Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Phyton (Buenos Aires)
versão On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.2 Vicente López dez. 2014
ARTÍCULOS ORIGINALES
Linking relative growth rates to biomass allocation: the responses of the grass Leymus chinensis to nitrogen addition
Relación entre las tasas relativas de crecimiento y la distribución de biomasa: respuestas de la gramínea Leymus chinensis a la adición de nitrógeno
Li1,2 YY, X-T Lü1, Z-W Wang1, C Zhou3,4, X-G Han1
1 State Key Laboratory of Forest and Soil Ecology, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110164, China.
2 Graduate University of Chinese Academy of Sciences, Beijing 100049, China
3 Key Laboratory for Vegetation Ecology, Institute of Grassland Science, Northeast Normal University, Changchun 130024, China.
4 College of Life Science, Liaoning University, Shenyang 110036, China.
Address Correspondence to: Dr. Zheng-Wen Wang, e-mail: wangzw@iae.ac.cn; liyy@iae.ac.cn
Recibido / Received 10.I.2014.
Aceptado / Accepted 17.III.2014.
Abstract. Relative growth rate (RGR) of plants is a key component of fitness. Theoretically, the RGR of plants would be closely related with biomass allocation. Our mechanistic understanding of the relationship between RGR and biomass allocation under global change scenarios is still limited. We examined the responses of RGR and biomass allocation of Leymus chinensis, a dominant grass in the temperate steppe of northern China, to a wide range of N addition. We found that N addition increased RGR of L. chinensis up to a threshold of 10 g N/m2. While leaf and stem weight ratios were positively correlated with N addition rates, root weight ratio showed a negative correlation. The RGR of total biomass was positively correlated with both leaf and stem weight ratios, but negatively correlated with root weight ratios. The positive responses of leaf and stem biomass allocation, or the negative responses of root biomass allocation to N addition determined enhancements or reductions, respectively, of RGR on L. chinensis. The increased RGR of L. chinensis to N addition gave a direct and robust explanation for the increased of dominance of this species under field N-addition studies. Our results have important implications for projecting the growth status of L. chinensis under scenarios of increased N deposition in northern China.
Keywords: Plant functional traits; Nitrogen deposition; Growth dynamics; Temperate steppe; Allometry.
Resumen. La tasa relativa de crecimiento (RGR) de las plantas es un componente clave en la adaptación de las plantas al ambiente. Teóricamente, la RGR de las plantas estaría muy relacionada con la distribución de biomasa. Nuestro entendimiento de los mecanismos de la relación entre RGR y la distribución de biomasa todavía es limitado en relación al cambio global. Las respuestas de la RGR y la distribución de biomasa a un amplio rango de adición de N se examinaron en Leymus chinensis, una gramínea dominante en la estepa templada del norte de China. La adición de N incrementó la RGR de L. chinensis hasta un límite de 10 g N/m2. Mientras las relaciones del peso de hojas y tallos se correlacionaron positivamente con las tasas de adición de N, la relación del peso de raíz mostró una correlación negativa. La RGR de la biomasa total se correlacionó positivamente con las relaciones del peso de hojas y tallos, pero negativamente con las relaciones del peso de raíz. Las respuestas positivas de la distribución de biomasa a hojas y tallos, o la respuesta negativa de distribución de biomasa a las raíces, a la adición de N determinó incrementos o reducciones, respectivamente, de la RGR en L. chinensis. La mayor RGR de L. chinensis a la adición de N dio una explicación directa y robusta por la mayor dominancia de esta especie en estudios de adición nitrogenada a campo. Nuestros resultados tienen importantes implicancias para proyectar el nivel de crecimiento de L. chinensis bajo un escenario de una mayor deposición de N en el norte de China.
Palabras clave: Características funcionales de las plantas; Deposición de N; Dinámica del crecimiento; Estepa templada; Alometría.
