L. (Nakatani & Kikuzaki, 1987) and exhibited important antioxidant activity. The structure can be clearly distinguished from those of the reported compounds origanol A and B (Rao et al., 2011) which are esters of 3,4- dihydroxybenzylic alcohol 4-O-ß-glucoside.
Many phenolic compounds of O. vulgare L. ssp. viridulum are favone glycosides. They can be pointed out by analyzing the online UV-DAD spectra (Mabry et al., 1970) as they appear in the optimized HPLC chromatogram. They were found to be mainly luteolin and apigenin derivatives (Fig. 2, Table 1). Together they represent a significant ratio of polyphenols in this variety. Many mono or di-glycosides of luteolin and apigenin have been reported in the genus, not only O-glycosides but also C-glycosides (Hussain & Markham, 1981; Hossain et al., 2010). Some of them are isomeric. The UV spectra does not ofer information about the glycosidation extent. Therefore, further HPLC-mass spectrometry studies are required to provide a more complete identification.
The profile presented in Figure 2 is characteristic of O. vulgare L. ssp. viridulum (= O. vulgare L. ssp. virens). The identified compounds were located in the liquid chromatogram by comparing retention times and superposed UV spectra of the compounds. Once the appropriate conditions are given, this method allows a rapid separation and characterization of phenols from hydroalcoholic extracts of this oregano.
The same profile was also found in different samples which, according to botanical descriptors, had been classified as O. vulgare L. ssp. viridulum [= O. vulgare L. ssp. virens (Hoffmannsegg et Link) Ietswaart]. Otherwise, some differences in the chromatographic profile, obtained under equal conditions, were found in oregano samples which had been classified as belonging to other taxonomical groups.
Further investigation is being conducted to fnd out whether polyphenols can or cannot be suitable chemical markers of taxonomical groups in O. vulgare subspecies and its hybrids, which are mainly the materials cultivated in Argentina.
ACKNOWLEDGEMENTS
We thank Ing. Agr. Otto Brutti for providing O. vulgare L. ssp. viridulum vegetal material, Ing. Agr. Cecilia Xifreda for reviewing our micrographic observations, Dr. Beatriz Varela for providing an O. vulgare L. ssp. virens sample, Laboratorio de Control de Calidad Melacrom S.C. for making the HPLC-DAD chromatograms, and the Departamento de Ciencias Básicas de la Universidad Nacional de Luján for financial support.
REFERENCES
1. Argüello, J.A., S.B. Núñez, V. Davidenco, D.A. Suárez, L. Seisdedos, M.C. Baigorria, N. La Porta, G. Ruiz & V. Yossen (2012). Sistema de producción y cadena de valor del cultivo de Orégano (Origanum sp.) en la Provincia de Córdoba (Argentina). Phyton, International Journal of Experimental Botany 81: 23-34. [ Links ]
2. Cai, B., S.P. Ong & X. Liu (2012). High Performance Liquid Chromatography Fingerprinting Technology of the commonly-used traditional Chinese medicine herbs Chemical Industry Press, World Scientific Publishing Co. New Jersey, London, Beijing, Shangai, Hong Kong, Taipei, Chenai. [ Links ]
3. Chatzopoulou, A., A. Karioti, C. Gousiadou, V. Vivancos, P. Kyriazopoulos, S. Golegou & H. Skaltsa (2010). Depsides and other polar constituents from Origanum dictmnus L. and their in vitro antimicrobial activity in clinical strains. Journal Agricultural and Food Chemistry 58: 6064- 6068. [ Links ]
4. Dambolena, J.S., M.P. Zunino, E.I. Lucini, R. Olmedo, E. Banchio, P.J. Bima & J.A. Zygadlo (2010). Total phenolic content, radical scavenging properties and essential oil composition of Origanum species from different populations. Journal of Agricultural and Food Chemistry 58: 115-1120. [ Links ]
5. Farías, G. O. Brutti, R. Grau, P. Di Leo Lira, D. Retta, C. van Baren, S. Vento & A.L. Bandoni (2010). Morphological, yielding and quality descriptors of four clones of Origanum spp. (Lamiaceae) from the Argentine Littoral region Germoplasm bank. Industrial Crops and Products 32: 472-480. [ Links ]
