Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Ciencia y Tecnología
On-line version ISSN 1851-7587
Rev. cienc. tecnol. no.25 Posadas June 2016
INGENIERÍA, TECNOLOGÍA E INFORMÁTICA
Bacterial and Antioxidant Activity of Commertial Essential Oils of Rosemary, Clove, Oregano and Sage
Actividad Antibacteriana y Antioxidante de los Aceites Esenciales Comerciales de Romero, Clavo de Olor, Orégano y Salvia
Mariane L. Ugalde1,2, Aline M. De Cezaro2, Aline Cenci2, Claudio V. Junior2, Natalia Paroul2, Geciane Toniazzo2, Juliana Steffens2,*, Rogerio L. Cansian2
1 Instituto Federal Farroupilha, Campus Julio de Castilhos, Sao Joao do Barro Preto s/n, PO Box 38, CEP 98130-000, Julio de Castilhos, RS, Brasil.
2 URI Erechim, Departamento de Ingenieria de alimentos, Av. Sete de Setembro, 1621- CEP 99709-910, Erechim, RS, Brasil.
* E-mail: julisteffens@uricer.edu.br
Abstract
This study aimed to compare the chemical composition and in vitro antibacterial and antioxidant activity of commercial essential oils of rosemary, clove, oregano, sage and binary combination of clove and oregano. Eugenol (89.58%) for clove, carvacrol (60.71%) for oregano, bornyl acetate (39.64%) for rosemary, linalool (39.26%) for sage and eugenol (56.42%) for binary combination were identified as the main components . In evaluation of antibacterial activity, the oregano showed the highest inhibition zones and the smallest minimum inhibitory concentration. The binary combination with IC50 of 6.40 μg/mL, followed by clove with IC50 of 11.79 μg/mL had excellent antioxidant potential.
Keywords: Carvacrol; Commercial essential oils; Eugenol; Antibacterial activity; Antioxidantactivity.
Resumen
Este estudio tuvo como objetivos comparar la composicion quimica y actividad antioxidante y antibacteriana in vitro de los aceites esenciales comerciales de romero, clavo de olor, oregano, salvia y combinacion binaria de clavo y oregano. Fueron identificados como componentes principales, eugenol (89,58%) para el clavo, el carvacrol (60,71%) para el oregano, el acetato de bornilo (39,64%) de romero, linalol (39,26%) de la salvia y el eugenol (56,42%) para la combinacion binaria. En la evaluacion de la actividad antibacteriana el oregano mostro las zonas de inhibicion mas altas y la concentracion inhibitoria minima mas pequena. La combinacion binaria con IC50 de 6,40 μg/mL, seguido de clavo de olor con IC50 de 11,79 μg/mL tiene un excelente potencial antioxidante.
Palabras clave: Carvacrol; Aceites esenciales comerciales; Eugenol; Actividad antibacteriana; Actividad antioxidante.
Introduction
Essential oils (EOs) are volatile aromatic liquids extracted by many parts of plants, i.e. flowers, leaves, bark, seeds, roots and resins. These natural compounds are defined as secondary metabolitesof plants and have relevant functionindefense of producing organismactingas antimicrobial, antiviral, antifungaland insecticides. Although the food industries use the EOs mainly as flavorings, they represent an important alternative source of natural antimicrobial and antioxidant and can be used for the preservation of food products. In Brazil, EOs and extracts are included within the class of additives such as natural flavoring (1).
The EOsare formed by several organiccompounds oflow molecular weight, with different antimicrobialactivities and may be divided into fourgroups according totheir chemical structure: terpenes, terpenoids, phenylpropenes and others (2). The presence of those compounds justifies its antimicrobial and antioxidant properties. The EOs of rosemary, clove, oregano and sage have antimicrobial and antioxidant activities described in the literature (3-6). However, there are few studies comparing these effects, mainly for commercial EOs, which can be easily used in the food industry. Given the importance of the search of natural antimicrobials and antioxidants with application in food industry, this study aimed to determine comparatively the chemical composition and in vitro antibacterial and antioxidant activity of commercial EOs of Rosemary (Rosmarinus officinalis L.), clove (Eugenia caryophyllata L.), oregano (Origanum vulgare L.), sage (Salvia sclarea L.) and of binary combination of EOs of clove and oregano, in proportion 1:1.
