Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.85 no.1 Vicente López jun. 2016
ARTÍCULOS ORIGINALES
Nitrogen deposition influences the response of Potentilla tanacetifolia to phosphorus addition
La deposición de nitrógeno influencia la respuesta de Potentilla tanacetifolia al agregado de fósforo
Yang G1,2, Z Zhang1, G Zhang3, H Zhang2, X Han3, CA Busso4
1 Shanghai Institute of Technology, Shanghai 201418, China.
2 Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 1100164, China.
3 State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China.
4 Departamento de Agronomía-CERZOS (CONICET), Universidad Nacional del Sur, 8000 Bahía Blanca, Prov. de Buenos Aires, Argentina.
Address correspondence to: Zhang Zhiguo, e-mail: zgzhang@sit.edu.cn
Received 28.IV.2016.
Accepted 30.IV.2016.
Abstract. Phosphorus is an essential macronutrient for all living plants and plant production. Simultaneously, atmospheric nitrogen deposition also affects plant productivity at a global scale. However, few studies have investigated how plants respond to P addition while simultaneously considering N deposition. We investigated plant biomass, nutrient status and stoichiometric ratios on Potentilla tanacetifolia in response to P fertilization under contrasting N addition rates in a typical meadow steppe in Inner Mongolia, China. Aboveground biomass of P. tanacetifolia increased under increasing levels of P fertilization under conditions of N addition. However, there was no significant change in biomass when only phosphorus was added. Plant leaf and stem P concentrations increased linearly with P addition when there was no N addition. Our results suggest that increased plant P nutrition under P addition will not turn into plant growth enhancement unless N demands are also satisfied. Nitrogen addition significantly increased leaf N concentrations, and leaf and seed N:P ratios, when there was no P fertilization. Nevertheless, the effects of N addition were weakened, and eventually disappeared when P fertilization rates increased. This indicates that N-induced alterations of the plant nutrition status and stoichiometric ratios were P availability-dependent. Overall, our results suggest that multiple-nutrient constraints and their interactions must be considered when assessing plant nutrient and growth responses to nutrient enrichment.
Keywords: Nitrogen; Phosphorus; N: P ratio; Plant organs.
Resumen. El fósforo es un macronutriente esencial para todas las plantas vivas y la producción vegetal. Simultáneamente, la deposición atmosférica de nitrógeno también afecta la productividad de las plantas a una escala global. Sin embargo, pocos estudios han investigado como responden las plantas a la adición de P mientras se considera la deposición atmosférica de N al mismo tiempo. Se investigaron la biomasa vegetal, el nivel de nutrientes y relaciones estequiométricas en Potentilla tanacetifolia en respuesta a la fertilización fosforada bajo varias tasas de adición de N en una estepa de pradera típica en el interior de Mongolia, China. La biomasa aérea de P. tanacetifolia se incrementó con mayores niveles de fertilización fosforada bajo condiciones de adición de N. Sin embargo, no hubo un cambio significativo en la biomasa cuando solo se agregó P. Las concentraciones de P en hoja y tallo se incrementaron linealmente con el agregado de P cuando no hubo adición de N. Nuestros resultados sugieren que una mayor nutrición fosforada a la planta bajo agregado de P no determinará un mejor crecimiento vegetal a menos que también sean satisfechas las demandas de N. El agregado de N incrementó significativamente las concentraciones de N foliares, y las relaciones N:P de la semilla y de las hojas, cuando no hubo fertilización fosforada. De todas maneras, los efectos de la adición de N fueron debilitados, y eventualmente desaparecieron, cuando se incrementaron las tasas de fertilización con P. Esto indica que las alteraciones inducidas por el N en el nivel de nutrición vegetal y las relaciones estequiométricas fueron dependientes de la disponibilidad de P. Nuestros resultados sugieren que limitaciones de varios nutrientes al mismo tiempo y sus interacciones deben ser consideradas cuando se estudian las respuestas de los nutrientes y del crecimiento de las plantas a un aumento en el nivel de nutrientes.
