Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.83 no.2 Vicente López dic. 2014
ARTÍCULOS ORIGINALES
Morphoanatomical functional traits in xerophytic species of a saline environment
Caracteres morfoanatómicos funcionales en especies xerofíticas de ambientes salinos
Pérez Cuadra V & V Cambi
Lab. de Plantas Vasculares. Dep. de Biología, Bioquímica y Farmacia. Universidad Nacional del Sur. San Juan 670. 8000. Bahía Blanca, Prov. Buenos Aires. Argentina.
Address Correspondence to: Vanesa Pérez Cuadra, e-mail: vperezcuadra@uns.edu.ar
Recibido / Received 19.X.2013.
Aceptado / Accepted 23.X.2013.
Abstract. The halophytic community of Salitral de la Vidriera (Buenos Aires Province, Argentina) has species with different morphoanatomical functional traits. The aim of this study was to compare these traits in four species, two Asteraceae (Baccharis spartioides and B. tenella) and two Frankeniaceae (Frankenia juniperoides and F. pulverulenta). Leaves and stems were treated under traditional techniques for anatomical study. Leaves the of Asteraceae and F. pulverulenta were amphistomatic while in F. juniperoides they were hypostomatic. All species showed trichomes and only the Frankeniaceae had salt glands. The mesophyll was isolateral in Asteraceae, and dorsiventral in Frankeniaceae; the number of foliar vascular bundles was variable. The stems of the Asteraceae showed subepidermal collenchyma or parenchyma which alternated with chlorenchyma; this tissue combination was not found in the Frankeniaceae stems. Baccharis tenella presented fibers in the cortex while B. spartioides had schysogenous ducts. The stems of the four species showed a complete vascular cylinder and parenchymatic pith. The anatomical differences among species most likely contribute to their survival and perpetuation in the study region
Keywords: Asteraceae; Frankeniaceae; Halophytic species; Functional traits; Anatomy.
Resumen. La comunidad halófila del Salitral de la Vidriera (Prov. Buenos Aires, Argentina) posee especies con diferentes caracteres morfoanatómicos funcionales; el objetivo de este estudio fue comparar este tipo de caracteres en cuatro especies, dos Asteraceae (Baccharis spartioides y B. tenella) y dos Frankeniaceae (Frankenia juniperoides y F. pulverulenta). Hojas y tallos fueron tratados con técnicas tradicionales para su estudio anatómico. Las hojas de Asteraceae y F. pulverulenta fueron anfestomáticas mientras que en F. juniperoides fueron hipostomáticas. Todas las especies presentaron tricomas y sólo las Frankeniaceae, glándulas de sal. El mesofilo fue isolateral en las Asteraceae y dorsiventral en las Frankeniaceae; el número de haces vasculares foliares fue variable. Los tallos de las Asteraceae tuvieron colénquima subepidérmico o parénquima que alternó con clorénquima, esta combinación de tejidos no fue encontrada en los tallos de las Frankeniaceae. Baccharis tenella presentó fibras en la corteza mientras que B. spartioides tuvo conductos esquizógenos. Los tallos de las cuatro especies mostraron un cilindro vascular completo y una médula parenquimática. Las diferencias anatómicas entre las especies seguramente contribuyen a la perpetuación y supervivencia de las mismas en la región de estudio.
Palabras clave: Asteraceae; Frankeniaceae; Especies xerófilas; Caracteres funcionales; Anatomía.
INTRODUCTION
Plants in their natural environments are exposed to a variety of ecological factors (foods, freezing, heat, salinity, dryness, etc.) that they must tolerate. Tolerance, the more specific term that describes the special adaptation of a plant to endure a relevant stress factor (Lauchli & Lüttge, 2002), might be related to the life cycle, relative growth rate, competitive ability, morphoanatomical features, defense against herbivory, etc., all functional traits that may determine the differential success of any species (Austin, 2005; Chase, 2005).
Traditionally, communities may contain one or a few dominant species or may be composed of a more or less similar number of different species (Shipley, 2010). In any case if the plant community is defined by several species, the community characteristics will be based on demographic rules that describe how individuals of different species interact with each other. On the other hand, if communities are interpreted as groups of plants that have different functional characteristics, the descriptive rules of those communities will generate an assessment of how the different traits affect positively or negatively the fitness of those groups (Shipley, 2010). The species with functional adaptive traits to environmental conditions are those that manage to cross "through natural filters" (selective forces imposed by the environment). Contrarily, those species that are poorly adapted turn out to be finally excluded. Thus, those species best adapted to selective forces will be more abundant than those less adapted (Shipley, 2010).