Materials and methods
To determine the chemical composition and in vitro antimicrobial and antioxidant activities, commercially EOs obtained by (Ferquima®) Rosemary (Rosmarinus officinalis L.), clove(Eugenia caryophyllata L.), oregano (Origanum vulgare L.) and sage(Salvia sclarea L.) were used.
Chemical composition
EOs samples were prepared by diluting in hexane (Merck®) (10.000 mg mL-1). For identification of the volatile compounds of EOs were made analyses by gas chromatography coupled to mass spectrometry(GC-MS) (Shimadzu QP5050A)using aDB-WAX capillary column (30m, 0.25mm, 0.25μm). The column temperature was programmed at 50°C for 3 minutes, increased 5°C per min until 130°C and then 1°C per min until 210°C per 5 min. Helium was used as carrier gás and the temperatures of the detector and injector were 250°C. This device was operated with a flow rate of 1 mL/min with an electronicimpact of 70 eVandsplit mode (split ratio1:3). The volume injected was 1.0 μL. The peaks were integrated by manual mode, and the compounds were identified by comparing their mass spectra with those available in the Wiley mass spectral database (330,000) and by comparing the retention times of standard compounds (eugenol and linalool). 90% was adopted as a minimum percentage of similarity between the mass spectra of the compounds of the samples and the library of the equipment for identification of them.
Antimicrobial activity
Twelve microorganisms were selected for the analysis of antibacterial activity, six Gram-positive bacteria Enterococcus faecalis (ATCC 29212), Micrococcus luteus (ATCC 10240), Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), Streptococcus mutans (ATCC25175), Listeriamonocytogenes (ATCC 7644) and six Gram negative bacteria Aeromonas sp. (microorganism obtained from the Biological Institute, Campinas- SãoPaulo), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Salmonella choleraesuis (ATCC 107008), Klebsiella pneumoniae (ATCC 10031), Proteusmirabilis (ATCC 25933), previously grownin Luria Bertani (LB) (10 g L-1 of tryptone, 5g L-1 of yeast extract and 5 g L-1 of NaCl) for 24 h at 36 ±1 °C. To evaluate the antimicrobial activity by the formation of the inhibition halo, Petri plates were used with culture médium Müeller-Hinton Agar(Merck®) and Whatman paper disks number 3, with 7 mm of diameter (7). The cultures of bactéria were inoculated by scattering on plates with the aid of a Drigalski hook, in a volume of 200 μL(108UFC mL-1). For each bacterium and EO tested, a plate was prepared in which three discs have been deposited with the volume being tested (5, 10 and 15 μL of pure oil), a negative controldisk (white) and a positive controldisk (30μgof chloramphenicol antibiotic). These volumes were determined from the previous tests, where the maximum volume of EO used was that the paper disc absorbed without overflowing. After incubationof the plates at36 ± 1°C for 48 hours, the results were analyzedby measuringthe diameter of the inhibition haloof bacteria's growth,including thediameter of the paperdisk.
Results were expressedin millimeters (mm) by the arithmetic averages of the values of halos obtained in three replicates (per volume used), and the averages where subjected to Analysisof Variance (ANOVA) and compared by Tukey test (p <0.05), using theASSISTAT® program. To determine the Minimum Inhibitory Concentration (MIC), the indirect method for bacterial growth by optical density in liquid culture (8) was used. After the results of the antibiograman alysis on solid media, eleven selected bacteria were grown inculture médium LB broth at 37°C for 24 h. The bacterium E.faecalis was not used in this step in view of the impossibility of its reactivating. After a period of growth, the cultures were inoculated into microtubes (Eppendorf)10 μL of pre-inoculum (108 UFC mL-1), 1 mL of LB broth, plus 1% of emulsifier dimethylsulfoxide (DMSO)(Nuclear®) containing different concentrations of EOs (0.01 to 2.50 mg mL-1) and the control without EO. All concentrations of each EO, with different bactéria were evaluated in triplicate. Subsequent to inoculation process, the microtubes were incubated in an electromagnetic stirrer (60 Hz) for 24h at 32°C. Before and after the incubation period, 0 and 24 h espectively, aliquots of 100 μL of bacterial culture were transferred to flat-bottom microplates, three readings for each repetition were performed. There were problems of turbidity of the sample that contained only oregano EO on the concentration of 2.50 mg mL- 1which cannot be used.