Palabras clave: Nitrógeno; Fósforo; Relación N:P; Organos vegetales.
INTRODUCTION
Human activities are substantially influencing the global nutrition cycle and strongly altering the original nutritional balance across diverse ecosystems. So far, global average N:P ratio of fertilizer inputs has increased by 51% since 1975 (Peuelas et al., 2013). In comparison to the well-known effects of N enrichment on plant nutrient status, we know relative less about the influences of P additions on plant nutrient uptake and productivity in terrestrial ecosystems. As one of the most limiting nutrients to plant growth in these ecosystems (Elser et al., 2007), P plays a direct role in afecting plant productivity (Vrede et al., 2004). However, a general notion holds that soil P content limits plant growth in tropical ecosystems or most woodlands, while N content does it in temperate regions, especially for grasslands (Elser et al., 2007). Therefore, most of the P addition studies have been conducted in forests and shrublands, whereas there is little understanding on how and to what extent plant growth and production respond to varying P supply in temperate grasslands.
In the past several decades, anthropogenic N deposition is increasing worldwide because of increased fossil fuel combustion, fertilizer application and legume plantation (Galloway et al., 2004). From 1995 to 2005, reactive nitrogen increased 20% (Galloway et al., 2008); with a similar trend, the mean inorganic N wet deposition over China increased from 11.11 kg/ha/yr in the 1990s to 13.87 kg/ha/yr in the 2000s (Jia et al., 2014). However, few studies have considered N deposition from the atmosphere when investigating plant responses to P addition. Because of the synergistic interaction between N and P in plant metabolism (Güsewell 2004; Niklas et al. 2005), P effects on plant nutrition should also be regulated by N addition. Specifically, deposited-N may enhance plant acquisition of P by either increasing soil P availability via decreasing soil pH or enhancing P uptake. This might be to maintain the N:P homeostasis when N concentrations are increased in plant tissue by N addition (Yu et al., 2011). Thereafter, plant responses to P addition should consider atmospheric N deposition simultaneously.
Besides plant productivity, whether P addition has a significant effect on plant N or P concentration remains controversial, especially in temperate drylands. A recent study found a positive relationship between P addition and P concentrations on Leymus chinensis in a typical steppe (Zhang, 2003). In a phosphorus-poor soil, however, P addition showed no main effects on plant either N and P pools or the N:P ratio (He et al., 2015). These results indicated that P cycling in terrestrial ecosystems has a more complicated pattern than previously thought. Another unknown, but important issue, is how plants allocate their nutrients to different tissues, especially to reproductive organs, under varying P conditions. Seed is an important organ of plant reproduction (O’Neill & Roberts, 2001). The environment might exert influence on plant responses that are later expressed in changes of seed characteristics (such as seed quality and size) (Valencia-Díaz & Montana., 2005). For example, N addition reduced seed size but increased seed N concentration on Phytolacca Americana L. (He et al., 2005).
Potentilla tanacetifolia is a perennial herb with a wide range of morphological and physiological plasticity under different environmental conditions (Ren et al., 2011). The present study was conducted on P. tanacetifolia to examine how (1) plant biomass, nutrient status, and their allocation patterns among diferent organs respond to varying P addition, and (2) these responses change when N deposition was further considered in a meadow grassland of northeast China.
MATERIALS AND METHODS
Study site. The experiment was conducted at the Erguna Forest-Steppe Ecotone Research Station (50° 10' 46.1'' N, 119° 22' 56.4'' E), which belongs to the Chinese Academy of Sciences. Mean annual precipitation at the site is approximately 375 mm, and mean annual temperature is -3 °C. The soil is a chernozem according to the US soil taxonomy classification (Cline, 1979). The site was fenced since 2013 to prevent grazing by large animals. The dominant plant species in the study area was Leymus chinensis, a perennial rhizomatous grass. Our model plant species, P. tanacetifolia, accounts for 4.5% of the total aboveground biomass in the studied plant community.