The vegetation of salt marshes is varied in terms of taxonomic diversity and physiognomy; this is related to the different strategies that plants have to cope with salinity as a limiting growth factor (Ragonese, 1951). With a rapid observation, one can detect a gradual change from cushion plants (that occur scattered in areas of maximum tolerable salinity) to shrubs (that appear as the salt concentration decreases making soil resources more available); at the same time, different species that are sheltered by shrub species also give way to less rustic ones (Ragonese, 1951). In this gradation each sector of the salt marsh (from the area of higher to that of the lower salinity) is part of a stage of the plant succession ending in a zone where no more changes, at least perceptible, are observed in the floristic composition, allowing to assume that the community has reached a stable structure (Begon et al., 1999).
The halophytic vegetation must support variations in water availability (physical and physiological drought), intense radiation, alternating temperatures, low soil fertility and salt stress (Ruthsatz, 1978; Wahid, 2003). These limitations are extremely detrimental to the growth and development of plants. However, there are species that survive successfully under these conditions, having particular functional characteristics of their morphoanatomy (Wahid, 2003).
The aim of this study was to compare the morphoanatomic functional traits of four halophytic species: two species of the Asteraceae, Baccharis spartioides (Hook. & Arn. Ex DC.) J. Remy and B. tenella Hook. & Arn., and two of the Frankeniaceae, Frankenia juniperoides (Hieron.) Correa and F. pulverulenta L.
The Asteraceae family, one of the best represented in the Salitral de la Vidriera (Buenos Aires Province, Argentina), is cosmopolitan in origin, with high concentration of species in the subtropics and warm regions. This family, characteristic of the Northern Hemisphere, has many endemic species in the Southern Hemisphere among which are the two Baccharis species studied here, both endemic of Argentina (Cabrera, 1963). Baccharis spartioides lives in areas of the Salitral de la Vidriera where vegetation is open while B. tenella (having a low frequency) is interspersed in places with open or closed vegetation.
The Frankeniaceae family, one of the least significant groups, comprises four genera and about 60 species distributed in brackish and steppe areas of worldwide subtropical and temperate regions. Frankenia juniperoides, endemic of Argentina, and F. pulverulenta, a naturalized species in the same country, vegetate in halophytic soils in diferent regions of Argentina (Correa, 1963). Particularly in the study area, the first species forms carpets in the dense bush, while the second one (weak) forms small patches in open areas.
MATERIALS AND METHODS
The studied species grow in the Salitral de la Vidriera (Partido Villarino), approximately 30 km from Bahía Blanca, Buenos Aires Province, Argentina. The partido of Villarino is located in the south of Buenos Aires Province, and occupies an area of 11400 km2; the region of saltmarshes is delimited by the parallels 38° 35´ and 38° 50´ S, and the meridians 62° 40´ and 63° 15´ W, extending approximately 1580 km2 (Bonorino, 1970). This is a natural halophytic environment where all four species vegetate spontaneously. Plant collection was conducted during March and May of 2009.
Samples, taken from the middle part of leaves, and the middle area of the third to the fourth mature stem internodes, were fixed in formalin-acetic acid-alcohol, dehydrated in an ethyl alcohol-tertiary butyl alcohol series and embedded in Paramat. The study plant sections (10 µm) were stained with safranin-fast green and mounted in Canada balsam. Other leaves and stems, cleared (Dizeo de Strittmatter, 1973) and stained with safranin, were mounted in gelatin-glycerin to determine their epidermal characteristics. Epidermal studies were not performed in B. spartioides (due to the small size of their leaves) and B. tenella (because trichome abundance precluded the observation of the epidermal characters). Observations were made using a compound microscope; photographs were obtained with a conventional digital camera.
Calcium oxalate crystals were classifed following Perez Cuadra & Hermann (in press).
RESULTS
Baccharis spartioides
Leaf. Anatomy. A thin, smooth cuticle covered the epidermis, being thicker towards the abaxial side. The epidermal cells were generally isodiametric (quadrangular or rounded), of thin walls (some of them slightly thickened) except the outer, tangential ones which were generally thickened. The stomata were at the same level of other epidermal cells or raised (Fig. 1 C), ultimate disposal was more frequent in the adaxial epidermis. The guard cells had cuticular projections on their tangential walls (external and internal) (Fig. 1 C). Substomatal chamber was of variable size (Fig. 1 C). Multicellular uniseriate glandular trichomes were observed, composed by thin-walled cells, increasing in size from the proximal to the distal zone; the terminal cell had one or two small druses of A1 type. The trichomes were arranged in an aggregated way, forming nests. The mesophyll is centric, there were four to five layers of chlorenchyma to adaxial and abaxial, containing some calcium oxalate crystals in the form of rectangular polyhedra. Rounded cells with thin walls containing tannins were observed delimiting the chlorenchyma and the sheath of each vascular bundle. There was a central collateral vascular bundle without sclerenchymatic tissue and with a parenchymatic sheath; there were one to two minor bundles (with similar characteristics) on each side of the major one, which had xylem projections to the adaxial side. Towards the abaxial position of the phloem in each vascular bundle (inside the parenchymatic sheath), there was a very large schizogenous duct, similar in size to the vascular bundle itself or in some cases slightly higher.