To evaluate the bacterial growth (optical density) and to determine the MIC of EO on bacterium, reading of the microplate was performed using the automated microplate reader(Bio-Tec InstrumentsInc., ModelEL800), coupled to one computer with Kcjuniorprogram, with preset length of 490 nm. Growth inhibition was determined by the difference between the readings taken at 24 and 0 h. The average optical density values were statistically analyzed by analysis of variance (ANOVA) and compared by Tukey's test (p <0.05), to determine the MIC, using theASSISTAT® program.
Antioxidant activityby free radicals capture with DPPH test
The methodology for evaluation of antioxidant activity, based on the measurement of the extinction of absorption of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical at 515 nm, was performed in triplicate by spectrophotometric method (9). The technique consisted of incubation for 30 min of 500 μL of at 0.1m Methanolic solution of DPPH with 500 μL of solutions containing increasing concentrations of EOs of rosemary, clove, oregano and sage(1.0; 2.5; 7.5; 10; 25; 50; 75; 100; 250; 500; 750 and 1000μg mL-1) in ethanol. We proceeded similarly to the preparation of the solution called “control”, replacing 500 μL of sample per 500 μL of ethanol. A solution called “white” was prepared with solutions at different concentrations of EOs and ethanol, without DPPH. The antioxidant compound BHT (3,5-di-tert-4-butylhydroxytoluene) was used as a positive control. The percent uptake of theDPPH radical was calculated in terms of percentage of antioxidant activity (AA %), according to Equation1.
![]()
The concentration of EO needed to capture 50% of the free radical DPPH (IC50) was calculated by linear regression analysis of the points plotted graphically (10). For the plot points, the average values obtained from triplicates performed for each concentration were used. The antioxidant capacity was calculated according to the equation of the curve of the antioxidant activity, where y is replaced by 50 and x is the value of IC50. Results were expressed as the arithmetic average of the values obtained in three replicates, where the averages were statistically analyzed by analysis of variance(ANOVA) and compared by the Tukey test (p <0.05), using the ASSISTAT® program.
Results and discussion
Chemical composition
The contents of the major components found in the EOs evaluated, including the binary 1:1 combination of EOs ofclove and oregano, determined by analysis by GC-MS, are shown in Table 1.
Table 1: Principal volatile compounds (% of area) found in rosemary(R. officinalis), clove E. caryophyllata), oregano (O. vulgare), sage (S. sclarea) commercial essential oils and binary combination of clove/ oregano 1:1.


Several publications have presented data on the chemical composition of different EOs, which may comprise more than sixty individual components (3). The major compounds may constitute up to 85% of the EO, while other sare present only astrace elements, the latter have akey role to playin biological activities, possibly producing a synergistic effect between other components (2). In the present study, bornyl acetate was identified as the major compound (39.64%) for the rosemary EO (Table 1). Some studies report the isolation and identification of different compounds with antioxidant and antimicrobial activity present in the rosemary EO, which consists of monoterpenichydrocarbons, terpenicesters, linalool, verbinol, terpineol, 3-octanoneandiso-bornylacetate, among other compounds(12). Pintore et al. (13) identified for the rosemary EO, percentages ranging from 3-89% of bornylacetate,2-25% of 1,8-cineole, camphor 2-14% and 2-25% α-pinene, like in this study.
The clove EO presented phenylpropeneeugenol as the major volatile compound (89.58%) (Table 1), where in similar result was obtained by Silvestri ET al. (7) with eugenol representing 90.30% of volatile compounds. The monoterpenoid carvacrol was the major compound found in orégano EO (60.71%) (Table 1), which presents known antimicrobial activity (3). Silva ET al., (14) evaluated Five distinct trademarks of EOs of oregano, and all chromatograms showed a single large peak at the same retention time. When compared to a standard, it was identified as carvacrol, in percentages ranging between 61.70 and 93.40% of the total volatile detected in each oil. Busatta et al. (15) found much lower percentage of carvacrol (11.67%), evaluating oregano EO obtained from leaves originating from Chile.
Sage EO had linalool as main volatile compound (39.26%) (Table 1).Pierozan et al.(8) determined the chemical composition of different species of sage, where the majority of volatile compounds obtained were α-thujone (40.37%), Salvia sclarea,β-thujone (19.96%) in the S.lavandulifolia, linalool(29.36%) inS.sclareaandα-thujone (22.39%) inS.triloba. The binary combination of clove and oregano EOs (1:1) qualitatively presented sum of both oils, but with quantitative changes, as expected.