Experimental design. The P addition experiment was carried out in May 2014. Phosphorus fertilization was made using KH2PO4 at rates of 0, 2, 4, 6, 8 or 10 g P /m2/yr. Five replicates were used for each fertilizing rate. We had 6 plots (one for each fertilization rate) on each of 5 randomly distributed blocks. Treatments of varying P addition were further nested either with or without N addition treatments. This is, 30 subplots received a constant N addition (NH4NO3) rate of 10g N/m2/yr. The other 30 sub-plots were only supplied with the various P fertilization rates. In this experiment, the effects of K added during the KH2PO4 fertilization process were balanced by adding appropriate levels of KCl. The effects of Cl were regulated by adding appropriate amounts of CaCl2. All of our study plots received the same levels of K and Cl.
Field sampling and measurements. In early August 2014, we randomly selected 3 individual plants of P. tanacetifolia in each plot. These plants were relatively young and with no obvious symptoms of pathogen or herbivore attack. They were manually clipped at the soil surface, and thereafter partitioned into leaves, stems, and seeds. These samples were first ovendried at 70 °C to a constant mass and then weighed.
After being ground in a ball mill (Retsch MM 400; Retsch, Haan, Germany), leaves were digested with H2SO4-H2O2 (Temminghof & Houba, 2004) to determine N and P concentrations using an auto discrete analyzer (Cleverchem 200+, DeChem-Tech. GmbH, Gemany).
Statistical analyses. Three-way ANOVA was used to test the effect of organs, N addition, P addition and their possible interactions on plant biomass and stoichiometry of P. tanacetifolia. When F values were significant (P<0.05 or lower) means were compared using the Duncan test. Regression models were used to determine the relationship between P addition rate and various plant variables on different organs. All analyses were conducted using R version 3.1.2 (R development core team, 2014).
RESULTS
N addition versus biomass. Stem and aboveground biomass of P. tanacetifolia increased (P<0.01) as N addition also increased (Table 1, Fig. 1).
Table 1. Results (F-values) of repeated measures ANOVAs for the effects of N (N) and P (P) additions on leaf biomass, stem biomass, stem:leaf (S:L) ratio and aboveground biomass.
Tabla 1. Resultados (valores F) de ANOVAs con medidas repetidas para los efectos de los agregados de N (N) y P (P) sobre la biomasa foliar, biomasa de tallos, relación tallo:hoja (S:L) y biomasa aérea.

** P<0.01

Fig. 1. Effects of N (N0 = 0 g N/m2/yr, N1 = 10 g N/m2/yr) and P addition on stem and leaf biomasses on the grassland plant Potentilla tanacetifolia in northeastern China. Data are means ± SE of n=5. Symbols with asterisks are significantly different (P<0.05) from those in the control treatments.
Fig. 1. Efectos de los agregados de N (N0 = 0 g N/m2/año, N1= 10 g N/m2/año) y P en las biomasas de tallo y hoja en la planta de pastizales naturales Potentilla tanacetifolia en el noreste de China. Los datos son promedios ± EE of n=5. Los símbolos con asteriscos son significativamente diferentes (P<0,05) de aquellos en los tratamientos control.
N addition altered plant stoichiometry parameters. There was a strong link (P<0.05) between P addition versus P concentration and N: P ratio under the scenario of no N addition (Table 2). P concentrations in leaves, stems and seeds all increased (P<0.001) as P addition rates also increased when there was no N addition (Fig. 2). Even more, P concentration in seeds was higher (P<0.001) under no N than N addition when no P was added to the substrate (Fig. 2). When N was added, however, leaf P concentrations increased (P<0.01) when P addition rates also increased (Fig. 2). Besides, P concentrations were significantly higher (P<0.05) in seeds than those on leaves and stems when there was no N addition (Table 2). Leaf and seed N:P ratios declined (P<0.05 or lower) with increasing P inputs when N was either added or not to the substrate (Fig. 2).
Table 2. Results (F-values) of repeated measures ANOVAs for the effects of N addition (N), P addition (P) and organs (O) on plant N and P concentrations (mg/g) and N:P ratios.
