Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.2 Vicente López dic. 2014
ARTÍCULOS ORIGINALES
Leaf photosynthetic characteristics in eight shaded Malaysian filmy ferns
Características fotosintéticas foliares en ocho himenofiláceas de ambientes sombreados en Malasia
Nurul Hafiza MR, KT Yong, N Osman, A Nasrulhaq-Boyce
Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia.
Address Correspondence to: Prof. Amru Nasrulhaq Boyce, e-mail: amru@um.edu.my
Recibido / Received 22.VIII.2013.
Aceptado / Accepted 10.XII.2013.
Abstract. The photosynthetic characteristics of eight Malaysian Hymenophyllaceae filmy ferns from shady habitats were investigated in this study. Chlorophyll content was highest in Trichomanes meifolium, followed by Cephalomanes obscurum, Hymenophyllum serrulatum, H. denticulatum, H. javanicum, H. acanthoides, H. exsertum and H. blandum, with values ranging from 3.3 to 8.6 mg/g fresh weight. Soluble protein content was remarkably high in H. serrulatum, with values of 53 ± 3.50 mg/g, followed by H. denticulatum, H. acanthoides, T. meifolium and the other species. Protein to chlorophyll ratios in the filmy ferns were low as expected, except for in H. serrulatum, H. acanthoides and H. denticulatum. Chloroplast number and size ranged between 34 to 138 per cell profile, and between 4.8 to 6.5 µm in diameter, in the Hymenophyllaceae. Quantum efficiency measurements in four Hymenophyllaceae spp. exhibited Fv/Fm values ranging between 0.73 to 0.81. The Hymenophyllaceae spp. also showed low in vivo CO2 assimilatory rates and light saturation points, ranging between 5 to 15 µmol CO2/m2/s and below 150 µmol/m2/s, respectively. The findings add further to our understanding on how the flmy ferns adapt and thrive in their humid and shady habitats.
Keywords: Plant science; Photosynthesis; Filmy ferns; Hymenophyllaceae; Shaded habitats.
Resumen. Las características fotosintéticas de ocho Malasia Hymenophyllaceae helechos membranosos de hábitats sombreados fueron investigados en este estudio. El contenido de clorofila fue mayor en Trichomanes meifolium, seguido por Cephalomanes obscurum, Hymenophyllum serrulatum, H. denticulatum, H. javanicum, H. acanthoides, H. y H. exsertum blandum con valores que van desde 3,3 hasta 8,6 mg/g peso fresco. El contenido de proteína soluble fue notablemente alta en H. serrulatum, con valores de 53 ± 3,50 mg/g, seguido por H. denticulatum, H. acanthoides, T. meifolium y las otras especies. La relación proteína:clorofila en los helechos fue baja como se esperaba, excepto en H. serrulatum, H. acanthoides y H. denticulatum. Número y tamaño de cloroplasto oscilaron entre las 34 y 138 por perfil de célula y entre 4,8 a 6,5 µm de diámetro, en el Hymenophyllaceae. Mediciones de la eficiencia cuántica de cuatro Hymenophyllaceae spp. mostraron valores de Fv / Fm que oscilan entre 0,73 a 0,81. El Hymenophyllaceae spp. también mostró baja en las tasas de asimilación de CO2 in vivo y los puntos de saturación, que oscila entre 5 a 15 µmol CO2/m2/s y por debajo de 150 µmol/m2/s, respectivamente. Los hallazgos se añaden a nuestra comprensión de cómo los helechos membranosos se adaptan y prosperan en sus hábitats húmedos y sombríos.
Palabras clave: Ciencia de las plantas; Fotosíntesis; Helechos vaporosos; Hymenophyllaceae; Hábitats sombreados.
INTRODUCTION
Filmy ferns from the Hymenophyllaceae family are aesthetically attractive ferns that live abundantly in the humid tropical rainforest. They show an amazing diversity in terms of morphology and the habitats they occupy, making Hymenophyllaceae a great model for studying evolutionary ecology and related adaptive survival strategies in pteridophytes (Dubuisson et al., 2003). They are small in size and some of them appear as very dark green or even black clumps and can be characterized by a single-cell thick lamina with lack of cuticle, giving them direct contact with air in their surroundings and are able to carry out rapid gas exchange. Their physiology, however, has received little attention despite the large amount of literature on their morphology and taxonomy.