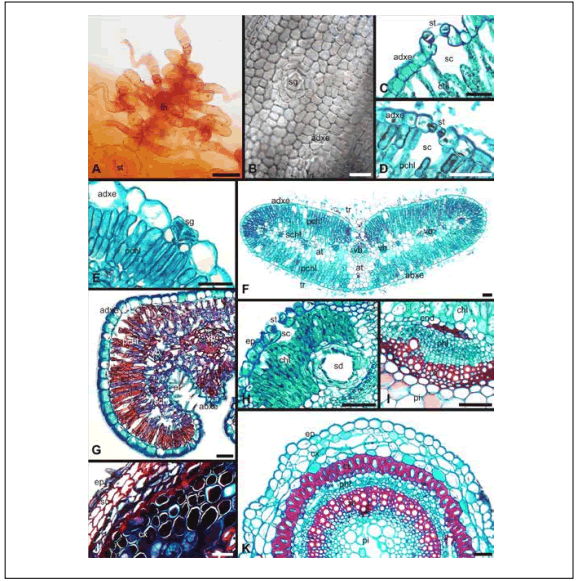
Fig. 1. Morphoanatomical functional traits. A, C, H: B. spartioides; D, F, I: B. tenella; B, G, J: F. juniperoides; E, K: F. pulverulenta. A: Stem epidermis. B: Adaxial foliar epidermis. C-D: Foliar stomata; E: Foliar salt gland. F-G: Leaf. H, J: Stem cortex. I: Stem vascular bundle. K: Stem. A-B: Surface view; C-K: Transversal section. Abbreviations: at, aqueous tissue; chl, chlorenchyma; cs, cortical sclereids; cx, cortex; abxe, abaxial epidermis; adxe, adaxial epidermis; end, endodermis; ep, epidermis; et, eglandular trichome; pchl, palisade chlorenchyma; phl, phloem; pi, pith; sc, substomatal chamber; schl, spongy chlorenchyma; sd, schysogenous duct; sg, salt gland; st, stomata; su, suber; tr, trichomes remains; tn, trichome nest; vb, vascular bundle; xil, xilem. Scale bars: 50 µm.
Fig. 1. Caracteres funcionales morfoanatómicos. A, C, H: B. spartioides; D, F, I: B. tenella; B, G, J: F. juniperoides; E, K: F. pulverulenta. A: Epidermis de tallo. B: Epidermis foliar adaxial. C-D: Estoma foliar; E: Glándula de sal foliar. F-G: Hoja. H, J: Corteza caulinar. I: Haces vasculares del tallo. K: Tallo. A-B: Vista en superficie; C-K: Corte transversal. Abreviaturas: at, tejido acuífero; chl, clorénquima; cs, esclereidas corticales; cx, corteza; abxe, epidermis abaxial; adxe, epidermis adaxial; end, endodermis; ep, epidermis; et, tricomas eglandular; pchl, clorénquima en empalizada; phl, foema; pi, médula; sc, cámara subestomática; schl, clorénquima esponjoso; sd, conducto esquizógeno; sg, glándula de sal; st, estoma; su, súber; tr, restos de tricomas; tn, nido piloso; vb, haz vascular; xil, xilema. Barras: 50 µm.
Stem. Epidermis in surface view. The cuticle was slightly striated. The epidermal cells were polygonal with thickened walls. Cyclocytic stomata were observed (Fig. 1 A), mostly located in rows. Mixed pilose nests (with glandular and eglandular trichomes) were seen (Fig. 1 A).