Tabla 2. Resultados (valores F) de ANOVAs con medidas repetidas para los efectos del agregado de N (N), agregado de P (P) y órganos (O) en las concentraciones (mg/g) de N y P, y relaciones N:P de la planta.
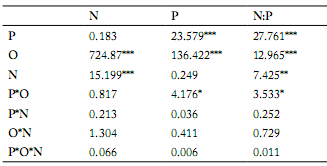
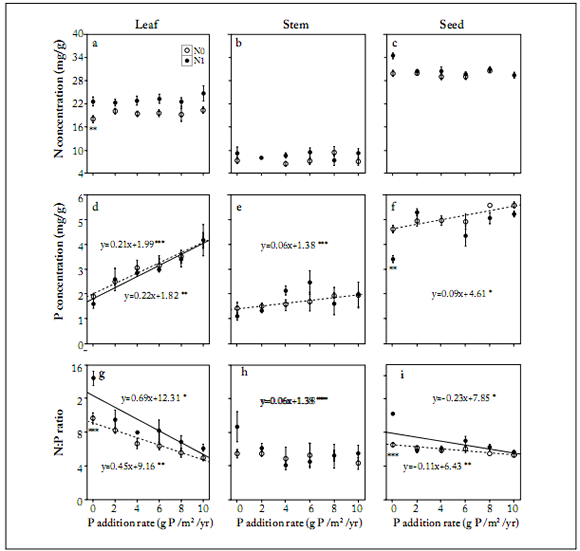
Fig. 2. Responses of leaf (a, d, g), stem (b, e, h) and seed (c, f, i) N and P concentrations, and N:P ratios to phosphorus addition under contrasting nitrogen treatments (N0 = 0 g N/m2/yr, N1 = 10 g N/m2/yr). Data are means ± SE of n=5. Symbols with asterisks are significantly different (P<0.05) from those corresponding to the control treatments.
Fig. 2. Respuestas de las concentraciones de N y P foliar (a, d, g), tallo (b, e, h), y semilla (c, f, i), y relaciones N:P al agregado de fósforo bajo tratamientos de nitrógeno contrastantes (N0 = 0 g N/m2/año, N1 = 10 g N/m2/año). Los datos son promedios ± EE of n=5. Los símbolos con asteriscos son significativamente diferentes (P<0,05) de aquellos en los tratamientos control.
It is important to highlight that N:P ratios on leaves and seeds were higher (P<0.001) when N was added than when it was not to the substrate (Fig. 2).
Leaf N concentrations were greater when N was added than when it was not added to the substrate under all P addition rates (Fig. 2). However, the difference was only significant (P<0.01) when there was no P addition (Fig. 2). Finally, plant N concentrations were greater (P<0.01) when there was N addition than when there was not on average for all P addition rates and plant organs (Table 2, Fig. 2).
DISCUSSION
The aboveground biomass of P. tanacetifolia, especially that on stems, increased with N input under various levels of P addition. We found that P addition had no significant effect on P. tanacetifolia biomass. This agrees with a study conducted in a typical steppe on the dominant species Leymuschinensis. In this study, the biomass of different organs, as well as the total biomass, showed no response to P fertilization (Zhang, 2003). Addition of P alone increased P concentrations but had negligible effects on biomass, indicating luxury consumption. These results suggest that plant growth might not be limited by P availability in our study grassland, at least during the frst few years of P fertilization. In the current study, for example, data came from the first year of P fertilization. The response of plants to P fertilization, however, might show up with time from this nutrient addition (Prietzel & Stetter, 2010). It means that if biomass would either increase or not after P addition on P. tanacetifolia requires further longterm studies.
Davidson (2004) found that grasses grew better in a N+P treatment, compared with only P addition. This indicated that N was the most important limiting nutrient for the grass (Oliveira, 2001; LeBauer, 2008). The biomass of P. tanacetifolia increased only when N was also added. This might be because when available N is supplied into the ecological system, phosphatase activity is substantially stimulated, and it increases levels of available phosphorus in the soil (Marklein & Houlton, 2012). The result is that adequate levels of N and P are supplied for plant and microbial growth, further enhancing the productivity of the ecosystems. Similarly, large amount of studies on grasslands had confirmed that aboveground net primary productivity increases as N addition also increases (Yu et al., 2009; Lü et al., 2012; Blanke et al., 2012; Zhang et al., 2015), even in arctic ecosystems (Jonasson et al., 1999).