Studies on sun and shade plants, particularly ferns, have been carried out for many years, since the early 1980s albeit infrequently. It has been well documented that shaded plants are incapable of achieving high photosynthetic rates although they perform more efficiently at low light irradiances (Boardman, 1977; Givnish, 1988). Sun and shade plants have been shown to exhibit obvious differences in their morphology, ultrastructure, physiology and biochemistry. Shade plants generally possess fewer, larger chloroplasts with larger grana, higher pigment content and lower photosynthetic rates. On the other hand, most plants that grow in high light intensities in their natural habitats show the opposite characteristics of shaded plants, with higher rates of photosynthesis at saturating light intensity. Similar studies have been reported, particularly on ferns and bryophytes (Nasrulhaq-Boyce & Mohamed, 1987; Nasrulhaq-Boyce & Duckett, 1991; Proctor, 2003; Marschall & Proctor, 2004). More recently, Wong et al. (2012) studied the photosynthetic rates of woody and fern species from different light regimes. They reported that the shade adapted plants showed low gross photosynthetic rate when the dark-adapted leaves were exposed to 500 or 2000 µmol/m2/s photosynthetic photon fux (PPF). Proctor (2003) studied and compared the ecophysiological characteristics, such as light response, water relations and dessication tolerance between the filmy ferns, Hymenophyllum peltatum (Poir.) Desv. [syn. H. wilsonii] and H. tunbrigense (L.) Sm. They reported that the CO2 uptake in the filmy ferns studied were low at low light irradiances. Several authors have reported similar fndings with other ferns species in terms of their structure and ecological adaptation (Hebant & Lee, 1984; Nasrulhaq-Boyce & Duckett, 1991; Johnson et al., 2000; Proctor, 2005; Proctor, 2012).
The aim of the present study was to investigate the chlorophyll and protein content, and chloroplast anatomy in selected Hymenophyllaceae species as well as their chlorophyll fluorescence and photosynthetic activity in an attempt to understand further photosynthesis in the shaded flmy ferns.
MATERIALS AND METHODS
Plant material. The filmy ferns were collected from Gunung (Mount) Ulu Kali, Pahang which is 34 miles northeast of Kuala Lumpur. Gunung Ulu Kali (1772 m.a.s.l.) is the southernmost tallest mountain in the Titiwangsa Range in Peninsular Malaysia, not far from the summit area where the renown hill resort, Genting Highlands is situated (Fig. 1). Although there are heavy anthropogenic activities in the resort area, the surrounding region is made up of large tracts of montane forests that remain untouched and house rich biological diversity. The plants investigated in the present study were obtained from these forests, with an elevation ranging between 1300 - 1700 m.a.s.l. and annual rainfall ranging between 2480-3780 mm. Irradiance, surrounding temperature and relative humidity measured ranged between 33 - 93 µmol/m2/s, 14-25.3 °C and 51% - 100%, respectively. The mean temperature was 17.9 °C, while the mean RH was 95.4%. The plants were collected from the ground and tree trunks in moist and shady areas, together with their underlying soil, placed in plastic bags, and brought back to the laboratory for work on the same day.
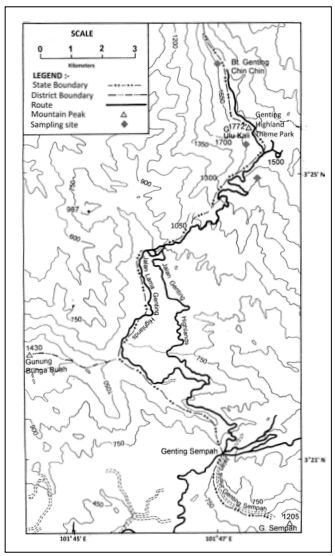
Fig. 1. Map of Gunung Ulu Kali, Genting Highlands, Pahang, Malaysia, where the ferns were collected from.
Fig. 1. Mapa de Gunung Ulu Kali, Genting Highlands, Pahang, Malasia, donde se recogieron los helechos.
Chlorophyll and protein analysis. Chlorophyll was extracted in 80% acetone from freshly collected leaves and its content was determined using the method of Arnon (1949) as outlined in Nasrulhaq-Boyce et al. (2011). Total protein content was estimated using the Lowry et al. (1951) method.
Measurement of light intensity, relative humidity and temperature. Light intensity was taken using a Lux-meter (Field scout quantum meter, USA) at each site where the fern species under study occur naturally. Readings were taken between 11.00 am to 2.00 pm in the early afternoon when it was certain that no dark clouds were present in the sky. Readings were taken between ten to ffteen times. The same procedure was followed for relative humidity and temperature.