INTRODUCTION
As a comprehensive and conventional trait of plants, relative growth rate (RGR) is an important component of fitness (Mcgraw & Garbutt, 1990), which integrates morphophysiological traits of plants (Li et al., 1998). The RGR is a key trait for explaining the distribution of species along environmental gradients (Poorter, 1999), and predicting species-specific aboveground net primary productivity (Lambers et al., 1998). Consequently, RGR plays an important role in determining plant competitive strategies (Biere, 1996; Scheepens et al., 2010; Gremer et al., 2013). There is increasing evidence for the inter-specific variation of RGR among different species (Poorter, 1989; Poorter et al., 1990; Villar et al., 1998; Ryser & Wahl, 2001; Ruiz-Robleto & Villar, 2005). This makes it an important issue in ecology to scale up from the properties of individual species to ecosystem processes. At the species level, the RGR is closely correlated with the trade-of between leaf area ratio and net assimilation rate (Poorter & Remkes, 1990; Shipley, 2002, 2006) or the changes of biomass allocation patterns (Poorter & Lambers, 1991; van den Boogaard et al., 1996; Villar et al., 1998; Ruiz-Robleto & Villar, 2005). However, studies focusing on the intra-specific variation in RGR have been largely ignored. Furthermore, improving our understanding of plant functional traits at the species-specific level will fulfill knowledge gaps on trait-based community ecology (Violle et al., 2012).
Biomass allocation, defined as the relative amount of biomass allocated to different organs at any one time (Poorter et al., 2012), varies considerably across environments. Numerous observations show a functional equilibrium of biomass allocation. It means that plants will allocate relatively more biomass to roots if their growth is limited by belowground factors (e.g., nutrients, water). However, they will allocate relatively more biomass to shoots if they are limited by aboveground factors (e.g. light, carbon) (Poorter et al., 2012). Biomass allocation is a strong driver for the capacity of plants to use resources (Evans, 1972), such as carbon, water and nutrients. Moreover, plant biomass allocation patterns are sensitive to global change factors. A quantitative understanding of biomass allocation patterns has not only theoretical implications in ecology and evolution but also practical implications in agricultural practices (Poorter et al., 2012).
It is theoretically established that there is a close relationship between biomass allocation and relative growth rate (Poorter, 1989; Poorter et al., 1990; Villar et al., 1998; Ruiz-Robleto & Villar, 2005). Our quantitative understanding of the relationship between biomass allocation to different plant organs and RGR, however, is still poor. It is generally assumed that plants could coordinate the biomass investment among their organs so that a tight balance could be achieved among different organs (Poorter & Nagel, 2000). In an inter-specific study based on 20 Aegilops species, it was shown that RGR was positively correlated with stem weight ratio, negatively correlated with root weight ratio, and not correlated with leaf weight ratio (Villar et al., 1998). Other studies, however, have shown that RGR was positively related with leaf weight ratio across various species (Walters et al., 1993a). While a negative correlation between biomass allocation to roots and growth rate is theoretically expected in resource-enriched environments (Gleeson & Tilman, 1992), there is rarely empirical evidence for this expectation (Gleeson & Tilman, 1994). The divergence between the theoretical prediction and the empirical evidence, and the mixed results from different studies, suggests that more studies are critically needed to get a better understanding of the relationship between biomass allocation and the relative growth rate of plants.
Nitrogen (N) is the primary limiting factor for plant growth in most terrestrial ecosystems (LeBauer & Treseder, 2008). With the increasing anthropogenic activities, more reactive N is available in soils through atmospheric N deposition (Gruber & Galloway, 2008). There is increasing evidence that the increasing N availability exerts an important control on community assemblage through its species-specific effects on plant growth (Clark et al., 2007). It is still unclear, however, whether the changes of N availability would alter the relationship between biomass allocation and relative growth rate. Stem weight ratios will increase under N-enriched condition due to the increases of light limitation (Hautier et al., 2009). At the same time, however, less biomass would be allocated to roots as a result (Tilman, 1988; Sims et al., 2012). In a greenhouse experiment focusing on wild rice, it was reported that the alteration of biomass allocation would partly account for the positive responses of RGR to N addition (Sims et al., 2012). Whether such changes will occur in other species, especially those growing in natural ecosystems, is still an open question.