Our results also indicated that increments in aboveground biomass were mostly the result of increases on stem rather than on leaf biomass. As the supporting organ of the plant, the stem plays an important role in light competition (Tilman, 1988). This suggests that as nutrient limitation is alleviated, plants start to allocate more C to stem growth which positively contributes in the process of competition for light. Many studies showed that plant productivity in grasslands had either a lower or no response to P addition (Huf & Potts, 2015). This happened when the interactive effects with N addition were not considered. However, our study indicated that the efects of P addition on plant growth might be enlarged under a N deposition scenario (e.g., Fig. 1).
Plant stoichiometry in P. tanacetifolia in response to nutrient addition. We found that leaf N concentration increased with N input (Table 2, Fig. 2). This was reported by various previous studies (Menge & Field, 2007; Blanke et al., 2012). Leaf is the main organ for plant photosynthesis (Jones, 1992), and it requires more N investment to reach higher rate of photosynthetic activity (Yang et al., 2014; Wright et al., 2004). As a result, high leaf N concentrations under N addition will significantly increase plant photosynthetic activity, and subsequent plant biomass production (Kerkhof et al., 2006; Marschnert et al., 2006). N addition in our study significantly alleviated N limitation; plant productivity, however, might also be limited by other elements such as P. Koerselman and Meuleman (2008) found that thresholds of foliar N:P ratios were <14 for N limitation and >16 for P limitation on European wetland plants. Our study found that the mean leaf N:P ratio was <14, indicating a scarcity of N in our research. Our results suggested that N addition without P supply might contribute to relieve N limitation in the study system (N:P =14.4). This agrees with results reported on other prairie grasslands (Clark & Tilman, 2008) under the scenario of N limitation.
Seed P concentration decreased with N addition. We found that N addition leads to decreases in seed P concentrations when no P was added. These results indicate that N input might trigger a relative shortage of P in the reproductive organs. Our results are similar to those found in an annual grassland where N fertilization decreased P concentrations in Avena. This might be because N addition increases plant growth, with a dilution effect on P concentrations under higher plant biomasses, which qualify N addition as the best candidate for an increasing P demand (Menge & Field, 2007).
Many studies suggested that N enrichment was the main driver for the loss of plant species diversity (Goldberg & Miller, 1990; Stevens et al., 2004; Clark & Tilman, 2008; Bai et al., 2010; Stevens et al., 2010; Roth et al., 2013). This may be because nitrogen addition could decrease germination rate of seeds (Luzuriaga et al. 2005). However, whether these reduced seed germination rates were caused by a relative shortage of P in seeds needs further investigation. Moreover, seeds, as the reproductive organs, should keep high nutrient concentrations to ensure plant reproduction. We showed that N and P concentrations in seeds were significantly higher than those in other vegetative organs. Our results provide further evidence that N deposition coupled with P nutrition may change plant vegetative and reproductive stoichiometry, which might be a potential mechanism for inducing the disappearance of plant species (Drenovsky & Richards, 2005).
CONCLUSIONS
Although leaf and stem P concentrations increased linearly with P fertilization without simultaneous N addition, these increased plant P levels might not stimulate plant growth if N demands are not satisfied. Nitrogen deposition alone (i.e, without P addition) can change plant stoichiometry. As the P addition rates increased, however, the effects of N fertilization were weakened. This suggests that N deposition-induced alteration of plant nutrition status and N:P ratio also rely on P availability. This study may improve our understanding of the tight and very complicated linking between the N and P cycling at a plant and ecosystem scales. Thereafter, multiplenutrient constraints and their interactions must be considered when assessing plant nutrients and growth responses to nutrient enrichment.
