Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.2 Vicente López dic. 2014
ARTÍCULOS ORIGINALES
Fruit development of two high oleic safflower (Carthamus tinctorius L.) cultivars
Desarrollo del fruto de dos cultivares de cártamo (Carthamus tinctorius L.) alto oleico
Franchini MC1, AC Flemmer1, LI Lindström1, MA David2, PA Fernandez3
1 Cátedra de Morfología Vegetal, Dpto. de Agronomía, UNSur, San Andrés 800, Bahía Blanca, 8000, Argentina.
2 Secretaría General de Ciencia y Tecnología, Dpto. de Agronomía, UNSur, San Andrés 800, Bahía Blanca, 8000, Argentina.
3 Comisión de Investigaciones Científicas, Provincia de Buenos Aires, Dpto. de Agronomía, UNSur, San Andrés 800, Bahía Blanca, 8000, Argentina.
Address Correspondence to: MC Franchini, e-mail: franchini@uns.edu.ar
Recibido / Received 23.VIII.2014.
Aceptado / Accepted 10.IX.2014.
Abstract. The purpose of this study was to describe fruit development in two high oleic safflower (Carthamus tinctorius L.) cultivars during four growing seasons. Pericarp histogenesis, and dynamics of pericarp and seed dry weight and fruit water content were studied. The dynamics of the pericarp and seed growth was similar between cultivars and years. The pericarp completed its growth before the seed. Pericarp potential size was already set at anthesis as no cell division was observed at this time. Maximum pericarp dry weight was achieved 8 days after anthesis, when cell wall lignification concluded. At this time, twinned prismatic simetric crystals had decreased in number and size respect to those observed at anthesis. Physiological maturity (maximum seed dry weight) was achieved between 17 and 25 days after anthesis. Similar pericarp growth rate and duration between cultivars and years were associated to similar maximum pericarp dry weight (17 mg), except in 2012. In this year, the higher maximum pericarp dry weight (20 mg) was only associated to a higher fruit volume (50 µL). Maximum seed dry weight (22 mg) was lower in CW88 OL than in CW99 OL, except in 2012. However, seed growth rate and time of physiological maturity were similar between cultivars. Fruit water content at physiological maturity (39%) was similar between cultivars. The recommended moisture (10-13%) at harvesting was achieved around 33 days after anthesis. The timing of the different morphological and histological events of safflower fruit development presented in this work sets a not-yet-existent conceptual framework, and constitutes an important tool for the interpretation and comparison of the effects of genotype, environment or agricultural management practices on crop yield and fruit quality.
Keywords: Carthamus tinctorius L.; Fruit development; Fruit water content; Physiological maturity.
Resumen. El objetivo de este trabajo fue describir el desarrollo del fruto de dos cultivares de cártamo (Carthamus tinctorius L.) alto oleico durante cuatro estaciones de crecimiento. Se estudió la histogénesis del pericarpio, y la dinámica del peso seco del pericarpio y de la semilla y del contenido de agua del fruto. La dinámica del crecimiento del pericarpio y de la semilla fue similar entre cultivares y años. El pericarpio completó su crecimiento antes que la semilla. El tamaño potencial del pericarpio ya estaba fijado en antesis ya que en ese momento no se observaron divisiones celulares. El máximo peso seco del pericarpio se alcanzó 8 días después de la antesis, momento en el que concluyó la lignificación de sus células. Al mismo tiempo, los pares de cristales prismáticos y simétricos habían disminuido en número y tamaño respecto de los observados en antesis. La madurez fisiológica (máximo peso seco de la semilla) se alcanzó entre los 17 y 25 días después de la antesis. La duración y tasa de crecimiento del pericarpio similares entre cultivares y años estuvieron asociadas a un similar máximo peso seco del pericarpio (17 mg), excepto en 2012. En este año, el mayor máximo peso seco del pericarpio (20 mg) estuvo únicamente asociado al mayor volumen del fruto (50 µL). El máximo peso seco de la semilla (22 mg) fue menor en CW88 OL respecto de CW99 OL, excepto en 2012. Sin embargo, la tasa de crecimiento de la semilla y el tiempo en que se alcanzó la madurez fisiológica fueron similares entre cultivares. El contenido de agua del fruto en madurez fsiológica (39%) fue similar entre cultivares. El porcentaje de humedad del fruto recomendado para la cosecha (10-13%) se alcanzó alrededor de los 33 días después de la antesis. El momento en que ocurren los diferentes eventos morfológicos e histológicos del desarrollo del fruto de cártamo presentados en este trabajo establece un marco conceptual inexistente, y constituye una herramienta importante para la interpretación y comparación del efecto del genotipo, el medio ambiente o las prácticas de manejo del cultivo sobre el rendimiento y la calidad del fruto de cártamo.
Palabras clave: Carthamus tinctorius L.; Desarrollo del fruto; Contenido de agua del fruto; Madurez fisiológica.
INTRODUCTION
Safflower (Carthamus tinctorius L.) is an annual oilseed crop belonging to the Asteraceae family that has attracted significant interest in the semiarid central region of Argentina as an alternative crop in rotation with wheat. This is because of its adaptation to dry climatic conditions (Weiss, 2000).
Safflower was traditionally grown to obtain its flowers as a source of dye for coloring food and fabrics (Sujatha, 2008). Today, it is mainly cultivated for its fruits, which are used for extraction of high quality, edible and industrial oil (Knowles, 1989; Johnson et al., 2007). Safflower fruits are also used in the birdseed market, especially for members of the parrot family and pigeons in Canada, USA, France, Egypt and Japan (Peterson, 1996).