Chlorophyll-fluorescence measurements. Chlorophyll fluorescence measurements were made using a modulated chlorophyll fluorometer (Hansatech Ltd. England). The leaves of the ferns were placed in a standard Hansatech leaf clip and chlorophyll fluorescence measured after dark adaptation for 10 minutes.
Photosynthetic activity and light response determination. Gas exchange measurements were made with a portable LICOR photosynthesis system (LI-6400XT, USA). Light response curve measurements were taken within a PAR range of 0-2000 µmol/m2/s.
Microscopy. Fresh leaves were cut into small pieces and then mounted on a slide using distilled water as medium. The slides were examined and photographed using a Leica DFC290 digital camera that attached to a compound microscope. Chloroplast size and number per cell were calculated from 20 different cells, with four cells each of fve different leaves.
Statistical analysis. The data obtained were pooled and analysed using Minitab Pro v16.1.0.0 and SPSS statistical software. A one-way ANOVA was applied to evaluate significant differences in the studied parameters. Least significant difference was calculated following a significance at p=0.05.
RESULTS
The Hymenophyllaceae species collected in the present study were generally from constantly moist, shaded highland habitats. Table 1 shows that the irradiances of their habitats were considerably low for all the eight species of Hymenophyllaceae, where the light intensities received by the plants were below 100 µmol/m2/s. Light intensity increased in the morning as a result of sunrise but remained low and fairly constant until early afternoon when it started to decrease. The temperature and relative humidity readings of the habitats of the Hymenophyllaceae species ranged between 19.8 - 23.8 °C and 69.9 - 91.4%, respectively.
Table 1. Summary of some environmental measurements of the habitats from which the species were collected.
Tabla 1. Resumen de algunas de las mediciones ambientales de los hábitats de los que se recogieron las especies.
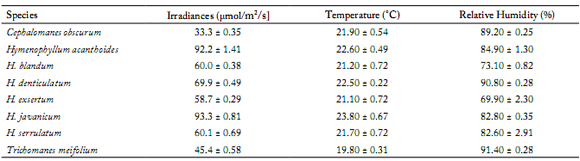
All values are expressed as mean ± S.E.
Todos los valores se expresan como promedio ± error estándar (E.E.).
Table 2 and Table 3 show the results of the determination of the chlorophyll and protein contents in the leaves of the eight shaded Hymenophyllaceae species. Chlorophyll content expressed on a firesh weight basis was notably very high in Trichomanes meifolium Bory (8.6 mg/g), followed by the other species ranging between 3.3-6.8 mg/g fresh weight (Table 2). Cephalomanes obscurum (Blume) K. Iwats., Hymenophyllum serrulatum C.Chr. and H. denticulatum Sw. showed chlorophyll values of 6.8, 6.3 and 5.8 mg/g fresh weight, respectively, whilst the other species exhibited chlorophyll values of 4.7 (H. javanicum Spreng.), 3.8 [H. acanthoides (Bosch) Rosenst.], 3.5 (H. exsertum Wall. ex Hook.), and 3.3 mg/g fresh weight (H. blandum Racib.). However, the opposite was observed with regard to the chlorophyll a/b ratio, where the Hymenophyllaceae species exhibited lower ratios, ranging between 0.9~1.9.
Table 2. Leaf chlorophyll content of Hymenophyllaceae species.
Tabla 2. Contenido foliar de clorofila de las especies Hymenophyllaceae.

All values are expressed as mean ± SE. Means that do not share a letter are significantly different at a=0.05.
Todos los valores se expresan como promedio ± error estándar (E.E.). Los promedios que no comparten una letra son significativamente diferentes a a=0,05.
Table 3. Leaf soluble protein content of Hymenophyllaceae species determined via the Lowry method.
Tabla 3. Contenido foliar de proteína soluble de especies Hymenophyllaceae determinado mediante el método de Lowry.

All values are expressed as mean ± S.E. Means that do not share a letter are significantly different at a=0.05.
Todos los valores se expresan como promedio ± error estándar (E.E.). Los promedios que no comparten una letra son significativamente diferentes a a=0,05.
Contrary to the level of chlorophyll, the soluble protein content determined using the Lowry method, was generally lower in all Hymenophyllaceae species, with the exception of H. serrulatum, (Table 3). Soluble protein content in H. serrulatum, however, was remarkably high, recording values of 53 mg/g fresh weight. H. denticulatum, H. acanthoides, T. meifolium, C. obscurum, H. javanicum, H. exsertum and H. blandum exhibited protein content ranging between 0.3 to 9.7 mg/g fresh weight. In addition to this, the protein/chlorophyll ratio in H. serrulatum was also higher (Table 3).