ACKNOWLEGEMENTS
We thank Lili Dong and Baixue Yang for laboratory assistance, and the Erguna Forest-Steppe Ecotone Research Station, China, for providing the experimental plots. This work was supported by Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15010401 and XDB15010403). C.A. Busso would like to thank the sabbatical leave by Universidad Nacional del Sur and the Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET), the Associateship awarded by the Third World Academy of Sciences (TWAS)-UNESCO, and housing, facilities and financial support from the Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, China.
REFERENCES
1. Bai, Y., J. Wu, C. Clark, S. Naeem, Q. Pan, J. Huang, L. Zhang & X. Han (2010). Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from Inner Mongolia grasslands. Global Change Biology 16: 358-372. [ Links ]
2. Bennett, E., S. Carpenter & N. Caraco (2001). Human impact on erodable phosphorus and eutrophication: A global perspective. Bioscience 51: 227-234. [ Links ]
3. Blanke, V., S. Bassin, M. Volk & J. Fuhrer (2012). Nitrogen deposition effects on subalpine grassland: The role of nutrient limitations and changes in mycorrhizal abundance. Acta Oecologica 45: 57-65. [ Links ]
4. Clark, C. & D. Tilman (2008). Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451: 712-715. [ Links ]
5. Cline, M.G. (1979). Soil classification in the United States. University of Pensylvania, Cornell University. [ Links ]
6. Davidson, E., C. de Carvalho, I. Vieira, R. Figueiredo, P. Moutinho, F. Ishida, M. dos Santos, J. Guerrero, K. Kalif & R. Saba (2004). Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecological Applications 14: 150-163. [ Links ]
7. Elser, J., M. Bracken, E. Cleland, D. Gruner, W. Harpole, H. Hillebrand, J. Ngai, E. Seabloom, J. Shurin & J. Smith (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135-1142. [ Links ]
8. Fritz, C., G. Dijk, A. Smolders, V. Pancotto, T. Elzenga, J. Roelofs & A. Grootjans (2012). Nutrient additions in pristine Patagonian Sphagnum bog vegetation: can phosphorus addition alleviate (the effects of) increased nitrogen loads?. Plant Biology 14: 491-499. [ Links ]
9. Galloway, J., F. Dentener, D. Capone, E. Boyer, R. Howarth, S. Seitzinger, G. Asener, C. Cleveland, P. Green, E. Holland, D. Karl, A. Michaels, J. Poeter, A.Townsend & C. Vrsmarty (2004). Nitrogen cycles: past, present, and future. Biogeochemistry 70: 153-226. [ Links ]
10. Galloway, J., A. Townsend, J. Erisman, M. Bekunda, Z. Cai, J. Freney, L. Martinelli, S. Seitzinger & M. Sutton (2008). Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 320: 889-892. [ Links ]
11. Goldberg, D. & T. Miller (1990). Effects of different resource additions on species diversity in an annual plant community. Ecology 71: 213-225. [ Links ]
12. Güsewell, S. (2004). N:P ratios in terrestrial plants: variation and functional signifficance. New Phytologist 164: 243-266. [ Links ]
13. Han, W., J. Fang, D. Guo & Y. Zhang (2005). Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytologist 168: 377-385. [ Links ]
14. Han, W., J. Fang, P. Reich., F. Ian Woodward & Z. Wang (2011). Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecology Letters 14: 788-796. [ Links ]
15. He, J., L. Wang, D. Flynn, X. Wang, W. Ma & J. Fang (2008). Leaf nitrogen:phosphorus stoichiometry across Chinese grassland biomes. Oecologia 155: 301-310. [ Links ]
16. He, M., & F. Dijkstra (2015). Phosphorus addition enhances loss of nitrogen in a phosphorus-poor soil. Soil Biology & Biochenistry 82: 99-106. [ Links ]