Anatomy. The stem was rounded to oval in cross section, with soft ribs. The cuticle was thin. The epidermal cells showed two morphologies: in the ribs, they were quadrangular to rectangular, and they were pear-shaped in the valleys; the latter ones were smaller than the formers. All had thickened outer tangential walls and may have one or more papillae. Stomata were placed at the same level as the remaining epidermal cells (Fig. 1 H). Guard cells had cuticular projections of the outer (larger) and inner tangential walls (Fig. 1 H). In general, the stomata were on the ribs presenting a large substomatal chamber. Trichomes, arranged in nests, were glandular and eglandular, both types multicellular. The glandular ones could be uni or biseriate presenting the apical cells small druses of A1 type (Fig. 1 A); the eglandular were uniseriate and of a lash type (Fig. 1 A). All cells that formed trichomes were thin walled. At a subepidermal level, in costal regions, there were chlorenchyma packages (Fig. 1 H) consisting of four to fve layers of rectangular cells (similar to the palisade chlorenchyma); a sheath of parenchyma cells delimited these packets at the sides and below (Fig. 1 H). At a similar level but in the valleys, there were one to two layers of angular collenchyma. In cortical deep position, there were large schysogenous ducts (Fig. 1 H) related to the rib areas. Inside the collenchyma (and in a position relatively closer to the epidermis), packages of undifferentiated cells with thin walls were observed. Approximately 11 to 15 collateral vascular bundles were recognized, being differentiated the vascular cambium (fascicular and interfascicular). The pith was large and consisted of parenchymatic thin walled cells, that increased in size centripetally, and with very few and small intercellular spaces. Some of these cells showed small blunt styloids, solitary rectangular and oval polyhedral crystals or gathered in small groups.
Baccharis tenella
Leaf. Epidermis in surface view. Both epidermis were fully covered by tricellular eglandular trichomes of lash type, which gave the leaf a woolly appearance by the great length of their terminal cells.
Anatomy. The epidermis had a thin cuticle with fine striations to the adaxial side,and a thicker one with noticeable striations near the margins to the abaxial leaf side. The epidermal cells were isodiametric to rectangular, with slightly thickened outer tangential walls. Stomata were pseudo-sunken (sunken stomata were considered those which had external tangential walls of the guard cells below the level of the inner tangential walls of epidermal cells; pseudo-sunken were those with the inner tangential walls of guard and epidermal cells at the same level, while the outer tangential walls of the guard cells were below the level of the outer tangential walls of epidermal cells, remaining protected by a small epistomatic chamber) (Fig. 1 D), with a small substomatal chamber (Fig. 1 D). Both sides of the leaf had a great amount of eglandular lash type trichomes, which were tricellular and thin-walled. The mesophyll was of isolateral type (Fig. 1 F); the palisade chlorenchyma consists of two to four layers of cells (Fig. 1 F). Rounded cells with few chloroplasts that constituted the spongy chlorenchyma were found below the palisade ones (Fig. 1 F). A central collateral vascular bundle and three to four minor collateral ones were observed on each side (Fig. 1 F). The vascular bundles had not schlerenchymatic tissue associated; the central one had a parenchymatic incomplete sheath (differentiated only on both sides) while the others showed a complete one. Towards the abaxial side of the phloem of the central vascular bundle, a small schysogenous duct was observed.
In the middle zone of the leaf, towards the adaxial and abaxial leaf surfaces, the palisade chlorenchyma was interrupted by parenchyma cells (Fig. 1 F). These cells, scarce to the adaxial side, continued in a deeper position with a low number of fibers, while to the abaxial side they were found in a greater quantity (arranged in approximately seven layers), and presented thickened walls.
Stem. Epidermis in surface view. Cuticle was smooth. The cells were polygonal with slightly thickened walls. Anomocytic stomata were arranged in rows. Eglandular trichomes of lash type were observed.
Anatomy. The stem was pentagonal, with five ribs. The cuticle was thick. Epidermal cells that were on the ribs were pyriform to quadrangular, in some cases presenting a central papilla; over valleys cells were generally quadrangular presenting one or more smaller papillae. All cells had the outer tangential wall thickener than the others. The pseudo-sunken stomata had cuticular projections of the external and internal tangential walls of the guard cells. Eglandular trichomes were observed with similar characteristics to those mentioned for the leaf. In subepidermal position of costal areas, one or two layers of thick walled parenchymatic cells were seen followed in depth by a fiber cap; in the intercostal areas there were five to six layers of chlorenchyma, consisting of rectangular cells similar in appearance to the palisade tissue. Surrounding the chlorenchyma, at the sides and below, there was a sheath of aqueous tissue, followed towards the interior of the stem with an endodermis (Fig. 1 I). The stem presented 10 to 12 collateral vascular bundles, each one with a phloem fiber cap (Fig. 1 I). Interfascicular vascular cambium was developed, and functioning secondary vascular tissues were seen. The pith, large and star-shaped, presented some sclerotic parenchymatic cells on the outside, in the contacting area with the xylem, intermingled with xylematic fibers (Fig. 1 I). The pith was composed by parenchymatic rounded cells which increase in size in centripetal direction; cells showed slightly thickened walls and delimited intercellular spaces.