It has been well documented that shaded ferns have larger and fewer chloroplasts but are richer in chlorophyll content when compared to sun ferns (Boardman, 1977; Nasrulhaq-Boyce and Mohamed, 1987; Nasrulhaq-Boyce & Duckett, 1991). Light microscopy observations of the leaves of all the Hymenophyllaceae species collected showed that C. obscurum possessed the highest number of chloroplast per cell profile, numbering 138, followed by H. acanthoides (63), H. serrulatum (54), H. exsertum (46), H. javanicum (43), H. denticulatum (42), and H. blandum (34) (Table 4). However, the chloroplasts were significantly larger in H. blandum (6.5 µm) and H. denticulatum (6.4 µm) leaves which together had the fewest chloroplast number per cell than the other species, namely C. obscurum, H. acanthoides, H. serrulatum, H. exsertum and H. javanicum. These results were not suprising as the species with the larger number of chloroplasts also had smaller chloroplasts. Nevertheless the chloroplast numbers were relatively low and the leaf parenchyma cells were closely packed with chloroplasts.
Table 4. Mesophyll chloroplast number per profile in the leaves of the Hymenophyllaceae species.
Tabla 4. Número de cloroplastos del mesófilo por perfil en las hojas de las especies de Hymenophyllaceae.

All values are expressed as mean ± SE. Means that do not share a letter are significantly different at a=0.05.
Todos los valores se expresan como promedio ± error estándar (E.E.). Los promedios que no comparten una letra son significativamente differentes a a=0,05.
Chlorophyll fluorescence in H. denticulatum, T. meifolium, H. javanicum and H. serrulatum exhibited Fv/Fm ratios or photosynthetic quantum yield values, ranging between 0.71 to 0.81 (Table 5) which are values within the range for a normal healthy leaf.
Table 5. Chlorophyll fluorescence in the leaves of the Hymenophyllaceae species collected.
Tabla 5. Fluorescencia de la clorofila en las hojas de las especies de Hymenophyllaceae recogidas.

All values are expressed as mean ± SE. Means that do not share a letter are significantly different at a=0.05.
Todos los valores se expresan como promedio ± error estándar (E.E.). Los promedios que no comparten una letra son significativamente differentes a a=0,05.
Figure 2 shows the in vivo light saturation curve for photosynthesic rates in the Hymenophyllaceae species determined in their natural habitats. Initial in vivo light saturation studies with a LICOR infrared gas analyzer on five Hymenophyllaceae species, namely, H. serrulatum, T. meiofolium, H. exsertum, H. denticulatum and H. javanicum showed that CO2 assimilatory rates for all these filmy ferns were low ranging between 5 to 15 µmol CO2/m2/s. Saturation of CO2 uptake occurred at low irradiances, between 100 µmol/m2/s to 150 µmol/m2/s. Photosynthetic activity increased with increasing light intensity until light saturation was reached, throughout the duration of the experiments. The diurnal photosynthetic rates fuctuated throughout the day depending on the light intensity that came through the canopy to the forest foor. The filmy ferns showed optimal photosynthesis at light intensities lower than 150 µmol/m2/s, a light intensity to which they are normally exposed to in their native environment.

Fig. 2. Photosynthetic light response curves of shaded Hymenophyllaceae species (A) Trichomanes meiofolium; (B) Hymenophyllum javanicum; (C) H. denticulatum; (D) H. exsertum, (E) H. serrulatum, at varying light intensities. Measurements were taken in the field using a portable infrared gas analyzer (LICOR, LI-6400XT). Data are expressed in terms of projected leaf area. Each point is an average of three readings.
Fig. 2. Curvas de respuesta de la fotosíntesis a la luz de las especies sombreadas Hymenophyllaceae (A) Trichomanes meiofolium; (B) Hymenophyllum javanicum; (C) H. denticulatum; (D) H. exsertum, (E) H. serrulatum, a diferentes intensidades de luz. Las mediciones se realizaron en el campo usando un analizador de gases infrarrojo portátil (LICOR, LI-6400XT). Los datos se expresan en términos de área foliar proyectada. Cada punto es un promedio de tres lecturas.