The fruit (grain) consists of the pericarp (hull), which represents about 33-60% of its dry weight, and the seed (40-67%) where the oil is synthesized and accumulated (Dajue & Mündel, 1996; Lyon et al., 2007). Traditional safflower oil contains about 6-8% palmitic acid, 2-3% stearic acid, 16-20% oleic acid and 71-75% linoleic acid. However, high oleic oil types that contain more than 75% of oleic acid have been developed and are widely used in North America (Velasco & Fernández-Martínez, 2001).
The weight and the structure of the safflower fruit affect both crop yield and the efciency of the industrial process for oil extraction (Smith, 1996; Fernandez et al., 2012a). Fruit weight is the last yield component to be fixed. The quality and cost of the byproducts obtained from the oil extraction process depend on the easiness with which the pericarp separates from the seed (Fernandez et al., 2012a; Figueiredo et al., 2013), and this in turn could be afected by pericarp structure (Denis et al., 1994).
Despite the importance of cereals and oilseeds as a human and animal food source, there is a scarcity of information regarding the development of their fruit. In some grain crops, pericarp growth ends when the seed is still very small, so the degree of development attained by the pericarp could limit further seed development. For example, ovary volume and weight at anthesis affect the final seed or fruit weight in wheat (Triticum aestivum L., Calderini et al., 2000; Lizana et al., 2007; Ugarte et al., 2007), barley (Hordeum vulgare L., Scott et al., 1983), soybean (Glycine max (L.) Mill., Egli, 1998) and sunflower (Helianthus annuus L., Lindström et al., 2006b; Mantese et al., 2006). In safflower, only some of the developmental stages of the safflower pericarp (Ebert & Knowles, 1968; Franchini et al., 2012) and seed (Franchini et al., 2012) have been described.
Defining the different stages of safflower pericarp development would be an important contribution to studies aimed at understanding the relationships and interactions between size and anatomical structure of the pericarp in different genotypes and environments, as well as their effect on fnal fruit weight and quality.
Seed development, from fertilization to the mature seed, can be divided into three phases. Phase I begins with fertilization and is characterized by a strong mitotic activity and a slow increase of dry weight (Egli et al., 1981; Guldan & Brun, 1985; Gao et al., 1992; Jones et al., 1996; Lindström et al., 2006b). In phase II (i.e., the linear phase of seed growth) the seed accumulates reserve materials such as fats and carbohydrates. This gives an economic value to the seed. Phase III begins when the accumulation of reserve materials declines prior to stopping at physiological maturity (Egli, 1998). To our knowledge, the dynamics of safflower seed dry weight has not yet been studied.
Physiological maturity represents the end of seed growth (maximum seed dry weight), hence the end of fruit growth and yield processes. Knowledge of the time of physiological maturity would allow to program harvest time to avoid seed sprouting in the heads (Shakeri-Amoughin et al., 2012), hail and insect damage (Mündel et al., 2004), and decisions concerning weed control (Egli, 1998). Usually, physiological maturity is estimated using indirect indicators based on visual changes (1) in fruit characters or in the color of some plant organs in soybean (e.g., the loss of green color from pods: Tekrony et al., 1979), (2) of the receptacle color from green to yellow in sunflower (Schneiter & Miller, 1981), or (3) when plants are quite dry but not brittle, and the capitulum bracts became brown in safflower (Mündel et al., 2004). Visual methods are subjective and can be afected by environmental conditions as has been determined in soybean (Gbikpi & Crookston, 1981), sunflower (Schneiter & Miller, 1981) and maize (Zea mays L., Hunter et al., 1991). Moreover, visual estimation of physiological maturity has not been contrasted with the achievement of maximum fruit dry weight in safflower.
A more objective approach to determine physiological maturity is establishing the fruit water content which coincides with the maximum fruit dry weight (Gambín et al., 2007; Rondanini et al., 2009). Fruit water content at physiological maturity is about 38% in sunflower (Rondanini et al., 2007), 37% in wheat (Calderini et al., 2000), 35% in maize (Sala et al., 2007) and 60% in soybean (Fraser et al., 1982). The relationship between fruit dry weight and its water content has only been studied in one safflower cultivar grown in Iran (Shakeri-Amoughin et al., 2012). These authors reported that fruit water content was about 13% at physiological maturity, which is the moisture suitable for grain storage and commercialization.
The purpose of this study was to describe pericarp histogenesis, and the dynamics of pericarp and seed dry weight and fruit water content in two high oleic safflower cultivars. This information is essential to evaluate the effects of genotype, environment and agricultural practices at different times of fruit ontogeny on crop yield and fruit quality.
MATERIALS AND METHODS
Experimental design and growing conditions. Two commercial high oleic safflower cultivars (CW88 OL and CW99 OL) provided by Oleaginosa Moreno Hermanos S.A. and developed by SeedTec, Woodland, California, were studied. These cultivars are currently grown in the semiarid central region of Argentina. Sowing was made during four growing seasons on 19 August 2008 and 26 August 2010, 2011 and 2012 at the experimental field of the Agronomy Department-UNSur, Bahía Blanca, Argentina (38° 45' S; 62° 11' W). The soil was a typical Ustipsamment (Soil Survey Staf, 1999). Sowing was performed manually at 3-4 cm depth, with 35 cm row spacing and 10 cm between plants. The experimental design was completely randomized with two replicates (plots) per cultivar. The size of the plot was 5 to 10 rows width and 2 to 3 m long, depending on the year. Plant density was adjusted to 36 plants/m2. Weeds were hand controlled. Total growing season rainfall (July-January) was 176, 170, 215 and 433 mm in 2008, 2010, 2011 and 2012, respectively. Mean daily temperatures from anthesis to harvest maturity were 23.1, 23.6, 22.4 and 22.5 °C during 2008, 2010, 2011 and 2012, respectively.