6. Fecka, I. & S. Turek (2008). Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chemistry 108: 1039-1053. [ Links ]
7. González, M.D. & A. Rolando (2005). Chemical study on a water extract of argentine commercial origanum. Journal of the Argentine Chemical Society 93: 225-231. [ Links ]
8. Hossain, M.B., N.P. Brunton, C. Barry-Ryan, A.B. Martin-Diana & M. Wilkinson (2010). Characterization of phenolics composition in Lamiaceae spices by LC-ESI-MS/MS, Journal Agricultural and Food Chemistry 58: 10576-10581. [ Links ]
9. Hussain, S.Z. & K.R. Markham (1981). The glycoflavone vicenin-2 its distribution in related genera within the Labiatae Phytochemistry 20: 1171-1173. [ Links ]
10. Hussain, S.Z., V.H. Heywood & K.R. Markham (1982). Distribution of flavonoids as chemotaxonomic markers in the genus Origanum L. and related genera in Labiatae. In: Margaris N., Koedam A. and Vokou D. (eds). Aromatic plants: basic and applied aspects. Martinus Nijhof Publishers, Hague, The Netherlands, pp. 141-152. [ Links ]
11. Hu, P., Q.L. Liang, G.A. Luo, Z.Z. Zhao & Z.H. Jiang (2005). Multi-component HPLC fingerprinting of radix Salviae miltiorrhizae and its LC-MS-MS identification. Chemical and Pharmaceutical Bulletin 53: 677-683. [ Links ]
12. Ibrahim, A.Y., N.M. Shaffie & H.M. Motawa (2010). Hepatoprotective and Therapeutic Activity of Origanum syriacum Aqueous Extract in Paracetmol Induced cell Damage in Albino Mice. Journal of American Science 6: 449- 458 [ Links ]
13. Ietswaart, J.H. (1980) A taxonomic revision of the genus Origanum (Labiatae), Leiden University Press, Leiden, The Netherlands. [ Links ]
14. Janicsak, G., I. Mathe, V. Miklossy-Vari & G. Blunden (1999). Comparative studies of the rosmarinic and caffeic acid contents of Lamiaceae species. Biochemical Systematics and Ecology 27: 733-738. [ Links ]
15. Kikuzaki, H. & N. Nakatani (1989). Structure of a new antioxidative phenolic acid from oregano (Origanum vulgare L.). Agricultural and Biological Chemistry 53: 519-524. [ Links ]
16. Koukoulitsa, C., A. Karioti, C. Bergonzi, G. Pescitelli, L.D. Bari & H. Skaltsa (2006). Polar constituents from the aerial parts of Origanum vulgare L. ssp. hirtum growing wild in Greece. Journal Agricultural and Food Chemistry 54: 5388-5392. [ Links ]
17. Mabry, T., K.R. Markham & M.B. Tomas (1970). The Systematic Identification of Flavonoids, Springer-Verlag, Berlin. [ Links ]
18. Nakatani, N. & H. Kikuzake (1987). A new antioxidative glucoside isolated from oregano (Origanum vulgare). Agricultural and Biological Chemistry 51: 2727-2732. [ Links ]
19. Petersen, M. & M.S. Simmonds (2003). Rosmarinic acid. Phytochemistry 62: 121-125. [ Links ]
20. Rao, G.V., T. Mukhopadhyay, T. Annamalai, N. Radhakrishnan & M.R. Sahoo (2011). Chemical constituents and biological studies of Origanum vulgare Linn. Pharmacological Research 3: 143-145. [ Links ]
21. Rouquaud, E. & M.E. Videla (2000). Oréganos de Mendoza (Argentina). Revista de la Facultad de Ciencias Agrarias de la Universidad Nacional de Cuyo 32: 23-32. [ Links ]
22. Seibl, J., W. Simon, E. Pretsch & T. Clerc (1998). Tablas para la determinación estructural por métodos espectroscópicos. Updated translation from the German 3rd Edition, Springer-Verlag Iberica Ed. [ Links ]
23. Shan, B., Y. Cai, M. Sun & H. Corke (2005). Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agricultural and Food Chemistry 53: 7749-7759. [ Links ]
24. Sztefanov, A., K. Szabó & J. Bernáth (2003). Comparative analysis of Hungarian Matricaria recutita (L.) Rausch. populations. International Journal of Horticultural Science 9: 81-85. [ Links ]
25. Torres, L.E., A.G. Chaves, G. Barboza, P. Brunetti, J.A. Bustos, Y. Massuh, S. Ocaño, N. Castillo & M.S. Ojeda (2010). Evaluation of the agronomic performance and taxonomic characterization of four clones of oregano (Origanum sp.). Molecular Medicinal Chemistry 21: 91-93. [ Links ]
26. Treutter, D. (2010) Managing Phenol Contents in Crop Plants by Phytochemical Farming and BreedingVisions and Constraints. International Journal of Molecular Science 11: 807-857. [ Links ]