DISCUSSION
As it is well known and documented, the eight species selected for this study, thrived in cool, moist habitats at high elevation with low light irradiances, as shown in Table 1. Similar readings were reported previously in the same area of study (Nasrulhaq-Boyce et al., 2011). There was relatively little difference in irradiance, temperature and relative humidity between the different habitats where the ferns were collected as mentioned above and in Table 1. As a result of this it was not possible to see any significant correlation between habitat light irradiance and protein content or habitat light irradiance and chloroplast number or the rate of photosynthetic activity, even though the two ferns with the highest chlorophyll content were collected from habitats with the lowest irradiances.
As shown in Table 2, the chlorophyll content expressed on a fresh weight basis was high in all the species studied. It was notably very high in the needle-like dark green leaves of Trichomanes meifolium. High chlorophyll values have been reported previously in Selaginella willdenowii (Desv. ex Poir.) Baker (Hebant & Lee, 1984), Cyclosorus multilineatus (Wall. ex Hook.) Tardieu & C. Chr. [syn. Abacopteris multilineata], Christensenia aesculifolia (Blume) Maxon, Tectaria singaporiana (Wall. ex Hook. & Grev.) Ching. [syn. Tectaria singaporeana], T. vasta (Blume) Copel. (Nasrulhaq-Boyce & Mohamed, 1987), and Teratophyllum rotundifoliatum (Bonap.) Holttum (Nasrulhaq-Boyce & Duckett, 1991), with values ranging between 2.0 ~ 5.8 mg/g fresh weight. However, the values between 6.3 to 8.6 mg/g fresh weight observed for Trichomanes meifolium, Cephalomanes obscurum and Hymenophyllum serrulatum were remarkably high. More recently, studies on the shaded Malaysian Pogonatum cirratum subsp. macrophyllum (Dozy & Molk.) Hyvönen moss species showed chlorophyll values of 4.79 mg/g fresh weight (Nasrulhaq-Boyce et al., 2011). The high chlorophyll content associated with the low irradiance level in the habitats in which the filmy ferns lived reflects the adaptation to living in a shady environment. Another observation made was the generally low chlorophyll a/b ratios in the Hymenophyllaceae leaves of the plants compared to the two ferns living in sunlit habitats, namely Dicranopteris linearis and Nephrolepis biserrata (Table 2). This is indicative of shady plants possessing a larger proportion of chl a/b-binding light harvesting complexes associated with photosystem II (PSII) and also of chloroplasts with more granal thylakoids where PSII resides. It can be regarded as an adaptation for plants adapted to living in low light environments (Anderson et al., 1988; Chow et al., 1988; Brach et al., 1993; Björkman & Demmig-Adams, 1995; Johnson et al., 2000; Marschall & Proctor. 2004; Nasrulhaq-Boyce et al., 2011; Proctor, 2012; Wong et al., 2012). A previous study on Trichomanes speciosum Willd. reported that the low chlorophyll a:b ratio observed was accompanied by an increase in the proportion of stacked thylakoids within the chloroplasts (Johnson et al., 2000). This was also reported for the deep shade fern Teratophyllum rotundifoliatum, which possesses large grana stacks with thylakoid membranes packing the stroma of the chloroplasts (Nasrulhaq-Boyce & Duckett, 1991). However, (generally) plants found in higher light environments tend to produce a higher chlorophyll a:b ratio (Ludlow & Wolf, 1975). More recently, Mathew et al. (2005) reported high chlorophyll a:b ratios in the sunny fern Onoclea sensibilis L. indicating a smaller light harvesting system with less stacking of thylakoid membranes.
Generally, plants living in shade environments have larger chloroplasts but lower chloroplast number per cell than plants thriving in an exposed area (Boardman, 1977). This was observed in Teratophyllum rotundifoliatum and the other ferns species mentioned above (Hebant & Lee, 1984; Givnish, 1988; Nasrulhaq-Boyce & Duckett, 1991; Proctor, 2004). In this study, the chloroplast numbers per profile observed in the leaves of the shaded H. acanthoides, H. serrulatum, H. exsertum, H. javanicum, H. denticulatum and H. blandum were relatively low, between 34 and 63, with significantly large chloroplast diameters ranging between 5.6 to 6.1 µm. This is consistent with previous research findings, with the exception of C. obscurum, which had a high numbers of chloroplast (138) and the smallest chloroplast size (4.8 µm). Not all shade plants exhibit similar anatomical structures. For instance, Fatsia japonica (Tunb.) Decne. & Planch. and Alocasia macrorrhiza (L.) Schott have been reported to exhibit low numbers of chloroplasts per cell whilst living in environments of high light irradiance (Chow et al., 1988). Trichomanes meifolium, which grows in shady habitats, possess lamina cells with 100-200 chloroplasts per cell, with diameter ranging between 3-6 µm, despite their needle-like structure (Nasrulhaq-Boyce & Duckett, unpublished data). Recently, Sheue et al. (2007) reported a single giant cup-shaped chloroplast, termed a bizonoplast found in the deep-shade spike moss Selaginella erythropus (Mart.) Spring. They suggested that the chloroplast structure may have an evolutionary significance in photosynthetic functionality in adaptation to low-light environments.