Plant sampling and measurement. Each year, 60 to 110 plants were tagged at anthesis per cultivar per plot (BBCH 61, Flemmer et al., 2014). Anthesis was defned when at least 50% of the plant stand reached this growth stage. The main capitulum of two of the tagged plants per cultivar per plot was harvested every three to five days from anthesis to harvest maturity (BBCH 97, Flemmer et al., 2014) and was immediately placed in a portable refrigerator. Only safflower main capitulum was sampled since we consider that the main capitulum development is representative of the plant development as a whole.
Pericarp histogenesis. To study pericarp histogenesis, fruits of both cultivars of all harvested capitula were fixed in formaldehyde, from anthesis to harvest maturity during 2008. Some of the fixed samples were embedded in paraffin and processed according to conventional histological techniques of cutting (12 µm) and staining (safranin-fast green; Ruzin, 1999) to obtain cross sections of the middle portion of the fruits. Other samples were included in Spurr's low viscocity resin (Spurr, 1969), cut (1-3 µm) on a LKB ultramicrotome and stained (toluidine blue; Ruzin, 1999). Pericarp hand cross sections from the middle portion of the remaining fixed fruits were also made to evaluate the evolution of pericarp thickness and tissue lignification (number of sclerified strata) in both cultivars. Presence of lignified tissues was detected by phloroglucinol-HCl staining (Ruzzin, 1999).
Observations and measurements were made with a Nikon Labophot-2 optical microscope equipped with a Nikon Coolpix 4500 camera and an ocular micrometer.
A preliminary description of the anatomical characteristics of CW99 OL pericarp observed during 2008 was already published (Franchini et al., 2012). Hence, in this work we present pericarp anatomy of CW88 OL and those anatomical characteristics of CW99 OL pericarp that provide new information in addition to that previoulsy published.
Fruit, pericarp and seed variables. Dynamics of pericarp, and seed dry weight and fruit water content (FWC) were studied in both cultivars from anthesis to harvest maturity during the four years. As soon as possible, 10-20 fruits of all harvested capitula were sealed into Petri dishes and, except for 2008, fresh weight was determined. After that, the pericarp was separated from the seed, and both were dried for 72 h at 60 °C and thereafter weighed. The percentage of FWC was then calculated on a fresh weight basis. In addition, the volume of 15 to 20 mature fruits per cultivar and year (2010, 2011 and 2012) was determined at harvest maturity using the water displacement method described by Wessel-Beaver et al. (1984).
A preliminary description of the dynamics of pericarp and seed dry weight of CW99 OL observed in 2008 and 2009 was already published (Franchini et al., 2012). We present the results of dry weight dynamics and fruit water content of CW88 OL, and those of CW99 OL, that provide new information to that previously published.
Data analysis. Relationships between pericarp dry weight, seed dry weight and pericarp thickness versus days after anthesis (DAA) were assessed using a bi-linear broken-stick function with an unknown breaking point (Di Rienzo et al., 2009). The conditional model fitted was y = a + b * x (for x < c) and y = b * c (for x > c) where y was the studied variable, x was DAA, a was the intercept, b was the slope which estimates the mean growth rate of the pericarp or the seed (mg/day), and c was the unknown breaking point (maximum value of the studied variable). If necessary, first sampling dates were not considered to evaluate the linear phase of growth and the maximum value of the studied variables.
Dynamics of FWC was described by fitting third order polynomials to the water content and DAA relationships during the whole observation period.
Data from cultivars and years were subjected to analysis of variance and mean differences were compared with the test LSD (Least Significant Difference test, Di Rienzo et al., 2009). Regression analyses were used to assess the degree of association between variables.
RESULTS
Pericarp histogenesis. At anthesis (Fig. 1A), pericarp transverse sections of CW88 OL showed an outer uniseriate epidermis covered by a cuticle (Fig. 1D). Immediately below, there were 8-10 strata of outer parenchymatic cells separated from the inner parenchyma (5-6 strata) by 4-5 strata of secretory cells (Fig. 1D). At this time, no dividing cells were observed in the pericarp. Twinned prismatic simetric crystals which shared a basal plane face were observed in many of the parenchymatic cells of the CW88 OL and CW99 OL pericarps (Figs. 1D and 2). These crystals, identified by rotation between crossed polarizers, were birefringent.

Fig. 1. External appearance of the main capitulum and pericarp transverse section of one of the fruits of the CW88 OL safflower cultivar at anthesis (A, D), at maximum pericarp dry weight (B, E) and at harvest maturity (C, F) during 2008. e: epidermis, ip: inner parenchyma, op: outer parenchyma, pl: phytomelanin layer, sc: secretory cells. Arrows in Fig. D indicate twinned prismatic simetric crystals. Scale bar: A-C = 10 mm; D-F = 40 µm.
Fig. 1. Apariencia externa del capítulo principal, y sección transversal del pericarpio de uno de los frutos del cultivar de cártamo CW88 OL en antesis (A, D), cuando el pericarpio alcanza su máximo peso seco (B, E) y en madurez de cosecha (C, F) durante 2008. e: epidermis, ip: parénquima interno, op: parénquima externo, pl: capa de fitomelanina, sc: células secretoras. Las flechas en la Fig. D señalan los cristales prismáticos, simétricos y apareados. Escalas: A-C = 10 mm; D-F = 40 µm.

Fig. 2. Scanning electron microscope image of CW88 OL pericarp at anthesis showing twinned prismatic simetric crystals (c) in the outer parenchyma. Each arrow indicates the shared surface for each crystal pair. Scale bar = 10 µm.