In this study, we examined the effects of N addition on biomass allocation and relative growth rate of L. chinensis, a dominant perennial grass in the Eurasian temperate steppe, in a greenhouse experiment using a wide range of N addition rates. Results from this study will improve our understanding of the growth and potential competition response of this dominant species under a scenario of an increasing N availability in the study region. We hypothesized that (1) allocation of biomass across the N addition gradient is greater to leaves and stems, and lower to roots; (2) the relationship between leaf or root weight ratio versus relative growth rate is positive or negative, respectively, and (3) RGR increases with increasing N availability.
MATERIALS AND METHODS
Experimental design. The experiment was conducted in a greenhouse at the Institute of Applied Ecology, Chinese Academy of Sciences in Shenyang (41.8° N, 123.4° E), China. The greenhouse was covered with shade cloth to minimize excessive heating and no artificial light was provided. The greenhouse was temperature-controlled; day/night temperatures averaged 25/20 °C for the duration of the experiment. The mean annual temperature is 8.3 °C, with the mean maximum monthly temperature of 36 °C in July and the mean minimum monthly temperature of -29 °C in January. Mean annual precipitation is 500 mm, occurring mainly from July to August (accounting for 62.5% of the total).
The experiment lasted 100 days, from July to November 2012. The experiment contained six treatments, corresponding to six levels of N addition rates (0, 2, 5, 10, 20 and 30 g N/m2, respectively). Soils were collected from natural grassland in Xilingol league, Inner Mongolia. All soils were passed through a 2-mm sieve to remove rocks and organic debris, and finally thoroughly mixed. Nitrogen was mixed into the soils in the form of NH4NO3 before soils were added to the pots, with the amount of N added in each pot being calculated following every treatment. Tree kilogram of fresh soil were added to each plastic pot (15-cm diameter, 36-cm depth) with foam plugs inserted into the drainage holes to keep soil within the pot.
Seeds of L. chinensis were collected from the field in autumn 2011. Sowing rates were based on previously determined germination rates. From 4 to 10 seeds were sown per pot on 21 July 2012. Pots were checked daily for seedling emergence. Seedlings began to emerge 10 d after sowing. Two weeks after the first seedling emergence, seedlings were thinned to a common density, leaving only one seedling per pot. All pots were watered as needed between treatment applications to maintain soils near feld capacity. The first harvest was conducted after the cotyledons had fully emerged and the frst true leaves were observed. Te other harvests were conducted at a 10-day interval after the first harvest. For each treatment, there were 60 pots, so that each harvest involved 10 pots. There were 360 pots in the whole experiment (6 treatments × 6 harvests × 10 replicates = 360 pots).
The experiment was conducted based on a randomized complete block design with N availability as the only factor. Ten replications for each harvest of all the six treatments were arranged in 10 blocks in the experiment. Pot positions were randomized within each block. To minimize effects of environmental differences in the growth room, pots within each block were rotated every two days.
Plant measurements. One pot from each of the 10 blocks was randomly selected for biomass measurements by harvesting at 10-day intervals from 20 August to 16 November. At each destructive harvest, the soil was carefully rinsed until the roots disentangled. Plant material in each pot was separated into leaves, stems, and roots. The plant samples were then oven-dried at 80 °C for 48 h, and finally weighed with a digital balance (Mettler AE 100).
Calculations and statistical analysis. Leaf weight ratio was quantified as the proportion of the total plant biomass allocated to leaf tissue. The same procedure was followed to calculate the stem and root weight ratios. The RGR is the quantity of biomass produced per unit of existing biomass at time (t1) per unit of time and was calculated as:
RGR=lnW2-lnW1/t2-t1 (Evans 1972),
where W2 is the weight of each of six individual plants at t2, and W1 is the mean weight of the six harvested plants at t1.