Antibacterial activity
The average values of inhibition zone (mm) obtained by disc diffusion are shown in Table 2. The clove EO showed action over all the bacteria tested, with the highest activity observed (33.33 mm) on K.pneumoniae. Other authors found that the clove EO showed strong antimicrobial activity when tested for microorganisms S.aureus, E.coli, C.jejuni, S.enteritidis, L.monocytogenesandS.epidermidis(5). The bacteriumK. Pneumoniae showed up not sensitive to oregano EO, which showed the highest activityon S.mutans(47.33 mm). Busatta et al. (15) obtained mean halos of 19.50 mm of oregano EO against K.pneumoniae. Sahin et al., (16) evaluating oregano EOs from Turkey, using10 μLper disc, also did not obtain action against K.pneumoniae, corroborating this research. The rosemary EO did not act on P. aeruginosa, when used 5 μL and presented highest activity on K. pneumoniae (31.33 mm) at 15 μL. High sensitivity of Gram-positive bacteria to EOs of rosemary and sage from Egypt was reported, including S. aureus, Micrococcus sp. And Sarcina sp.as well as S.cerevisiae. However, no or very little effect was observed against Gram-negative bacteria P.fluorenscens, E.coli and S.marcescens(17).
Table 2: Average values ofinhibition zone (mm) ofessentail oils (5, 10and15μL) of clove(Eugenia caryophyllata L.), oregano (Origanum vulgare L.), rosemary (Rosmarinus officinalis L.) and sage (Salvia sclarea L.) front Gram-positive and Gram-negative bacteria.

The sage EO showed the lowest performance among the oils tested, with activity on lyagainst S.choleraesuis, among Gram-negative bacteria. Pozzo et al. (18) evaluated the antimicrobial activity of EOs of condiments against Staphylococcusspp. and did not observeantibacterial activity of EOs of ginger, basil, rosemary and sage. In contrast to these authors, Delamare et al., (19) observed activity of sage EO against some isolates of Staphylococcus sp. There was atendency to increase the efficiency of EOs when it wasincreased thevolume usedfrom 5 to15 μL, but thiseffect was notstatisticallysignificant (p>0.05) for allmicroorganisms and EOstested(Table 2). Although there are exceptionsin the literature,in generalEOsare more effectiveagainst Gram-positive thanGramnegative bacteria, this low efficiency can be attributed to the fact that Gram-negative bacteria possess an outer membrane, which restricts diffusion of hydrophobic compounds through its lipopolysaccharide covering(3). Thisfact canbe observed inEOsof oregano and sage testedin this study, forall concentrations used. The clove EO showed similar behavior against the Gram-positive and negative bacteria, while the rosemary EO was more effective in relation to Gram-negative bacteria. The plates methodology has great importance to provide initial data of the antimicrobial action of natural products, by its easyand quick execution (20). However, it is considered essential to continue the studies to obtain values of MIC, which was performed in the second part of this study. In the evaluating of MIC, it was observedthat theoregano EOexhibited the bestperformance among thetested oils, being effective against all microorganisms evaluated (Table 3), reaching an average of 0.016 mg mL-1 for Grampositive bacteria and 0.020 mg mL-1 for Gram-negative bacteria. Busatta et al. (15) evaluated in vitro antimicrobial activity of oregano EO found average values for MIC of 0.460 mg mL-1, i.e.,lower performance than that found in the present study.
Table 3: Minimum Inhibitory Concentration (MIC) of oregano EO (Origanumvulgare L.), clove EO (Eugenia caryophyllata L.) and mixing of oregano and clove (1:1).

There are reports that the carvacrol and thymolare the main components and those responsible for the antimicrobial activity of the oregano EO (14). Furthermore, there are evidences that some components present in small amounts, such as γ-terpinene and ρ-cymene, affect in the antimicrobial activity by producing a synergistic effect between the others components (3, 21).