Fig. 2. Imagen de microscopía electrónica de barrido del pericarpio de CW88 OL en antesis mostrando los cristales prismáticos, simétricos y apareados (c) en el parénquima externo. La flecha señala la superficie en común a cada par de cristales. Escala = 10 µm.
Dynamics of pericarp thickness was similar (p>0.25) between cultivars during 2008. Maximum pericarp thickness was achieved at 7 and 8 DAA being 250 µm ± 10.53 and 246 µm ± 12.81 in CW88 OL and CW99 OL, respectively. At this time, the phytomelanin layer was discontinuous in both cultivars. At 8 DAA (Fig 1B), lignification of the pericarp cells of CW88 OL was complete including all cells of the inner parenchyma and 4-8 layers of cells of the outer parenchyma (Fig. 1E). By the time lignification was complete, the phytomelanin layer was continuous and the number and size of the crystals had decreased considerably in both cultivars; it was difficult to observe them in detail by light microscopy (Fig. 1E). Pericarp thickness and anatomical structure of CW88 OL did not change between 8 DAA (Fig. 1E) and harvest maturity (Fig. 1C and F). At harvest maturity, pericarp thickness and number of sclerified strata of the outer parenchyma were similar between cultivars and years (Table 1). Mean fnal pericarp thickness and number of sclerified strata in the outer parenchyma, averaged over the four years, were 231 µm ± 8.09 and 6 ± 0.40 in CW88 OL, and 265 µm ± 6.59 and 6 ± 0.14 in CW99 OL, respectively.
Table 1. Pericarp variables of CW88 OL and CW99 OL safflower cultivars during four years.
Tabla 1. Variables del pericarpio de los cultivares de cártamo CW88 OL y CW99 OL evaluadas durante cuatro años.

When Y x C interaction is non-significant (ns), mean values within a column followed by the same letter are similar (p>0.05). When Y x C interaction is significant (* p<0.05, ** p<0.01), mean values within each variable followed by the same letter are similar (p>0.05). MDW: maximum dry weight, NSS: number of sclerified strata.
Cuando la interacción Y x C es no significativa (ns), los valores promedio dentro de una columna seguidos por la misma letra son similares (p>0,05). Cuando la interacción Y x C es significativa (* p<0,05, ** p<0,01), los valores promedio dentro de cada variable seguidos por la misma letra son similares (p>0,05). MDW: máximo peso seco, NSS: número de estratos esclerificados.
Pericarp and seed variables. Dynamics of pericarp and seed dry weight showed a similar pattern for both cultivars and years, with the pericarp reaching its maximum dry weight before the seed (Fig. 3).
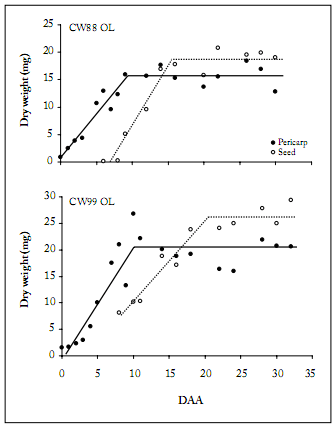
Fig. 3. Dynamics of the pericarp () and seed (○) dry weights (mg) of the CW88 OL and CW99 OL safflower cultivars during 2011. DAA: days after anthesis.
Fig. 3. Dinámica del peso seco (mg) del pericarpio () y de la semilla (○) de los cultivares de cártamo CW88 OL y CW99 OL durante 2011. DAA: días después de la antesis.
Pericarp growth rate and duration were similar between years and cultivars except in 2012 (Table 1). The highest pericarp growth duration was observed in CW99 OL in 2012, and it was associated with one of the lowest pericarp growth rates. Consistently, a negative relationship was found between pericarp growth rate and growth duration (R2 = 0.67; p<0.01). Maximum pericarp dry weight was similar between cultivars: it was 15.77 mg ± 0.87 in CW88 OL, and 18.28 mg ± 0.57 in CW99 OL. Comparing years, the highest maximum pericarp dry weight was observed in 2012 (Table 1).
Seed growth rate and time of physiological maturity were similar between cultivars: they were 1.48 mg/day ± 0.16 and 21 days ± 1.56 in CW88 OL, and 1.86 mg/day ± 0.21 and 21 days ± 1.11 in CW99 OL, respectively. These variables were only affected by years (Table 2). Except in 2012, maximum seed dry weight was lower in CW88 OL than in CW99 OL (Table 2). Averaged over cultivars, physiological maturity (PM) was delayed from three to six days in 2008 and 2012 with respect to the other years, and this was associated with one of the lowest seed growth rates. As it was observed for the pericarp, a negative relationship was found between seed growth rate and time of PM (R2 = 0.70; p<0.01). When the fruit reached PM, around 70% of the bracts of the main capitulum had turned yellow.
Table 2. Seed variables of CW88 OL and CW99 OL safflower cultivars during four years.
Tabla 2. Variables de la semilla de los cultivares de cártamo CW88 OL y CW99 OL evaluadas durante cuatro años.

When Y x C interaction is non-significant (ns), mean values within a column followed by the same letter are similar (p>0.05). When Y x C interaction is significant (* p<0.05, ** p<0.01), mean values within each variable followed by the same letter are similar (p>0.05). MDW: maximum dry weight, PM: physiological maturity.
Cuando la interacción Y x C es no significativa (ns), los valores promedio dentro de una columna seguidos por la misma letra son similares (p>0,05). Cuando la interacción Y x C es significativa (* p<0,05, ** p<0,01), los valores promedio dentro de cada variable seguidos por la misma letra son similares (p>0,05). MDW: máximo peso seco, PM: madurez fisiológica.