17. Huff, L.M. & D.L. Potts (2015). Ecosystem CO2 exchange in response to nitrogen and Phosphorus addition in a restored temperate grassland. The American Midland Naturalist 173: 73-87. [ Links ]
18. Jia, Y., G. Yu, N. He, X. Zhan, H. Fang, W. Sheng, Y. Zuo, D. Zhang & Q. Wang (2014). Spatial and decadal variations in inorganic nitrogen wet deposition in China induced by human activity. Scientifc Reports 4: 3763. [ Links ]
19. Jonasson, S., A. Michelsen, I. Schmidt & E. Nielsen (1999). Responses in microbes and plants to change temperature, nutrient, and light regimes in the arctic. Ecology 80: 1828-1843. [ Links ]
20. Jones, H.E. (1992). Plants and microclimate. A quantitative approach to environmental plant physiology. Cambridge University Press, Cambridge. [ Links ]
21. Kerkhoff, A., W. Fagan, J. Elser & B. Enquist (2006). Phylogenetic and Growth Form Variation in the Scaling of Nitrogen and Phosphorus in the Seed Plants. The American Naturalist 168: 103-122. [ Links ]
22. Koerselman, W. & A. Meuleman (1996). The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. Journal of Applied Ecology 33: 1441-1450. [ Links ]
23. LeBauer, D. & K. Treseder (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89: 371-379. [ Links ]
24. Lhuillier-Soundl, A., N. Munier-Jolain & B. Ney (1999). Influence of Nitrogen Availability on Seed Nitrogen Accumulation in Pea. Crop Science 39: 1741-1748. [ Links ]
25. Liu, X.,Y. Zhang, W. Han, A. Tang, J. Shen, Z. Cui, P. Vitousek, J. Erisman, K. Goulding, P. Christie, A. Fangmeier & F. Zhang (2013). Enhanced nitrogen deposition over China. Nature 494: 459-463. [ Links ]
26. Luzuriaga A., A. Escudero & F. Garcia-Perez (2005). Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae). Weed Research 46: 163-174. [ Links ]
27. Lü, X., D. Kong, Q. Pan, M. Simmons & X. Han (2012). Nitrogen and water availability interact to afect leaf stoichiometry in a semi-arid grassland. Oecologia 168: 301-310. [ Links ]
28. Lü, X., S. Reed, Q. Yu, N. He, Z. Wang & X. Han (2013). Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiarid grassland. Global Change Biology 19: 2775-2784. [ Links ]
29. Marklein, A. & B. Houlton (2012). Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytologist 193: 696-704. [ Links ]
30. Marschnert, H., E. Kirkby & C. Engels (1997). Importance of Cycling and Recycling of Mineral Nutrients within Plants for Growth and Development. Botanica Acta 110: 265-273. [ Links ]
31. Menge, D. & C. Field (2007). Simulated global changes alter phosphorus demand in annual grassland. Global Change Biology 13: 2582-2591. [ Links ]
32. Niklas, K., T. Owens, P. Reich & E. Cobb (2005). Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecology Letters 8: 636-642. [ Links ]
33. Oliveira, O., C. de I. Oliveira, E. Ferreira, B. Alves, C. Miranda, L. Vilela, S. Urquiaga & R. Boddey (2001). Response of degraded pastures in the Brazilian Cerrado to chemical fertilisation. Pasturas Tropicales 23: 14-18. [ Links ]
34. O’Neill, Sh.. & J.A. Roberts (2001). Plant Reproduction. University of California: Taylor & Francis.
35. Ordoñez, J., P. Bodegom, J. Witte, I. Wright, P. Reich & R. Aerts (2009). A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecology and Biogeography 18: 137-149. [ Links ]
36. Peñuelas, J., B. Poulter, J. Sardans, P. Ciais, M. Velde, L. Bopp, O. Boucher, Y. Godderis, P. Hinsinger, J. Llusia, E. Nardin, S. Vicca, M. Obersteiner & I. Janssens (2013). Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nature Communications 4: 1-10.