Frankenia juniperoides
Leaf. Epidermis in surface view. The cuticle was smooth. The epidermal cells were polygonal with thickened walls (Fig. 1 B). There were salt glands located in depressions at the adaxial side (Fig. 1 B) and at the same level of the epidermal cells at the abaxial surface; glands were bounded by a ring of epidermal cells. Paracyitic stomata were randomly distributed and eglandular trichomes were observed only at the abaxial epidermis.
Anatomy. Cuticle was thick (Fig. 1 G). Te epidermis was composed of rectangular cells with thickened outer tangential walls (Fig. 1 G). The epidermal cell size decreased towards the edges of the leaf, where they acquired a rounded shape, thickening of their walls decreased following the same pattern (Fig. 1 G). No stomata were observed (Fig. 1 G), but there were salt glands typical of the Frankeniaceae family (Metcalfe & Chalk, 1957), located in the margins of the leaves. The mesophyll was dorsiventral (Fig. 1 G); the palisade chlorenchyma, constituted by two to three layers of cells, extended to the edges of the blade (Fig. 1 G). All this chlorenchyma cells possessed large amounts of tannins (Fig. 1 G). The spongy chlorenchyma (interrupted in the region of the midrib where it was replaced by tannic cells), was composed of rounded or irregular cells (Fig. 1 G). A central collateral vascular bundle and five to seven, also collateral, minor ones at both sides of the former constituted the vascular system of the leaves (Fig. 1 G). The vascular bundles were small, the medium one being larger than the others. The central bundle had a cap of phloem fibers and a parenchymatic sheath (poorly differentiated) with large amount of tannins (Fig. 1 G), while the minor ones had neither caps nor sheaths (Fig. 1 G).
Stem. Epidermis in surface view. The cuticle was smooth, epidermal cells were rectangular with thin walls. Paracytic stomata were located approximately in rows parallel to the major axis of the organ. Eglandular unicellular trichomes had thickened walls and sharp ends.
Anatomy. The stem had a rounded cross section. The cuticle was thin. The epidermal cells were quadrangular, of thin walls except the external tangential ones which were thickened (similar to that described for leaves), and with their lumen filled with tannins. Stomata were not observed. Eglandular trichomes were unicellular, similar to those found in the abaxial surface of leaves, but with thick and sclerotic walls. Salt glands were absent. The cortex was composed of seven to eight layers of rounded parenchyma cells with thickened walls, most of which contained tannins (Fig. 1 J). However, only some of them presented type A1 and B druses (of diferent sizes), particularly those founded in the internal regions of the cortex. Limiting the stele there was a complete ring of fbrosclereids in mature stems, usually consisting of one or two layers of cells, among which there were some tannic cells. Inside the fibrosclereids sheath, one or two layers of parenchyma cells with tannins developed, being generally smaller than similar cells in an outermost position of the cortex. The secondary xylem and phloem were observed forming a complete cylinder, xylem rays were not observed. The pith, small, consisted of parenchymatic cells which increased in size centripetally, showing intercellular spaces. These cells also had tannins, although in a fewer proportion than the cells of the cortex, and some druses of similar type to the cortical ones, but larger; the pith tended to hollow out in mature stems. In stems with more advanced secondary growth, four to five layers of cork were seen at a subepidermal position (Fig. 1 J). They consisted of rectangular cells with thickened walls and a great content of tannins. Both phellogen and felodermis had tannins, the latter being a few layers thick.
Frankenia pulverulenta
Leaf. Epidermis in surface view. The cuticle was smooth; towards the adaxial surface, epidermal cells were irregular, while to the abaxial one they were rectangular, with thin and undulated walls. Anomocytic stomata were observed randomly distributed. Salt glands were at an epidermal level surrounded by radiated epidermal cells with similar characteristics to the typical ones. The abaxial epidermis had eglandular trichomes.
Anatomy. The cuticle was thin. The epidermis was composed of isodiametric to rectangular cells, with the outer, tangential walls slightly thickened; those of the abaxial surface were smaller in the area of the midrib and generally rounded. The stomata were pseudo-sunken, and the sub-stomatal chamber was small. There were scarce unicellular eglandular trichomes with rounded tip. The salt glands were similar to those described for F. juniperoides (in some cases it was noted that the glands reached the similar level of the adjacent epidermal cells, being slightly protected) (Fig. 1 E). The mesophyll was dorsiventral; there was palisade chlorenchyma composed of two layers of cells. Towards the abaxial surface, spongy chlorenchyma was composed of variable shaped cells (elongated, rounded) of loose arrangement, which became uniformly rounded below the central vascular bundle. A central collateral vascular bundle and seven minor, on both sides of it, formed the vascular system of the leaves. There was not sclerenchymatic tissue associated; only the central vascular bundle had a poorly defined sheath consisting of parenchyma cells with chloroplasts. It is noteworthy that the smaller vascular bundles were formed by only a few conducting cells.