Fruit variables. FWC dropped slowly at frst and then more rapidly, from anthesis to harvest maturity for both cultivars and years (Fig. 4). FWC at anthesis and at the time when the pericarp achieved its maximum dry weight were 77.21% ± 0.87 and 70.09% ± 1.41 in CW88 OL, and 76.63% ± 0.65 and 67.45% ± 2.99 in CW99 OL, respectively (Table 3). At anthesis, FWC was 4% higher (p<0.05) in 2012 than in the other years. At PM, no differences were detected in FWC between years and cultivars; the exception was CW99 OL which showed a higher FWC at PM in 2012 than in 2011 (Table 3). Recommended moisture level of the fruit at harvesting (12%) was achieved around 27 DAA in both cultivars in 2011 (Fig. 4), and 37 DAA in 2012 (data not shown). Harvest moisture level could not be determined in 2010: plants had to be harvested earlier to avoid fruit germination in the plant due to significant rainfall. At harvest maturity, all bracts of the main capitulum had turned yellow (Fig. 1C).

Fig. 4. Dynamics of the fruit water content (FWC, %) of the CW88 OL and CW99 OL safflower cultivars during 2011. DAA: days after anthesis, MPDW: maximum pericarp dry weight, PM: physiological maturity.
Fig. 4. Dinámica del contenido de agua del fruto (FWC, %) de los cultivares de cártamo CW88 OL y CW99 OL durante 2011. DAA: días después de la antesis, MPDW: máximo peso seco del pericarpio, PM: madurez fisiológica.
Table 3. Fruit variables of CW88 OL and CW99 OL safflower cultivars during four years.
Tabla 3. Variables del fruto de los cultivares de cártamo CW88 OL y CW99 OL evaluadas durante cuatro años.

When Y x C interaction is non significant (ns), mean values within a column followed by the same letter are similar (p>0.05). When Y x C interaction is significant (* p<0.05, ** p<0.01), mean values within each variable followed by the same letter are similar (p>0.05). FWC: fruit water content, MPDW: maximum pericarp dry weight, PM: physiological maturity.
Cuando la interacción Y x C es no significativa (ns), los valores promedio dentro de una columna seguidos por la misma letra son similares (p>0,05). Cuando la interacción Y x C es significativa (* p<0,05, ** p<0,01), los valores promedio dentro de cada variable seguidos por la misma letra son similares (p>0,05). FWC: contenido de agua del fruto, MPDW: máximo peso seco del pericarpio, PM: madurez fisiológica.
Averaged over years, fruit volume was 15% higher in CW99 OL (47.25 µL ± 2.62) (Table 3). Comparing years, fruit volume was 19 to 27% higher in 2012 than in the other years. In general, higher fruit volume was associated with higher maximum pericarp dry weight (Table 1 and 3).
DISCUSSION
Until now, studies of the histogenic development of the safflower pericarp have been reported in some cultivars (Ebert & Knowles, 1968; Franchini et al., 2012). The results reported here provide the first complete description of the safflower fruit development relating pericarp histogenesis and thickness, fruit dry weight and water content dynamics from anthesis to harvest maturity. In particular, our work provides the frst report regarding the evolution of safower pericarp thickness.
In general, our results in CW88 OL pericarp histogenesis during 2008 were similar to those in (1) a safflower normal line (Ebert & Knowles, 1968) and (2) CW99 OL safflower cultivar (Franchini et al., 2012). CW88 OL pericarp potential size was already fixed at anthesis, as no more cell divisions were observed in the ovary wall, and pericarp lignification was complete at 8 DAA.
The pericarp could play a critical role in the control of final seed size. In sunflower, the growing seed can compress the inner parenchymatic tissue of the pericarp reducing its thickness almost by 50% at PM (Lindström et al., 2007). Conversely, we observed that the inner parenchyma was completely lignified and this tissue could not be compressed by the developing seed in the safflower pericarp. According to this, maximum pericarp thickness was achieved at the same time secondary cell wall lignification was completed in CW88 OL and CW99 OL. These results lead us to suggest the hypothesis that the available space for the maximum development of the embryo (embryo potential size) is set when pericarp lignification ends.
A phytomelanin layer was observed in the pericarps of the CW88 OL and CW99 OL safflower cultivars as it was stated by Ebert & Knowles (1968) in other safflower lines. Roth (1977) reported that in safflower, the phytomelanin layer was originated by lysis of the walls of the secretory cells. Otherwise, this layer would be secreted to the esquizogenic space generated between the hypodermis and the sclerenchyma in sunfflower (Hanasusek, 1902 cited by Roth, 1977; Pandey & Dhakal, 2001). It is well known the deffense role of the phytomelanin layer in protecting the seed from (1) predation, primarily by insects, and (2) extreme environmental conditions (Pandey & Dhakal, 2001).
We observed twinned prismatic crystals in the parenchymatic tissue of the pericarp of CW88 OL and CW99 OL at anthesis. These crystals were birefringent and similar to one of the calcium oxalate crystals described by Dormer (1961) in the ovary wall of safflower, suggesting that they could be calcium oxalate crystals. The occurrence of calcium oxalate crystals in Asteraceae has been reported by Zarembo and Boyko (2008). Accumulation of calcium in the cell wall of young cells could interfere with the normal process of cell expansion. Thus, the presence of calcium oxalate crystals in young cells may serve as a calcium sink to reduce apoplastic calcium concentration (Franceschi & Nakata, 2005). In soybean seeds, calcium oxalate crystals increased in number from fertilization until 16 days later. From this moment onwards, they started to decrease up to seed maturation (Ilarslan et al., 1997). These authors hypothesized that as seeds complete maturation, there is a lowering of the pH inside the cell that could cause crystal disruption and liberation of calcium and oxalate. The same process may explain the decrease in the number and size of safflower pericarp crystals by 8-9 DAA when cell wall lignification was complete. Our results constitute the first reference about the dynamics of the pericarp crystals in safflower.