In this study, we quantified the RGR of L. chinensis based on the data from the fifth and sixth harvest. Root-to-shoot dry mass ratio was also determined. The relationship between biomass allocation, relative growth rate, and N addition rates was studied by regression analysis. We set p<0.05 as the significance level.
RESULTS
Biomass allocation. Total biomass of L. chinensis was significantly increased across the N addition gradient, from 0.32 ± 0.03 g in the treatment with no N addition to 0.69 ± 0.09 g in the treatment with the highest rate of N addition (Fig. 1). Both aboveground and belowground biomasses were increased across the N addition gradient (Fig. 1). The aboveground biomass was more sensitive to N addition than the belowground biomass. This was indicated by the greater slope of the regression between aboveground biomass and N addition rates. The root: shoot ratio was negatively correlated with N addition (Fig. 2). The root:shoot ratio showed a high sensitivity to N addition as indicated by the sharp decrease at the low N addition rates (Fig. 2). Leaf weight ratio and stem weight ratio were positively correlated with N addition rates, whereas root weight ratio was negatively correlated with N addition rates (Fig. 3). In the treatment receiving no N addition, the relative contribution of root to total biomass was higher than that for leaves. In contrast, the relative contribution of leaves to total biomass was significantly higher than that of roots in the treatment receiving the highest rate of N addition (Fig. 3). Across the whole N addition gradient, the relative contribution of stems to total biomass was much lower than that of leaves and roots (Fig. 3).
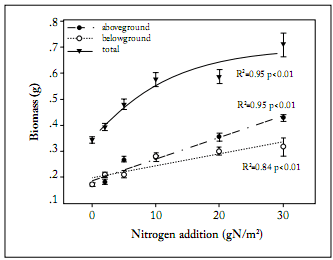
Fig. 1. Changes of aboveground, belowground, and total biomasses with N addition in Leymus chinensis. Each value is the mean ±1 S.E. of n=10.
Fig. 1. Cambios en las biomasas aérea, radical y total con la adición de N en Leymus chinensis. Cada valor es el promedio ±1 E.E. de n=10.
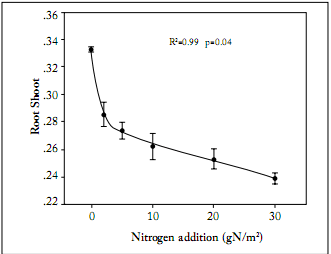
Fig. 2. Effects of nitrogen addition on root:shoot ratios of Leymus chinensis. Each value is the mean ±1 S.E. of n=10.
Fig. 2. Efectos de la adición de N en las relaciones raíz:tallo en Leymus chinensis. Cada valor es el promedio ±1 E.E. de n=10.

Fig. 3. Effects of N addition on leaf (LWR), stem (SWR) and root (RWR) weight ratios in Leymus chinensis.
Fig. 3. Efectos de la adición de N en las relaciones de peso de hojas (LWR), tallos (SWR) y raíces (RWR) en Leymus chinensis.
Relative growth rate. Nitrogen addition significantly enhanced the relative growth rate of leaf, stem and root tissue, and total biomass of L. chinensis (Fig. 4), up to a threshold of 10 g N/m2. Across the N addition gradient, RGR of leaves was the highest, and that of stem the lowest, among the three organs examined in this study (Fig. 4). The relative growth rate of total biomass was positively correlated with both leaf and stem weight ratios, but negatively correlated with root weight ratios (Fig. 5).

Fig. 4. Effects of N addition on relative growth rates of leaf, stem, root, and total biomass in Leymus chinensis.
Fig. 4. Efectos de la adición de N en las tasas relativas de crecimiento de la biomasa de hojas, tallos, raíces y total en Leymus chinensis.