Stem. Epidermis in surface view. The cuticle was smooth; the cells were rectangular with thin and smooth walls. Paracytic stomata were observed, distributed in rows parallel to the major axis of the organ; the ostiole was perpendicular to this axis. Eglandular trichomes and salt glands were present.
Anatomy. The cuticle was thin. The epidermal cells are pyriform or quadrangular (Fig. 1 K), with thickened and papillose outer tangential walls. There were some pseudo-sunken stomata, substomatal chamber being small. There were scarce eglandular unicellular trichomes with thin walls (sometimes sclerotic) and blunt ends (Fig. 1 K). The salt glands were located at the epidermal level or slightly sunken. The cortex was composed of three to four layers of rounded cells (those in the first layer had chloroplasts) which increased in size centripetally (Fig. 1 K). In the inner zone of this region, some cells contained starch granules forming an incomplete amylifere sheath. A complete ring of fibrosclereids which generally consisted of a single layer of cells (eventually formed by two or three layers of cells) was found bordering externally the stele (Fig. 1 K). The vascular tissue formed a complete cylinder with an incipient secondary growth (Fig. 1 K). The pith, large, consisted of isodiametric parenchyma cells with somewhat thickened walls increasing in size centripetally (Fig. 1 K). The central part of this zone had a tendency to break originating a hollow pith (Fig. 1 K).
DISCUSSION
Exploring the Salitral de la Vidriera, many stress factors (dry air and soil, high solar radiation, high concentration of salts in the soil, etc.) are detected which prevent the introduction or development of some plants and yet, allow the existence of others, such as those studied here. Despite the poverty of the environment, many species use their particular functional characteristics to develop in it. These environments are less popular so there are fewer competitors, since resources are scarce or of a lower availability to plants (Pyykkö, 1966).
A large number of species belonging to the families Nyc-taginaceae, Polygonaceae, Solanaceae and Verbenaceae present a few leaves reduced in size (microfly) and arranged in evasive positions like adppressed to the stem and embedded in dense rosettes (Ancibor, 1992; Ragonese, 1990). These last foliar characters are of a valuable adaptive feature and, apparently, are the result of evolutionary convergence. Particularly in B. spartioides very few leaf excrescences develop, whereby the foliar photosynthetic function is performed by the stem. This is a very common strategy among xerophytes: reduction of water loss to a single organ, the stem. Both species of Frankeniaceae (mainly F. juniperoides) presented another modification: leaves with revolute margins. This special morphology protects the abaxial surface of the leaf where there are the stomata, to reduce the risk of water loss through transpiration.
A common condition among xerophytes is that the epidermal cells present straight walls in surface view, a feature that is emphasized in the adaxial side. This characteristic helps to maintain cell turgor and make cells more resistant to breakdown by wilting (Dickinson, 2000). Epidermal cells also tend to have their outer tangential walls thickened to a greater or lesser degree, providing rigidity to the leaf, an important factor of resistance to drying (Ragonese, 1990). The four species here studied have these characters.
There are two general strategies for the protection of stomata, one related to their location in marginal folds (at adaxial or abaxial surfaces), and the other due to their sunken location in different levels with respect to the overall position of the rest of epidermal cells; both strategies avoid excessive loss of water (Ancibor, 1980; Garcia et al., 2008). Among the studied species, the two species of Frankeniaceae showed leaves with curved margins towards the abaxial surface. This was more noticeable in F. juniperoides that in F. pulverulenta, while B. tenella had their stomata protected by a pseudo-sunken position besides a dense cover of trichomes. The four species studied here, although with diferent strategies, had their stomata protected from direct exposure to the environment.
Trichomes, especially eglandular ones, form an air layer on the surface of the leaf which generates a gradient for some conditions reducing transpiration (Johnson, 1975; Dickinson, 2000; Cutler et al., 2007). When the amount of hairs is not sufficient to produce that effect, they often act conversely increasing the area of the leaf, and hence increase the surface exposed to transpiration (Johnson, 1975; Dickinson, 2000; Cutler et al., 2007). In these cases, it is common that the walls of the trichomes become sclerotic and do not constitute an escape route for water (Johnson, 1975). In addition, recent studies (Skelton et al., 2012) found a direct relationship between pubescence and stomata density, the greater the surface coverage by trichomes, the higher is the stomata density. All studied species have trichomes. The Frankeniaceae showed glandular trichomes, being more frequent in F. juniperoides and sporadic in F. pulverulenta. Both Asteraceae possessed glandular trichomes. In B. spartioides these trichomes presented small druses of calcium oxalate in the terminal cells. Baccharis tenella developed many trichomes that gave a grayish color to their leaves; they generate a boundary layer that helps to reduce water loss. Skelton et al. (2012) reported that when the water becomes a limiting factor, and the plant can not reduce the temperature through transpiration, the increasing refection of light caused by pubescence may be important in reducing its absorption which in fact favors the decrease of the internal temperature.