It is well documented that the amount of soluble protein content or ratio of soluble protein to chlorophyll content in shade plants is considerably lower than that in the sun species (Boardman, 1977; Givnish, 1988; Nasrulhaq-Boyce & Mohamed, 1987; Nasrulhaq-Boyce et al., 2011). It has been suggested that sun plants have more protein, in particular the major protein in the leaf, the photosynthetic enzyme ribulose bisphosphate carboxylase (rubisco), to enable them to photosynthesize more rapidly and eficiently. As shown in Table 3 the protein content determined by the Lowry method was low in all the shaded ferns, with values ranging between 0.3-9.7 mg/g fresh weight, compared to the sun ferns which registered values ranging between 12-20 mg/g. The exception was H. serrulatum which posted a remarkably high value, above 50 mg/g fresh weight. The latter observation runs contrary to the general rule for sun and shade plants. Nevertheless, all but one of the Hymenophyllaceae species studied exhibited a lower soluble protein to chlorophyll ratio (Table 3) than do the sun ferns Dicranopteris linearis and Nephrolepis biserrata.
Chlorophyll fluorescence in H. denticulatum, Trichomanes meifolium and H. serrulatum exhibited maximal fluorescence (Fv/Fm) ratios or photosynthetic quantum yield values, ranging between 0.73 to 0.81 (Table 5). These values are generally slightly lower than what has been reported for a normal healthy plant (0.82 ~0.83) (Peter Horton, personal communication). The low light intensities in which these plants thrive makes it difcult to get an accurate estimate of the maximum quantum yield using fluorescence. Our data suggest the maximum value of the ratio of variable to maximal fluorescence (Fv/Fm) to be ~0.8. Previous studies on T. speciosum showed Fv/Fm ratios of around 0.75, lower than the Fv/Fm ratios of T. meifolium and H. denticulatum studied (Johnson et al., 2000). They suggested that T. speciosum possessed a limited ability to quench chlorophyll fluorescence. However, Proctor (2003) has reported Fv/Fm ratios of about 0.82 in H. peltatum and H. tunbrigense, which is close to the values obtained for the Malaysian Hymenophyllaceae ferns and T. speciosum. Recent studies by Wong et al. (2012) similarly reported Fv/Fm for the dark adapted-fern leaves of Pyrrosia lingua (Tunb.) Farw., Asplenium nidus L. [syn. A. antiquum], Diplazium donianum (Mett.) Tardieu and Angiopteris somae (Hayata) Makino & Nemoto [syn. Archangiopteris somai] to be in the region of ~0.8. The lower chlorophyll fluorescence values observed in H. denticulatum, T. meifolium, H. javanicum and H. serrulatum probably indicate their limited ability to quench chlorophyll fluorescence.