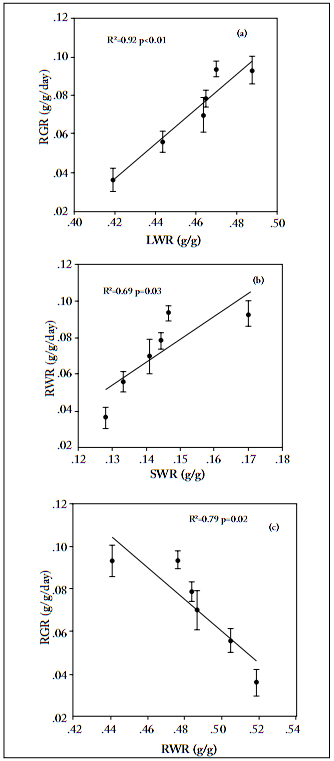
Fig. 5. Relationship between leaf weight ratio (LWR), stem weight ratio (SWR) or root weight ratio (RWR) versus relative growth rate (RGR) of Leymus chinensis. Each value is the mean ±1 S.E. of n=10.
Fig. 5. Relación entre las relaciones del peso de hojas (LWR), tallos (SWR) o raíces (RWR) y la tasa relativa de crecimiento (RGR) en Leymus chinensis. Cada valor es el promedio ±1 E.E. de n=10.
DISCUSSION
As the primary limiting factor for plant growth in the temperate steppe, N availability exerts strong impacts on both biomass allocation and relative growth rate of the dominant species, L. chinensis. Growth dynamics and biomass allocation patterns are quite sensitive to the changes of soil N availability. Our study presented robust evidence for the effects of increased N availability on the plasticity of plant growth, with great implications for grassland management under a scenario of increasing atmospheric N deposition.
Our results clearly demonstrated strong positive efects of N addition on both aboveground and belowground biomasses, and thus on total biomass of L. chinensis. A positive relationship between aboveground biomass and N addition was also found for Echinolaena infexa (C3grass) in a Brazilian savanna (Bustamante et al., 2012). A meta-analysis of data from 304 studies across 456 terrestrial plant species showed that plant biomass significantly increases with N availability in herbaceous species (Xia & Wan, 2008). The positive responses of both aboveground and belowground biomasses of L. chinensis to N addition (as found in this study) suggest that growth of this dominant species in the temperate steppe is strongly limited by N availability. Although the N limitation level has been well established for temperate steppes at a community level (Bai et al., 2010), our results present direct evidence for N limitation at a population level in L. chinensis.
Models of optimal biomass allocation in plants predict increased leaf weight ratios, and decreased root weight ratios, with increasing nutrient availability (Bloom et al., 1985; Fichtner & Schulze, 1992; Muller, 2000). One possible reason is that nitrogen deficiencies result in higher levels of carbon allocated to roots (Hermans et al., 2006). Cnsistently with our first hypothesis, we found that the fraction of biomass in leaves and stems increased, and that of roots decreased, with increasing N availability. Consequently, root: shoot ratios were lowered by high N availability. It is generally assumed that plants would modify their morphology to forage "optimally" for nutrients and light (Bloom et al., 1985; Tilman, 1988). At low N availability, plants allocate more biomass to roots to absorb more nutrients. In contrast, plants would allocate more biomass aboveground to compete for light at high N availability. Therefore, N is a strong environmental modulator for plant biomass allocation.
Many studies have examined the RGR of different plant species under various nutrient availability conditions (Chapin, 1980). Generally, enhanced soil N availability would increase plant growth and productivity (Frink et al., 1999). The positive responses of RGR of L. chinensis to N addition, especially to N addition with rates lower than 10 g N/m2, have great implications for its competition with neighboring species. Consequently, our results present additional evidence for the finding from the field experiment, that N addition would increase the dominance of L. chinensis in the temperate steppe (Bai et al., 2010). However, there was a threshold for the positive effects of N addition on RGR of L. chinensis. Our results showed that the positive effects of N addition would be diminished if rates of N addition were higher than 10 g N/m2. Tis threshold has great implications for grassland management, especially for the fertilization of artificial L. chinensis grasslands.