The clove EO was also effective in inhibiting all microorganisms (Table 3), with mean values for 0.70 mg mL-1 for Gram-positive bacteria and 0.49 mg mL-1for Gram-negative bacteria. Silvestri et al. (7), obtained values of MIC similar to clove EO, 0.50mg mL-1 for Grampositive bacteria and 0.58mg mL-1 for Gram-negative bacteria. The clove EO has eugenolas major substance, responsible for the analgesic, anti-inflammatory and antioxidant activity (6). The Eos of rosemary and sage had the worst performance, with a mean value ofMIC of 2.5 mg mL-1 for all microorganisms used in the study (data not found). Fu et al. (22) found activity of rosemary EO against S.epidermidis (ATCC 12228) and S.aureus (ATCC 6538). Delamare et al., (19) observed the activity ofsage EOagainst someisolates of Staphylococcusspp.
Given the better performance of Eos of clove and oregano in the MIC evaluation, it was decided to perform a binary combination of both, in the proportion 1:1, against to the same microorganisms. There was a reductionof MIC presented by clove EO individually for all bacteria tested, except for M.luteus, which remained unchanged (Table 3). Evaluating the chemical composition of the binary combination 1:1 of clove and oregano EOs (Table 1), can be observed that the eugenol, present in the clove essential oil, is the major component (56.42%), and the other six components present in the oregano essential oil presented minor percentual area, suggesting synergist effect of these compounds, which can justify the improvement of the MIC when compared to the clove EO with the mixture of oils (Table 3). The Origanumvulgare EO has demonstrated good bactericidal and fungicidal activity against different pathogens, due to the compounds carvacrol and thymol, which are phenolic components present in greater quantity (23).
Antimicrobial activity of na EO can depend only on one or two of the major components that constitute it. However, an increasing amount of evidence indicates that the inherent activity of the EOs may be a function of the interaction between its minor constituents. Several synergistic antimicrobial activities were reported to components or fractions of EOs, using binary or tertiary combinations (3). Doing a parallel between the results obtained in the diffusion plates and MIC, of the EOs tested, it can be observed that the EOs of clove and oregano remained as the best performers, with some particularities. In disc diffusion test, the oregano EO was ineffective against the bactéria K.pneumoniae, whereas a value of 0.025mg mL-1was obtained when performing their MIC (Table 3). This may be due to a possible difficulty of the oil to migrate to the agar in the disc diffusion test, whereas when performed the determination of MIC, the oil is in direct contact with the microorganism.
Antioxidant activity by capture radicals with DPPH test
Theantioxidant activity (AA%) of the oils tested was calculatedfrom the percent uptake of DPPH radical. The EO of clove showed the most AA%, 89.38% at the concentration of 50 μL mL-1, followed by rosemary oil 77.90% at a concentration of 750 μL mL-1 and 75.11% at a concentration of 250 μL mL-1 to oregano EO.With the BHT, used as a reference synthetic antioxidant, was observed an activity of 89.92% at a concentration of 100 μL mL-1. Silvestri et al. (7), evaluating the clove EO in different concentrations, obtained a maximum AA% of 95.6% at a concentration of 10,000 μL mL-1, which confirms the high antioxidant activity of this oil. Scherer et al. (5), analyzed the volatile composition of the clove EO where eugenol was identified as the major compound, with 83.75% of the total area. The same was observed in the present study (Table 1), which is a phenolic compound with strong antioxidant action that has been proven both in vitro and in vivo (24). The sage EO presented the lowest performance among the oils analyzed, with an antioxidant activity of 19.70% at a concentration of 1,000 μL mL-1, although this condiment was extensively studied and recognized for its antioxidant capacity related to their phenolic compounds (25). From the correlation between the antioxidant activity (AA%) and the concentration of the oils used, linear equations were obtained, which provide the data to calculate the IC50, which corresponds to the sample required to reduce by 50% the initial concentration of DPPH (Table 4).
Table 4: Linear equations and IC50 of EOs tested.