It has been reported in higher vascular plants that plants grown under high light intensities show a greater photosynthetic capacity at light saturation than shade plants, but lower rates at low light intensities compared to shade plants (Boardman, 1977; Nasrulhaq-Boyce & Mohamed, 1987; Givnish, 1988; Johnson et al. 2000; Proctor, 2012; Wong et al., 2012). Previous studies on the filmy ferns Hymenophyllum wilsonii and Hymenophyllum tunbrigense have shown that saturation of photosynthetic electron flow and CO2 uptake were lower at low light irradiances (Proctor, 2003). It has been reported that isolated chloroplasts from sun Malaysian ferns showed greater in vitro photochemical activity at saturating irradiance than chloroplasts from shade ferns (Nasrulhaq-Boyce & Mohamed, 1987). They suggested the greater capacity for electron transport might be attributed to the observed higher level of electron transport carriers (photosynthetic cytochromes f, b559, b563) found in the sun ferns. Marschall and Proctor (2004) studied 39 species of mosses and 16 liverworts for their ratios of chlorophyll and total carotenoids and light saturation of photosynthetic electron flow. These authors concluded that total chlorophyll, chlorophyll a:b and chlorophyll:carotenoids ratios correlated significantly with photosynthetic photon flux density. The in vivo CO2 assimilation activities in the leaves of the filmy ferns in this study revealed low rates consistent with those observed in other ferns and bryophytes. Similar recent studies by Proctor (2012) showed that the photosynthetic rates of the Hymenophyllaceae species from Trinidad, Venezuela and New Zealand also saturated at low light intensities, where all four of the shade-adapted species had PPFD95%= 51 µmol/m2/s. Another study on four fern species, Pyrrosia lingus, Asplenium antiquum, Diplazium donianum and Archangiopteris somai, by Wong et al. (2012), showed low gross photosynthetic rate when the dark-adapted leaves were exposed to 500-2000 µmol/m2/s of light irradiance. The shadeadapted H. javanicum recorded the lowest photo-assimilatory rates [~5 µmol CO2/m2/s] followed by H. serrulatum [~9 µmol CO2/m2/s], H. denticulatum [~10 µmol CO2/m2/s], T. meiofolium [~11 µmol CO2/m2/s] and H. exsertum [~15 µmol CO2/m2/s]. These Hymenophyllaceae species showed optimal photosynthesis at light intensities below 100 µmol/m2/s, the highest light intensity to which they are normally exposed to in their natural environment. Proctor (2012) proposed that the filmy ferns (Hymenophyllaceae) are a rare example of an evolutionary shift of adaptive strategy from typical vascular plant adaptation to an integrated package adapted to more or less constantly shade humid environments, where its photosynthesis saturates at low irradiance and generally low levels of desiccation tolerance. The results on the flmy ferns are consistent with previous reports on studies with sun and shaded living ferns and show their ability to adapt to their habitats, particularly their need to utilize periodic sun flecks (Boardman, 1977; Givnish, 1988; Alfredo et al., 2010; Huang et al., 2011; Johnson et al., 2000; Proctor, 2003; Proctor, 2012; Wong et al., 2012). As expected one cannot draw any correlation between the differences in photosynthetic parameters between the species studied with their habitat light irradiances as the latter was relatively similar and all the species were from shaded habitats. Nevertheless, the fndings of this study showed that the Hymenophyllaceae species were able to adapt well to their shaded, cool and moist environment, and possessed the ability to make an efficient use of what little light is available to them for photosynthesis and growth.
ACKNOWLEDGEMENTS
This work was financially supported by the University of Malaya Research University Grant scheme no. RG060/11BIO and the Fundamental Research Grant Scheme, Ministry of Higher Education, Malaysia no. FP006/2010A.
REFERENCES
1. Anderson, J.M., W.S. Chow & D.J. Goodchild (1988). Thylakoid membrane organisation in sun/shade acclimation. Australian Journal of Plant Physiology 15: 11-26. [ Links ]
2. Alfredo, O.S., C. Hernandez, E.C. Rafael, A.B. Leon & L.J. Corcuera (2010). Differences in light usage among three fern species of genus Blechnum of contrasting ecological breadth in a forest light gradient. Ecological Research 25: 273-281. [ Links ]
3. Arnon, D.L (1949). A copper enzyme is isolated chloroplast polyphenol oxidase in B. vulgaries. Plant Physiology 24: 1-15. [ Links ]