Dynamics of pericarp dry weight was similar between cultivars and years, and to the one described by other authors in safflower (Franchini et al., 2012) and in sunflower (Connor & Hall, 1997; Mantese et al., 2006; Rondanini et al., 2006; Lindström, 2012). Pericarp weight of a dry fruit is mainly composed by secondary cell walls (Roth, 1977; Lindström, 2012). In agreement with this, safower maximum pericarp dry weight was achieved at the same time lignification was completed. Maximum pericarp dry weight was mainly associated to fruit volume (Lindström et al., 2006b).
Final seed weight can be described as a function of the rate and duration of dry weight accumulation (Egli, 1998). In our work, differences in maximum seed dry weight between cultivars were not explained by time of PM (duration). Although no significant differences were detected (p=0.10) between cultivars, seed growth rates were 26% higher in CW99 OL than in the other cultivar. In turn, differences in seed growth rates could be associated to the number of cells fixed in the seed as it was observed by Lindström et al. (2006b) in sunflower.
Safflower seed growth rates (mean=1.8 mg/day) was within the values observed in cereals (1.7 to 2.4 mg/day, Egli, 1998) and in two sunflower hybrids (1.1-2.1 mg/day, Lindström et al., 2006b). In other sunflower cultivars, Mantese et al. (2006) observed higher values of seed growth rate (1.8 to 3.7 mg/day). Also, the number of days between anthesis and PM was similar to that observed by Koutroubas & Papakosta (2010) in other safflower cultivars grown under mediterranean environments.
FWC at PM depends on the species. For example, FWC at PM is 21% in castor bean (Ricinus communis L., Vallejos et al., 2011), 60% in soybean (Fraser et al., 1982), 38% in sunflower (Rondanini et al., 2007) and 37% in wheat (Calderini et al., 2000). In safflower, we observed that mean FWC at PM was 39% ± 2.24. At this time, 70% of capitulum bracts were yellow. This stage corresponds to BBCH 87 (Flemmer et al., 2014). Shakeri-Amoughin et al. (2012) observed that safflower fruits reached PM 42 days after flowering with 13% moisture. It is surprising that they have observed that PM is achieved with the FWC recommended for harvest. In soybean (Fraser et al., 1982) and sunflower (Rondanini et al., 2007), FWC at PM appears to be constant across genotypes. This should be verifed in other safflower genotypes, besides those studied here, to develop a predictive model of the time when PM is reached based on the moisture content of the fruits as in sunflower (Rondanini et al., 2007).
Safflower pericarp water content is the dominant component of fruit water dynamics since 75% of the whole-fruit water is contained within the pericarp when the fruit reaches its maximum water content (David, M.A, personal communication). Similar results were observed in sunflower (Rondanini et al., 2009). Considering that the water in the pericarp would be located in the protoplast of living cells, and that the parenchymatous tissue of safower pericarp is located beneath the epidermis, drying of safflower fruits at the field would be faster than that in sunflower, where the arrangement of the parenchymatic and the lignified tissues is reversed (Lindström et al., 2006a). Hence, safower fruit would continue to lose water from the time it is harvested at the field (10-13% of FWC) until it is received in the laboratory (5-7% of FWC; Fernandez et al., 2012b).
CONCLUSIONS
The safflower fruit development presented here provides novel information about pericarp histogenesis, and the dynamics of pericarp and seed dry weight, pericarp thickness and fruit water content. This information can be used to evaluate and understand how genotype, environment (temperature, solar radiation, water availability) or agricultural management practices (crop density, sowing date) could affect safflower fruit weight and morphology as well as pericarp anatomy, parameters directly associated with crop yield and fruit quality.
The description of fruit drying in both studied cultivars would allow farmers to determine the right time for harvesting once PM (39% of FWC) is reached. Therefore, farmers will be able to avoid environmental conditions that could cause yield losses. A chemical herbicide application could be necessary before the harvest to desiccate those parts of the plant which were still green. This herbicide would also contribute to reach the FWC suitable for grain storage.
ACKNOWLEDGMENTS
This work was supported by grants from Oleaginosa Moreno Hermanos S.A. and the Secretaría General de Ciencia y Tecnología (Universidad Nacional del Sur), Argentina.