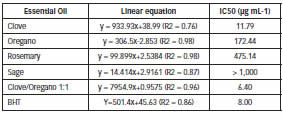
As shown in Table 4, the clove EO presented an IC50 of 11.79 μL mL-1, which can be considered good compared with antioxidant of excellence as ascorbic acid (IC50 = 2.15 μL mL-1) and BHT (IC50 = 5.37 μL mL-1) (26). Pérez-Rosés et al. (27) reached an IC50 of 13.20 μg mL-1 for the clove EO obtained from the leaves of the plant. Sahin et al. (16), evaluating EOs of oregano from plants of Turkey, had IC50 values greater than those obtained in this study with average values of 8,900 μg mL-1, where the major compounds present in the oils were caryophyllene (14.40%) and spathulenol (11.60%). Zaouali et al. (28) evaluating rosemary EOs from Tunisian, had IC50 values ranging between 6.00 and 28.50 μL mL-1, higher than those found in this study, and this activity being associated with high levels of camphor, linalool acetate and α-tujene found in the oil. For BHT, the IC50 obtained was 8.00 μL mL-1 (Table 4). Aiming future application in food products, the binary combination (1:1) of clove and oregano EOs was also evaluated with respect to antioxidant activity. This combination showed a maximum AA% of 85.16% at the concentration of 100.00 μL mL-1 and a IC50 of 6.40 μL mL-1 (Table 4), higher than that obtained for the reference substance, the BHT, used in this research as well as for both EOs when used in isolated, possibly as a result of different chemical composition obtained from this combination (Table 1). Together, the different compounds present in the EOs, produce an array of antioxidants that may act by different mechanisms to confer an effective system of defense against the attack of free radicals (29). The eugenol and carvacrol were the major volatile compounds found in binary combination of EOs of clove and oregano (1:1) (Table 1), which justifies the IC50 obtained of the mixture (Table 4).
Conclusion
From the assessed parameters, it can be concluded that the clove EO and oregano EO and the binary combination of EOs of clove and oregano (1:1) showed excellent potential to be used as antioxidant and antibacterial, which are justified by the major volatile compounds identified, carvacrol (60.71%), eugenol (89.58%), and, carvacrol (15.39%)/eugenol (56.42%), for oregano, clove and their combination, respectively. Evaluating the overall performance achieved by EOs in vitro tests, it can be inferred that they present a promising alternative in food preservation, such as component of bioactive packaging.
Acknowledgment
The authors thanks to CAPES, FAPERGS, SCIT-RS and CNPq for financial support.
1. ANVISA. 2007. Agência Nacional de Vigilância Sanitária. Regulamento técnico sobre aditivos aromatizantes. Resolução - RDC nº 2. Brasil. [ Links ]
2. Hyldgaard, M; Mygind, T y Meyer, RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Frontiers Microbiol. 3:p. 1. 2012. [ Links ]
3. Burt, S. Essential oils: their antibacterial properties and potential applications in foods. J. Food Microbiol.94: 223-253. 2004. [ Links ]
4. Oliveira, A. C; Valentim, I. B; Goulart, M. O. F; Silva, C. A; Bechara, E. J. H y Trevisan, M. T. S. Vegetals as natural sources of antioxidants. Quím. Nova. 32: 689-702. 2009. [ Links ]
5. Scherer, R; Wagner, R; Duarte, M. C. T y Godoy, H.T. Composition and antioxidant and antimicrobial activities of clove, citronella and palmarosa essential oils. Rev. Brasil. Plantas Medic. 11: 442-449. 2009. [ Links ]
6. Duke, J. A. Biologically-active compounds in important spices. In: Charalambous, G. Spices, herbs and edible fungi. Elsevier Publishers (Ed.) p. 225. Amsterdam, 1994. [ Links ]
7. Silvestri, J. D. F; Paroul, N; Czyewski, E; Lerin, L; Rotava, I; Cansian, R.L; Mossi, A; Toniazzo, G; Oliveira, D y Treichel, H. Profile of the chemical composition and antibacterial and antioxidant activities of the essential oil of clove (Eugenia caryophyllata Thunb.). Rev. Ceres. 57:589-594. 2010. [ Links ]
8. Pierozan, M. K; Pauletti, G. F; Rota, L; Santos, A. C. A; Lerin, L. A; Di Luccio, M; Mossi, A. J; Atti-Serafini, L; Cansian, R. L y Oliveira, J. V. Chemical characterization and antimicrobial activity of essential oils of salvia L. species. Food Sci. Tech. 29: 764-770. 2009. [ Links ]