The relative growth rate of L. chinensis ranged 2.3-fold across the whole N addition gradient. We found that LWR was closely correlated with RGR in L. chinensis, which contrast with the results of Lambers et al. (1998) but is consistent with those of Poorter & Lambers (1991). Our results suggest that variation in LWR should play an important role in determining RGR. Poorter et al. (1990) found a positive correlation between massbasis plant photosynthetic rate and RGR. Although leaf-level photosynthetic rate cannot represent whole plant photosynthetic rate, it might be considered as a potential index refecting photosynthetic capacity (Villar et al., 1998). Thus, seedlings allocating more biomass to leaves would have higher photosynthetic rates, and consequently higher RGR.
Our results showed a positive relationship between RGR and stem weight ratio in L. chinensis. Stem is generally considered to be a storage and support organ (White & Weidlich, 1974). It has been estimated that the stem weight ratio would not be an important factor to correlate with RGR (den Hertog et al., 1998). However, some studies found a positive correlation between RGR and stem weight ratio (Van den Boogaard & Villar, 1998). Moreover, Shipley & Peters (1990) found that species with stems had a higher relative growth rate than stem-less species. In our study, leaf sheaths were included in the stem faction. Plants that invest more in leaf blades would also invest more in leaf sheaths. Another reason for the positive correlation between RGR and stem weight ratio might be that plants with higher stem weight ratios were much taller, indicating an advantage for light interception.
Many studies indicate a positive relationship between nutrient uptake rates and RGR (Poorter et al., 1990, Walters et al., 1993b). The root biomass of L. chinensis was positively correlated with N addition rate, implying that nutrient uptake rates would be increased across the whole N addition gradient in the present study. However, the relative increase of roots was much lower than that of shoots with increasing N availability. Consequently, we found lower root weight ratios with higher N addition rates. It has been suggested that fast-growing species generally allocate less biomass to roots (Villar et al., 1998). This is supported by our findings since RGR of L. chinensis was negatively correlated with root weight ratio.
ACKNOWLEDGEMENTS
We are grateful to Min Long, Xiao-Guang Wang and Meng-Zhou Liu for the help in laboratory works, Linyou Lv for seed collection, and Qiang Yu for his comments on an earlier version of this manuscript. This study was supported by the Natural Science Foundation of China (31170433), Post-doctor Science Foundation of China under Grant (2012M520653) and Open fund of Key Laboratory of Vegetation Ecology, Ministry of Education.
REFERENCES
1. Bai, Y.F., J.G. Wu, C.M. Clark, S. Naeem, Q.M. Pan, J.H. Huang, L.X. Zhang & X.G. Han (2010). Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from Inner Mongolia Grasslands. Global Change Biology 16: 358-372. [ Links ]
2. Biere, A. (1996). Intra-specific variation in relative growth rate: Impact on competitive ability and performance of Lychnis fos-cuculi in habitats differing in soil fertility. Plant and Soil 182: 313-327. [ Links ]
3. Bloom, A.J., F.S. Chapin & H.A. Mooney (1985). Resource limitation in plants - an economic analogy. Annual Review of Ecology and Systematics 16: 363-392. [ Links ]
4. Brouwer, R. (1963) Some aspects of the equilibrium between overground and underground plant parts. Meded Inst Biol Scheik Onderz Landb Gewas 213: 31-39. [ Links ]