Tannins are frequent ergastic substances in plants exposed to high solar radiation preventing the destruction of chlorophyll by UV rays (Dickinson, 2000). Furthermore, some types of tannins act as deterrent against herbivores, generating an unpleasant taste (Dickinson, 2000; Salminen & Karonen, 2011). Among the species studied, only F. juniperoides has a great quantity of tannins; possibly the presence or absence of this character is related to the lineage of families rather than to adaptative evolution.
In both Frankeniaceae species a secondary early growth was observed; this would be a common feature on species in hostile environments, because it gives a greater rigidity and, in many cases, protects the conducting tissues (Dickinson, 2000; Carlquist, 2007).
Possession of a ring of sclereids in the stems with secondary growth is cited as a strong indication that the plant possesses anomalous secondary growth (Carlquist, 2007); in this study, both Frankeniaceae species presented it, although developing normal secondary growth. Probably in these cases, the presence of the ring of sclereids is related with the stem need to generate a major support due to their loss of turgor by excessive transpiration.
The Frankeniaceae species studied here shared a major quantity of similar functional anatomical characters than the species of Asteraceae. This can be due to the fact that the characteristics showed by the Frankeniaceae are more closely related to the phylogenetic lineage that in case of the Asteraceae.
In agreement with other authors (Bianco et al., 2004; Garcia et al., 2008), a greater amount of functional features was found in leaves than in stems. Stems develop important functional characters under special conditions like those when microfily is extreme (as in B. spartioides); therefore they may perform several leaf functions (i.e., gas exchange, photosynthesis); these features have also been found on leafess species growing in other environments (Poblete et al., 1991).
Only those characteristics that are related to environmental conditions of the habitat can be considered as functional traits. Characteristics may vary between species of different places even when identification of the environment is similar (e.g., xerophytic, halophytic, etc.). This definition restricts the generalization performed on certain traits believed to be common for all species growing in similar environments (Vendramini et al., 2002). Depending on the particular constitution of vegetation, certain characters would be more or less represented being functionally important in each particular habitat for certain species. Possession of salt glands in halophytic species is often considered important, when in fact only few botanical families have this feature. Therefore although the presence of salt glands can be considered a functional character, their occurrence is not so general as to characterize a whole group of species of saline habitats.
The differential capacities of colonization and survival of each studied species can be related to morphological and anatomical specific features, so that anatomical studies of plant species contribute with valuable data not only for basic and/or purely descriptive studies but also for ecological ones. They may assist in the understanding of certain features of the dynamics of the species, currently explained only from demographic and/or population parameters.
The change in the way of interpretation of morphological and anatomical characters from an adaptative to a functional view resolves many of the conflicts related to the presence of certain xerophytic characteristics in plants belonging to other environments (e.g., hygrophilous). That is, the same character may be associated with different functions in different plants (Pyykö, 1966). However, a character must only be considered of functional importance for the plant when it is effectively related to a selective force of the environment; otherwise, it should be considered as a character linked to the phylogenetic heritage.
Anatomical studies, as those proposed here, where structural aspects were related with ecological and environmental factors act as starting point for further studies, giving an added value that has been lost in recent times under modern molecular and ultrastructural studies. These anatomical studies also expand the number of parameters that can be characterized in plant populations, and revalue the importance of the interdisciplinary research approach, which contributes with a more detailed characterization and understanding of how plant communities work.
ACKNOWLEDGMENTS
Financial support for this work was provided by PGI 24/B154 and a CONICET fellowship awarded to V. Pérez Cuadra. We thank Dr. Carlos Villamil for the identification of the specimens used for this study.