37. Prietzel, J. & U. Stetter (2010). Long-term trends of phosphorus nutrition and topsoil phosphorus stocks in unfertilized and fertilized Scots pine (Pinus sylvestris) stands at two sites in Southern Germany. Forest Ecology and Management 259: 1141-1150. [ Links ]
38. Ren, H., Z. Xu, J. Huang, C. Clark, S. Chen & X. Han (2011). Nitrogen and water addition reduce leaf longevity of steppe species. Annals of Botany 107: 145-155. [ Links ]
39. Roth, T., L. Kohli, B. Rihm & B. Achermann (2013). Nitrogen deposition is negatively related to species richness and species composition of vascular plants and bryophytes in Swiss mountain grassland. Agriculture, Ecosystems and Environment 178: 121-126. [ Links ]
40. Stevens, C., N. Dise, J. Mountford & D. Gowing (2004). Impact of Nitrogen Deposition on the Species Richness of Grasslands. Science 303: 1876-1879. [ Links ]
41. Stevens, C., C. Duprè, E. Dorland, C. Gaudnik , D. Gowing, A. Bleeker, M. Diekmann, D. Alard, R. Bobbink, D. Fowler, E. Corcket, J. Mountford, V. Vandvik, P. Aarrestad, S. Muller & N. Dise (2010). Nitrogen deposition threatens species richness of grasslands across Europe. Environmental Pollution 158: 2940-2945. [ Links ]
42. Stevens, C. & D. Gowing (2013). Effect of nitrogen addition, form and clipping on competitive interactions between grassland species. Journal of Plant Ecology 7: 220-230. [ Links ]
43. Stcklin, J., K. Schweizer & C. Krner (1998). Effects of elevated CO2 and phosphorus addition on productivity and community composition of intact monoliths from calcareous grassland. Oecologia 116: 50-56. [ Links ]
44. Tilman, D. (1988). Plant strategies and the dynamics and structure of plant communities. Princeton: Princeton University Press. [ Links ]
45. Valencia-Díaz, S. & C. Montana (2005). Temporal variability in the maternal environment and its effect on seed size and seed quality in Flourensia cernua DC. (Asteraceae). Journal of Arid Environments 63: 686-695. [ Links ]
46. Vrede, T., D. Dobberfuhl, S. Kooijman & J. Elser (2004). Fundamental connections among organism C:N:P stoichiometry, macromolecular composition, and growth. Ecology 85: 1217-1229. [ Links ]
47. Wright, I., P. Reich, M. Westoby, D. Ackerly, Z. Baruch, F. Bongers, J. Cavender-Bares, T. Chapin, J. Cornelissen, M. Diemer, J. Flexas, E. Garnier, P. Groom, J. Gulias, K. Hikosaka, B. Lamont, T. Lee, W. Lee, C. Lusk, J. Midgley, M. Navas, Ü. Niinemets, J. Oleksyn, N. Osada, H. Poorter, P. Poot, L. Prior, V. Pyankov, C. Roumet, S. Thomas, M. Tjoelker, E. Veneklaas & R. Villar (2004). The worldwide leaf economics spectrum. Nature 428: 821-827. [ Links ]
48. Yang, X., Z. Tang, C. Ji, H. Liu, W. Ma, A. Mohhamot, Z. Shi, W. Sun, T. Wang, X. Wang, X. Wu, S. Yu, M. Yue & C. Zheng (2014). Scaling of nitrogen and phosphorus across plant organs in shrubland biomes across Northern China. Scientific Reports 4: 5448. [ Links ]
49. Yu, Z., D. Zeng, F. Jiang & Q. Zhao (2009). Responses of biomass to the addition of water, nitrogen and phosphorus in Keerqin sandy grassland, Inner Mongolia, China. Journal of Forestry Research 20: 23-26. [ Links ]
50. Yu, Q., J. Elser, N. He, H. Wu, Q. Chen, G. Zhang & X. Han (2011). Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 166: 1-10. [ Links ]
51. Zhang, L. (2003). Plant N:P stoichiometry: variations of Chinese higher plants and a preliminary test by field experimentation. Dissertation, Institute of Botany, Chinese Academy of Sciences. [ Links ]
52. Zhang, Y., J. Feng, F. Isbell, X. Lü & X. Han (2015). Productivity depends more on the rate than the frequency of N addition in a temperate grassland. Scientific Reports 5: 12558. [ Links ]