4. Björkman, O. & B. Demmig-Adams (1994). Regulation of photosynthetic light energy capture, conversion and dissipation in leaves of higher plants. In: Schulze E-D, Caldwell MM (Eds). Ecophysiology of Photosynthesis Vol. 100. Berlin, Germany: Springer Verlag, pp. 7-47. [ Links ]
5. Boardman, N.K. (1977). Comparative photosynthesis of sun and shade plants. Annual Review of Plant Physiology 28: 355-377. [ Links ]
6. Brach, A.R., S.J. McNaughton & D.J. Raynal (1993). Photosynthetic adaptibility of two fern species of a Northern Hardwood Forest. American Fern Journal 83: 47-53. [ Links ]
7. Chow, W.S., L. Qian, D.J. Goodchild & J.M. Anderson (1988). Photosynthetic acclimation of Alocasia macrorrhiza (L.) G. Don to growth irradiance: Structure, function and composition of chloroplast. Australian Journal of Plant Physiology 15: 107-122. [ Links ]
8. Dubuisson, J.Y., S. Hennequin, F. Rakotondrainibe & H. Schneider (2003). Ecological diversity and adaptive tendencies in the tropical fern Trichomanes L. (Hymenophyllaceae) with special refference to climbing and epiphytic habits. Botanical Journal of the Linnean Society 142: 41-63. [ Links ]
9. Givnish, T.J. (1988). Adaptation to sun and shade:A whole-plant perspective. Plant Physiology 15: 63-92. [ Links ]
10. Hebant, C. & D.W. Lee (1984). Ultrastructure basis and development control of blue iridescence in Sellaginella leaves. American Journal of Botany 71: 216-219. [ Links ]
11. Huang, D., L. Wu, J.R. Chen & L. Dong (2011). Morphological plasticity, photosynthesis and chlorophyll fuorescence of Athyrium pachyphlebium at different shade levels. Photosynthetica 49: 611-618. [ Links ]
12. Johnson, G.N., F.J. Rumsey, A.D. Headley & E. Sheffield (2000). Adaptations to extreme low light in the fern Trichomanes speciosum. New Phytologist 148: 423-431. [ Links ]
13. Lowry, O.H., N.J. Rosebrough, A.L. Farr & R.J. Randall (1951). Protein measurement with the Folin reagents. Journal of Biological Chemistry 193: 265-275. [ Links ]
14. Ludlow, C.J. & F.T. Wolf (1975). Photosynthesis and respiration rates of ferns. American Fern Journal 65: 43-48. [ Links ]
15. Mathew, W.R., P.S. Joshua, B. Xu, A. Cunkelman & A.B. Ronald (2005). A comparison of physiological and morphological properties of deciduous and wintergreen ferns in Sounthern Pennsylvania. American Fern Journal 95: 45-56. [ Links ]
16. Marschall, M. & M.C.F. Proctor (2004). Are Bryophytes Shade Plants? Photosynthetic Light Responses and Proportions of Chlorophyll a, Chlorophyll b and Total Carotenoids. Annals of Botany 94: 593-603. [ Links ]
17. Nasrulhaq-Boyce, A. & M.A.H. Mohamed (1987). Photosynthetic and respiratory characteristics of Malayan sun and shade ferns. New Phytologist 105: 81 -88. [ Links ]
18. Nasrulhaq-Boyce, A. & J.G. Duckett (1991). Dimorphic epidermal cell chloroplasts in the mesophyll less leaves of an extreme shade tropical fern, Teratophyllum rotundifoliatum (R.Bonap.) Holtt.: a light and electron microscope study. New Phytologist 119: 433-44. [ Links ]
19. Nasrulhaq-Boyce, A., M.A.H. Mohamed, A.L. Lim, S.S. Barakbah, K.T. Yong & D.M. Nor (2011). Comparative morphological and photosynthetic studies on three Malaysian species of Pogonatum from habitats of varying light irradiance. Journal of Bryology 33: 35-41. [ Links ]
20. Proctor, M.C.F. (2003). Comparative ecophysiological measurements on the light responses, water relations and dessiccation tolerance of the filmy ferns Hymenophyllum wilsonii Hook. and H. Tunbrigense (L.) Smith. Annals of Botany 91: 717-727. [ Links ]
21. Proctor, M.C.F. (2005). Why do Polytrichaceae have lamellae? Journal of Bryology 27: 221-229. [ Links ]
22. Proctor, M.C.F. (2012). Light and desiccation responses of some Hymenophyllaceae (filmy ferns) from Trinidad, Venezuela and New Zealand: poikilohydry in a light-limited but low evaporation ecological niche. Annals of Botany 109: 1019-1026. [ Links ]
23. Sheue, C.R., V. Sarafis, S. Kiew, H.Y. Liu, A. Salino, L.L. Kuo-Huang, Y.P. Yang, C.C. Tsai, C.H. Lin, J.W.H. Yong & M.S.B. Ku (2007). Bizonoplast, a unique chloroplast in the epidermal cells of microphylis in the shade plant Selaginella erythropu (Selaginellaceae). American Journal of Botany 94: 1922-1929. [ Links ]
24. Wong, S.L., C.W. Chen, H.W. Huang & J.H. Weng (2012). Using combined measurements for comparison of light induction of stomatal conductance, electron transport rate and CO2 fixation in woody and fern species adapted to diferent light regimes. Tree Physiology 32: 535-544. [ Links ]