5. Bustamante, M.M.C., D.Q. de Brito, A.R. Kozovits, G. Luedemann, T.R.B. de Mello, A.D. Pinto, C.B.R. Munhoz & F.S.C. Takahashi (2012). Effects of nutrient additions on plant biomass and diversity of the herbaceous-subshrub layer of a Brazilian savanna (Cerrado). Plant Ecology 213: 795-808. [ Links ]
6. Chapin, F.S. (1980). The mineral nutrition of wild plants. Annual Review of Ecology and Systematics 11: 233-260. [ Links ]
7. Clark, C.M., E.E. Cleland, S.L. Collins, J.E. Fargione, L. Gough, K.L. Gross, S.C. Pennings, K.N. Suding & J.B. Grace (2007). Environmental and plant community determinants of species loss following nitrogen enrichment. Ecology Letters 10: 596-607. [ Links ]
8. Den Hertog, J., I. Stulen, F. Posthumus & H. Poorter (1998). Interactive effects of growth-limiting N supply and elevated atmospheric CO2 concentration on growth and carbon balance of Plantago major. Physiologia Plantarum 103: 451-460. [ Links ]
9. Evans, G.C. (1972) The Quantitative Analysis of Plant Growth. Blackwell, Oxford. [ Links ]
10. Fichtner, K. & E.D. Schulze (1992). The effect of nitrogen nutrition on growth and biomass partitioning of annual plants originating from habitats of different nitrogen availability. Oecologia 92: 236-241. [ Links ]
11. Frink, C.R., P.E. Waggoner & J.H. Ausubel (1999). Nitrogen fertilizer: Retrospect and prospect. Proceedings of the National Academy of Sciences of the United States of America 96: 1175-1180. [ Links ]
12. Gleeson, S.K. & D. Tilman (1992). Plant allocation and the multiple limitation hypothesis. American Naturalist 139: 1322-1343. [ Links ]
13. Gleeson, S.K. & D. Tilman (1994). Plant allocation, growth-rate and successional status. Functional Ecology 8: 543-550. [ Links ]
14. Gremer, J.R., S. Kimball, K.R. Keck, T.E. Huxman, A.L. Angert & D.L. Venable (2013). Water-use efficiency and relative growth rate mediate competitive interactions in Sonoran desert winter annual plants. American Journal of Botany 100: 2009-2015. [ Links ]
15. Gruber, N. & J.N. Galloway (2008). An earth-system perspective of the global nitrogen cycle. Nature 451: 293-296. [ Links ]
16. Hautier, Y., P.A. Niklaus & A. Hector (2009). Competition for light causes plant biodiversity loss after eutrophication. Science 324: 636-638. [ Links ]
17. Hermans, C., J.P. Hammond, P.J. White & N. Verbruggen (2006). How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science 11: 610-617. [ Links ]
18. Lambers, H., H. Poorter & M.M.I. Van Vuuren (1998). Research on variation in plant growth rate - introduction. In: H. Lambers, H. Poorter & M.M.I. Van Vuuren (eds). Inherent Variation in Plant Growth; Physiological Mechanisms and Ecological Consequences. Backhuys Publishers, Leiden, The Netherlands, pp. 1-4. [ Links ]
19. LeBauer, D.S. & K.K. Treseder (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89: 371-379. [ Links ]
20. Li, B., J.I. Suzuki & T. Hara (1998). Latitudinal variation in plant size and relative growth rate in Arabidopsis thaliana. Oecologia 115: 293-301. [ Links ]
21. Mcgraw, J.B. & K. Garbutt (1990). Demographic growth analysis. Ecology 71: 1199-1204. [ Links ]
22. Poorter, H. (1989). Plant-growth analysis - towards a synthesis of the classical and the functional-approach. Physiologia Plantarum 75: 237-244. [ Links ]
23. Poorter, H. & H. Lambers (1991). Is interspecific variation in relative growth-rate positively correlated with biomass allocation to the leaves. American Naturalist 138: 1264-1268. [ Links ]
24. Poorter, H. & O. Nagel (2000). The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology 27: 1191-1191. [ Links ]
25. Poorter, H., K.J. Niklas, P.B. Reich, J. Oleksyn, P. Poot & L. Mommer (2012). Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193: 30-50. [ Links ]