9. Kulisic, T; Radonic, A; Katalinic, V. y Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 85: 633-640. 2004. [ Links ]
10. Vanin, A. B; Orlando, T; Piazza, S. P; Puton, B. M. S; Cansian, R. L; Oliveira, D y Paroul, N. Antimicrobial and antioxidant activities of clove essential oil and eugenyl acetate produced by enzymatic esterification. App. Biochem. Biotechnol. 174: 1286-1298. 2014. [ Links ]
11. Adams, R. P. Identification of essential oil components by gas cromatography/mass spectroscopy. 4° Ed., Allured Publ. Corp: Carol Stream. 2007. [ Links ]
12. Alonso Junior, R. Tratado de fitomedicina: bases clínicas y farmacológicas. Buenos Aires: Isis Ediciones. SRL. 1998. [ Links ]
13. Pintore, G; Usai, M; Bradesi, P; Juliano, C; Boatto, G; Tomi, F; Chessa, M; Cerri, R y Casanova, J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flav. Frag. J. 17:15- 19. 2002. [ Links ]
14. Silva, J. P. L; Duarte-Almeida, J. M; Perez, D. V y Franco, B. D. G. M. Oregano essential oil: influence of the chemical composition on the inhibitory activity against Salmonella Enteritidis. Food Sci. Tech. 30: 136-141. 2010. [ Links ]
15. Busatta, C; Mossi, A. J; Alves, R. M. R; Cansian, R. L y De Oliveira, J. V. Evaluation of Origanum vulgare essential oil as antimicrobial agent in sausage. Braz. J. Microbiol.38: 610-616. 2007. [ Links ]
16. Sahin, F; Gulluce, M; Daferera, D; Sokmen, A; Sokmen, M; Polissiou, M; Agar, G y Ozer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. Vulgare in the Eastern Anatolia region of Turkey. Food Contr. 15: 549-557. 2004. [ Links ]
17. Farag, R. S; Badei, A. Z. M. A;Hewedi, F. M y El-Baroty, G. S. A. Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. J. American Oil Chemist's Soc. 66: 792-799. 1989. [ Links ]
18. Pozzo, M. D; Viégas, J; Santurio, D. F; Rossatto, L; Soares, I. H; Alves, S. H y Costa, M. M. Antimicrobial activities of essential oils extracted from spices against Staphylococcus spp isolated from goat mastitis. Ciênc. Rural. 41: 667-672. 2011. [ Links ]
19. Delamare, A. P. L; Moschen-Pistorello, I. T y Artico, L. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 100: 603-608. 2007. [ Links ]
20. Klancnik, A; Piskernik, S; Jersek, B y Mozina, S. S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol Meth.81: 121-126. 2010. [ Links ]
21. Paster, N; Menasherov, M; Ravid, U y Juven, B. Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J. Food Protect. 58: 81-85. 1995. [ Links ]
22. Fu, Y; Zu, Y; Chen, L; Shi, X; Wang, Z; Sun, S y Efferth, T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytotherapy Res. 21: 989- 994. 2007. [ Links ]
23. Kačániová, M ; Vukovič, N; Horská, E; Salamon, I; Bobková, A; Hleba, L; Fiskelová, M; Vatľák, A; Petrová, J y Bobko, M. Antimicrobial and antiradicals activity of Origanum vulgare L.and Thymus vulgaris essential oils. J. Microbiol. Biotechnol. Food Sci. 2: 263-271. 2012. [ Links ]
24. Ito, M. M. K y Yoshino, M. Antioxidant action of eugenol compounds: role of metal ion in the inhibition of lipid peroxidation. Food Chem. Toxicol. 43: 461-466. 2005. [ Links ]
25. Cuppett, S. L. y Hall, C. A. Antioxidant activity of the Labiatae. Adv. Food Nutr. Res. 42: 245-251. 1998. [ Links ]
26. Cansian, R. L; Mossi, A. J; De Oliveria, D; Toniazzo, G; TreichelH; Paroul, N; AstolfI, V y Serafini, L. A. Antimicrobial and antioxidant activities of ho-sho (Cinnamomum camphora Ness e Eberm Var. Linaloolifera fujita) essential oil. Food Sci. Technol.30: 378-384. 2010. [ Links ]
27. Pérez-Rosés, R; Risco, E; Vila, R; Peñalver, P y Cañigueral, S. Antioxidant and complement modulating activities of five essential oils. Planta Medica. 73:489-. 2007. [ Links ]
28. Zaouali, Y; Bouzaine, T y Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem.Toxicol. 48: 3144-3152. 2010. [ Links ]
29. Shahidi, F. Natural Antioxidants - Chemistry, Health Effects and Applications. Champaign, AOCS Press, Illinois. 1997. [ Links ]
Recibido: 30/04/2015
Aprobado: 07/10/2015