REFERENCES
1. Ancibor, E. (1980). Estudio anatómico de la vegetación de la Puna de Jujuy. II. Anatomía de las plantas en cojín. Boletín de la Sociedad Argentina de Botánica 19: 157-202. [ Links ]
2. Ancibor, E. (1992). Anatomía ecológica de la vegetación de La Puna de Mendoza. I. Anatomía foliar. Parodiana 7: 63-76. [ Links ]
3. Austin, M.P. (2005). Vegetation and environment: discontinuities and continuities. In: van der Maarel, E. (ed.), pp 52-84. Vegetation ecology. Blackwell Publishing, Conrwall. 411 p. [ Links ]
4. Begon M., J.L. Harper & C.R. Townsend (1999). Ecología: Individuos, Poblaciones y Comunidades. Ediciones Omega, Barcelona. 1148 p. [ Links ]
5. Bianco, C.A., T.A. Kraus & A.C. Vegetti (2004). La hoja: morfología y anatomía. Universidad Nacional de Río Cuarto, Córdoba. 199 p. [ Links ]
6. Carlquist, S. (2007). Succesive cambia revisited: ontogeny, histology, diversity, and functional significance. Journal of the Torrey Botanical Society 134: 301-332. [ Links ]
7. Cabrera, A.L. (1963). Compositae. In: Cabrera, A.L. (ed.), pp. 1-443. Flora de la Provincia de Buenos Aires. Colección Científica del INTA, Buenos Aires. 648 p. [ Links ]
8. Correa, M.N. (1963). Frankeniaceae. In: Cabrera, A.L. (ed.), pp. 229-233. Flora de la Provincia de Buenos Aires. Colección Científica del INTA, Buenos Aires. 648 p. [ Links ]
9. Cutler, D.F., T. Botha & D.W. Stevenson (2007). Plant anatomy, an applied approach. Blackwell Publishing, Singapore. 313 p. [ Links ]
10. Dickison, W.C. (2000). Integrative Plant Anatomy. Academic Press, San Diego. 532 p. [ Links ]
11. Dizeo de Strittmatter, C.G. (1973). Nueva técnica de diafanización. Boletín de la Soiedad Argentina de Botánica 15: 126-129. [ Links ]
12. Chase, M. (2005). Relationship between the families of flowering plants. In: Henry, R.J. (ed.), pp 7-23. Plant diversity and evolution. CAB International, Cambridge. 341 p. [ Links ]
13. García, M., D. Jáuregui & E. Medina (2008). Adaptaciones anatómicas foliares en especies de Angiospermas que crecen en la zona costera del estado Falcón (Venezuela). Acta Botánica de Venezuela 31: 291-306. [ Links ]
14. Johnson, H. (1975). Plant pubescense: an ecological perspective. The Botanical Review 41: 233-258. [ Links ]
15. Läuchli, A. & U. Lüttge (2002). Salinity: environment-plants-molecules. Kluwer Academic Publishers, New York. 551 p. [ Links ]
16. Metcalfe, C.R. & L. Chalk (1957). Anatomy of the Dicotyledons. Clarendon Press, Oxford. 1550 p. [ Links ]
17. Pérez Cuadra, V. & P. M. Hermann (in press). Characterization and macropattern of calcium oxalate phytoliths in argentinean endemic species of Chenopodioideae (Amaranthaceae). Quaternary International. (Accepted for publication October 2011). [ Links ]
18. Pyykkö, M. (1966). The leaf anatomy of East Patagonian xeromorphic plants. Annales Botanici Fennici 3: 453-622. [ Links ]
19. Poblete, V., V. Campos, L. González & G. Montenegro (1991). Anatomical leaf adaptations in vascular plants of a salt marsh in the Atacama Desert (Chile). Revista Chilena de Historia Natural 64: 65-75. [ Links ]
20. Ragonese, A.E. (1951). La vegetación de la República Argentina. II. Estudio Fitosociológico de las Salinas Grandes. Revista de Investigaciones Agrícolas 5: 1-234. [ Links ]
21. Ragonese, A.M. (1990). Caracteres xeromorfos foliares de Nassauvia lagascae (Compositae). Darwiniana 30: 1-10. [ Links ]
22. Ruthsatz, B. (1978). Las plantas en cojín de los semi-desiertos andinos del Noroeste Argentino. Darwiniana 21: 491-539. [ Links ]
23. Salminen J.P. & Karonen M. (2011) Chemical ecology of tannins and other phenolics: we need a change in approach. Functional Ecology 25: 325-338. [ Links ]
24. Skelton, R.P., J.J. Midgley, J.M. Nyaga, S.D. Johnson & M.D. Cramer (2012). Is leaf pubescence of Cape Proteaceae a xeromorphic or radiation-protective trait. Australian Journal of Botany 60: 104-113. [ Links ]
25. Shipley, B. (2010). From plant traits to vegetation structure. Cambridge University Press, Cambridge. 291 p. [ Links ]
26. Vendramini, F., S. Díaz, D.E. Gurvich, P.J. Wilson, K. Tompson & J.G. Hodgson (2002). Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytologist 154: 147-157. [ Links ]
27. Wahid, A. (2003). Physiological significance of morpho-anatomical features of halophytes with particular reference to chloristan flora. International Journal of Agriculture and Biology 5: 207-212. [ Links ]