26. Poorter, H. & C. Remkes (1990). Leaf-area ratio and net assimilation rate of 24 wild-species differing in relative growth-rate. Oecologia 83: 553-559. [ Links ]
27. Poorter, H., C. Remkes & H. Lambers (1990). Carbon and nitrogen economy of 24 Wild-Species differing in relative growth-rate. Plant Physiology 94: 621-627. [ Links ]
28. Poorter, L. (1999). Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Functional Ecology 13: 396-410. [ Links ]
29. Ruiz-Robleto, J. & R. Villar (2005). Relative growth rate and biomass allocation in ten woody species with different leaf longevity using phylogenetic independent contrasts (PICs). Plant Biology 7: 484-494. [ Links ]
30. Ryser, R. & S. Wahl (2001). Interspecific variation in RGR and the underlying traits among 24 grass species grown in full daylight. Plant Biology 3: 426-436. [ Links ]
31. Scheepens, J.F., J. Stocklin & A.R. Pluess (2010). Unifying selection acts on competitive ability and relative growth rate in Scabiosa columbaria. Basic and Applied Ecology 11: 612-618. [ Links ]
32. Shipley, B. (2002). Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance. Functional Ecology 16:682-689. [ Links ]
33. Shipley, B. (2006). Net assimilation rate, specific leaf area and leaf mass ratio: which is most closely correlated with relative growth rate? A meta-analysis. Functional Ecology 20: 565-574. [ Links ]
34. Shipley, B. & R.H. Peters (1990). A test of the Tilman Model of plant strategies - relative growth-rate and biomass partitioning. American Naturalist 136: 139-153. [ Links ]
35. Sims, L., J. Pastor, T. Lee & B. Dewey (2012). Nitrogen, phosphorus and light effects on growth and allocation of biomass and nutrients in wild rice. Oecologia 170: 65-76. [ Links ]
36. Tilman, D. (1988). Plant Strategies and the Dynamics and Structure of Plant Communities. Princeton University Press, Princeton. [ Links ]
37. Van den Boogaard, R. & R. Villar (1998). Variation in growth and water-use efficiency - a comparison of Aegilops L. species and Triticum aestivum L. cultivars. In: H. Lambers, H. Poorter and M.M.I. van Vuuren (eds.). Inherent Variations in Plant Growth: physiological mechanisms and ecological consequences, pp. 289-308, Bachuys, Leiden, The Netherlands. [ Links ]
38. Van den Boogaard, R., M. deBoer, E.J. Veneklaas & H. Lambers (1996). Relative growth rate, biomass allocation pattern and water use efciency of three wheat cultivars during early ontogeny as dependent on water availability. Physiologia Plantarum 98: 493-504. [ Links ]
39. Villar, R., E.J. Veneklaas, P. Jordano & H. Lambers (1998). Relative growth rate and biomass allocation in 20 Aegilops (Poaceae) species. New Phytologist 140: 425-437. [ Links ]
40. Violle, C., B.J. Enquist, B.J. McGill, L. Jiang, C.H. Albert, C. Hulshof, V. Jung & J. Messier (2012). The return of the variance: intraspecific variability in community ecology. Trends in Ecology and Evolution 27: 244-252. [ Links ]
41. Walters, M.B., E.L. Kruger & P.B. Reich (1993a). Growth, biomass distribution and CO2 exchange of northern hardwood seedlings in high and low light - relationships with successional status and shade tolerance. Oecologia 94: 7-16. [ Links ]
42. Walters, M.B., E.L. Kruger & P.B. Reich (1993b). Relative growthrate in relation to physiological and morphological traits for northern hardwood tree seedlings - species, light environment and ontogenic considerations. Oecologia 96: 219-231. [ Links ]
43. White, R.A. & W. Weidlich (1974). Relationship between stem anatomy and growth habit in tree ferns (Cyatheaceae) and other ferns with erect stems. American Journal of Botany 61: 39-40. [ Links ]
44. Xia, J.Y. & S.Q. Wan (2008). Global response patterns of terrestrial plant species to nitrogen addition. New Phytologist 179: 428-439. [ Links ]