REFERENCES
1. Calderini, D.F., L.G. Abledo & G.A. Slafer (2000). Physiological maturity in wheat based on kernel water and dry matter. Agronomy Journal 92: 895-901. [ Links ]
2. Connor, D.J. & A.J. Hall (1997). Sunflower physiology. In: Schneiter, A.A, Seiler, A.A. (eds), pp. 113-182. Sunflower Technology and Production. Agronomy Monograph, no. 35. ASA, Madison, Wisconsin, 834 p. [ Links ]
3. Dajue, L. & H.H. Mündel (1996). Safflower (Carthamus tinctorius L.). Promoting the conservation and use of underutilized and neglected crops. Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute, Rome, Italy, 83 p. [ Links ]
4. Denis, L., V. Coelho & F. Vear (1994). Pericarp structure and hullability in sunflower inbred lines and hybrids. Agronomie 14: 453-461. [ Links ]
5. Di Rienzo, J.A., F. Casanoves, M.G. Balzarini, L. Gonzalez, M. Tablada & C.W. Robledo (2009). InfoStat, Software estadístico. http://www.infostat.com.ar [ Links ]
6. Dormer, K.J. (1961). The crystals in the ovaries of certain compositae. Annals of Botany 25: 241-256. [ Links ]
7. Ebert, W.W. & P.F. Knowles (1968). Developmental and anatomical characteristic of thin hull mutants of Carthamus tinctorius (Compositae). American Journal of Botany 55: 421-430. [ Links ]
8. Egli, D.B. (1998). Seed Biology and the Yield of Grain Crops. CAB Int., Wallingford, 196 p. [ Links ]
9. Egli, D.B., J. Fraser, J.E. Leggett & C.G. Poneleit (1981). Control of seed growth in soya beans [Glycine max (L.) Merrill]. Annals of Botany 48: 171-176. [ Links ]
10. Fernandez, P.A., L.I. Lindström, M.C. Franchini & L.F. Hernández (2012a). Hull anatomy and hullability in safflower (Carthamus tinctorius L.). Journal of Oilseeds Research 29:130-132. [ Links ]
11. Fernandez, P.A., L.I. Lindström, M.A. David & L.F. Hernández (2012b) Procesamiento industrial del cártamo (Carthamus tinctorius L.). Aceites y Grasas 3: 88-94. [ Links ]
12. Flemmer, A.C., M.C. Franchini & L.I. Lindström (2014). Description of safflower (Carthamus tinctorius L.) phenological growth stages according to the extended BBCH-scale. Annals of Applied Biology (In Press). [ Links ]
13. Figueiredo, A.K., L.M. Rodríguez, L.I. Lindström, I.C. Riccobene & S.M. Nolasco (2013). Performance analysis of a dehulling system for safflower grains. Industrial Crop and Products 43: 311-317. [ Links ]
14. Franceschi, V.R. & P.A. Nakata (2005). Calcium oxalate crystals in plants. Formation and function. Annual Review of Plant Biology 56: 41-71. [ Links ]
15. Franchini, M.C., A.C. Flemmer & L.I. Lindström (2012). Grain yield, yield components and oil content of safflower (Carthamus tinctorius L.) growing under semiarid conditions in Argentina. Journal of Oilseeds Research 29: 130-132. [ Links ]
16. Fraser, J., D.B. Egli & J.E. Leggett (1982). Pod and seed development in soybean cultivars with differences in seed size. Agronomy Journal 74: 81-85. [ Links ]
17. Gambín, B.L., L. Borrás & M.E. Otegui (2007). Kernel water relations and duration of grain filling in maize temperate hybrids. Field Crops Research 101: 1-9. [ Links ]
18. Gao, X., D. Francis, L.C. Ormrod & M.D. Bennett (1992). Changes in cell number and cell division activity during endosperm development in allohexaploid wheat (Triticum aestivum). Journal of Experimental Botany 43: 1063-1610. [ Links ]
19. Gbikpi, P.J. & R.K. Crookston (1981). Effect of flowering date on accumulation of dry matter and protein in soybean seeds. Crop Science 21: 652-655. [ Links ]
20. Guldan, S.J. & W.A. Brun (1985). Relationship of cotyledon cell number and seed respiration to soybean seed growth. Crop Science 25: 815-819. [ Links ]
21. Hunter, J.L., D.M. Tekrony, D.F. Miles & D.B. Egli (1991). Corn seed maturity indicators and their relationship to uptake of carbon-14 assimilate. Crop Science 31: 1309-1313. [ Links ]
22. Ilarslan, H., R.G. Palmer, J. Imsande & H.T. Horner (1997). Quantitative determination of calcium oxalate and oxalate in developing seeds of soybean (Leguminosae). American Journal of Botany 84: 1042-1046. [ Links ]
23. Johnson, R.C, T.J. Kisha & M.A. Evans (2007). Characterizing safflower germplasm with AFLP molecular markers. Crop Science 47: 1728-1736. [ Links ]
24. Jones, R.J., B.M.N. Schreiber & J.A. Roessler (1996). Kernel sink capacity in maize genotypic and maternal respiration. Crop Science 36: 301-306. [ Links ]
25. Knowles, P.F. (1989). Safflower. In: Röbbelen, G., Downy, R.K. and Ashri, A., (eds.), pp. 363-374. Oil crops of the world. McGraw-Hill, New York, 367 p. [ Links ]
26. Koutroubas, S.D. & D.K. Papakosta (2010). Seed filling patterns of safflower: Genotypic and seasonal variations and association with other agronomic traits. Industrial Crops and Products 31: 71-76. [ Links ]
27. Lindström, L.I. (2012). Histogénesis del fruto de girasol (Helianthus annuus L.): su aplicación al análisis del efecto de la radiación incidente sobre el peso y la aptitud al descascarado de los frutos y sus variables subyacentes. PhD Tesis, Universidad Nacional del Sur, Bahía Blanca. [ Links ]
28. Lindström, L.I., M.E. García, C.N. Pellegrini & L.F. Hernández (2006a). Aptitud al descascarado de frutos de girasol desarrollados bajo condiciones de estrés lumínico. Proceedings of the XXVI Reunión Argentina de Fisiología Vegetal, Chascomús, Argentina, p.162. [ Links ]
29. Lindström, L.I., C.N. Pellegrini, L.A.N. Aguirrezábal & L.F. Hernández (2006b). Growth and development of sunflower fruits under shade during pre and early post-anthesis period. Field Crops Research 96: 151-159. [ Links ]
30. Lindström, L.I., C.N. Pellegrini & L.F. Hernández (2007). Histological development of the sunflower fruit pericarp as affected by pre- and early post-anthesis canopy shading. Field Crops Research 103: 229-238. [ Links ]
31. Lizana, C., M. Cuba & D. Calderini (2007). Relación entre el peso potencial de los granos de trigo y el número de células del endosperma y el pericarpio. Proceedings of the 1st International Workshop: Ecofisiología Vegetal Aplicada al Estudio de la Determinación del Rendimiento y la Calidad de los Cultivos de Granos, Mar del Plata, Argentina, pp. 18-19. [ Links ]
32. Lyon, D., P. Burgener, R. Harveson, G. Hein & G. Hergert (2007). Growing Safflower in Nebraska. University of Nebraska-Lincoln Extension, Institute of Agriculture and Natural Resources. http://www.ianrpubs.unl.edu/ epublic/live/g1702/build/g1702.pdf [ Links ]
33. Mantese, A.J., D. Medan & A.J. Hall (2006). Achene structure, development and lipid accumulation in sunflower cultivars differing in oil content at maturity. Annals of Botany 97: 999-1010. [ Links ]
34. Mündel, H.H., R.E. Blackshaw, J.R. Byers, H.C. Huang, D.L. Johnson, R. Keon, J. Kubik, R. McKenzie, B. Otto, B. Roth & K. Stanford (2004). Safflower Production on the Canadian Prairies. http://safflower.wsu.edu/ SafflowerProduction_Canada.pdf [ Links ]
35. Pandey, A.K. & M.R. Dhakal (2001). Phytomelanin in Compositae. Current Science 80: 933-940. [ Links ]
36. Peterson, R. (1996). Birdseed market outlook. Proceedings of the North American Safower Conference, Mündel, H.H., Braun, J. & Daniel, C. (eds.), Great Falls, USA, p.15. [ Links ]
37. Rondanini, D.P, A. Mantese, R. Savin & A.J. Hall (2006). Responses of sunflower yield and grain quality to alternating day/night temperature regimes during grain filling: effects of timing, duration and intensity of exposure to stress. Field Crops Research 96: 48-62. [ Links ]
38. Rondanini, D.P., R. Savin & A.J. Hall (2007). Estimation of physiological maturity in sunflower as a function of fruit water concentration. European Journal of Agronomy 26: 295-309. [ Links ]
39. Rondanini, D.P., A.I. Mantese, R. Savin & A.J. Hall (2009). Water content dynamics of achene, pericarp and embryo in sunflower: Associations with achene potential size and dry-down. European Journal of Agronomy 30: 53-62. [ Links ]
40. Roth, I. (1977). Fruits of Angiosperms. Gebrüder Borntraeger, Berlín, 675 p. [ Links ]
41. Ruzin, S.E. (1999). Plant Microtechnique and Microscopy. Oxford University Press, Oxford, 322 p. [ Links ]
42. Sala, R.G., F.H. Andrade & M.E. Westgate (2007). Maize kernel moisture at physiological maturity as affected by the source-sink relationship during grain filling. Crop Science 47: 711-714. [ Links ]
43. Schneiter, A.A. & J.F. Miller (1981). Description of sunflower growth stages. Crop Science 21: 901-903. [ Links ]
44. Scott, R.W., M. Appleyard, G. Fellowes & E.J.M. Kirby (1983). Effect of genotype and position in the ears on carpel and grain growth and mature grain weight of spring barley. Journal of Agricultural Science 100: 383-391. [ Links ]
45. Shakeri-Amoughin, R., A. Tobeh & S. Jamaati-e-Somarinm (2012). Evaluation of some qualitative and quantitative traits, strength and quality of safflower seeds obtained from water and dry growing conditions and different plant densities in greenhouse conditions. International Research Journal of Applied and Basic Sciences 3: 545-555. [ Links ]
46. Smith, J.R. (1996). Safflower. AOCS Press, Champaign, 83 p. [ Links ]
47. Soil Survey Staf, USDA (1999). Soil taxonomy: A basic system for classifying soils. Soil Survey Staf, Agriculture, Homework, 436 p. [ Links ]
48. Spurr, A.R. (1969). A low viscocity resin embedding medium for electron microscopy. Journal of Ultrastructure Research 26: 31-53. [ Links ]
49. Sujatha, M. (2008). Biotechnological interventions for genetic improvement of safflower. Proceedings of the 7th International Safflower Conference, Knights, S.E. & Potter, T.D. (eds.), Wagga Wagga, Australia, 30 p. [ Links ]
50. Tekrony, D.M., D.B. Egli, J. Balles, T. Pfieffer & R.J. Fellows (1979). Physiological maturity in soybean plants. Agronomy Journal 71: 771-775. [ Links ]
51. Ugarte, C., D.F. Calderini & A. Slafer (2007). Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crops Research 100: 240-248. [ Links ]
52. Vallejos, M., D. Rondanini & D.F. Wassner (2011). Water relationships of castor bean (Ricinus communis L.) seeds related to fnal seed dry weight and physiological maturity. European Journal of Agronomy 30: 53-62. [ Links ]
53. Velasco, L. & J.M. Fernandez-Martinez (2001). Breeding for oil quality in safflower. Proceedings of the 5th International Safflower Conference, Bergman, J.W. & Mündel, H.H. (eds.) Williston, North Dakota, USA, pp. 133-137. [ Links ]
54. Weiss, E.A. (2000). Safflower. In: Oilseed Crops. 2nd edition, Blackwell Sci. Ltd, Oxford, pp. 93-129. [ Links ]
55. Wessel-Beaver, L., R.H. Beck & R.J. Lambert (1984). Rapid method for measuring kernel density. Agronomy Journal 76: 307-309. [ Links ]
56. Zarembo, E.V. & E.V. Boyko (2008). Carpology of some East Asian Cardueae Asteraceae. Anales del Jardín Botánico de Madrid 65: 129-134. [ Links ]














