Introduction
In December 2019, there were the first outbreaks in China due to the novel coronavirus (SARS-COV-2). With the growing number of cases in several countries, on March 11, 2020, the world health organization declared the 2019 coronavirus disease pandemic (COVID-19). Since then, scientists from different countries share the same goal: to develop treatments, vaccines, diagnostics, and products against COVID-19.[1,2]
The different technological strategies against COVID-19 allowed scientific and technological development also in the field of nanotechnology, presenting a great need to better understand and explore this novel nano-virus (60-140 nm in diameter).[3-5]
Nanotechnology provides the development of systems under a dimension of fewer than 100 nanometers, with potential for both diagnosis, treatment, and prevention of diseases, through nanoparticles that have unique properties, presenting better solubility, biocompatibility, conductivity, reduced toxicity, and multifunctionality.[6, 7]
Nanotechnology applied to the medical field is known as nanomedicine, where nanomaterials are used for treatments, vaccines, diagnostics, or disease prevention products.[8]
Nanomedicine, for example, is capable of being used to improve immunogenicity with prophylactic approaches. Nanoparticles can work basically by increasing the activation of immunity so that it protects against disease.[9]Due to their size, 1-100 nm, nanotransporters such as liposomes, nanoemulsions, synthetic polymeric nanoparticles, proteasomes, nano-granules, inorganic nanomaterials, as well as biological polymeric nanoparticles (exosome, bacteriophage) have been widely tested and used in the prevention of infectious and non-infectious diseases, as they can be captured by cells by endocytosis, and thus release biologically active compounds.[10,11]
Another important feature of nanotechnology in the medical field is the ability to modify the surface and effectively co-deliver adjuvants, which makes nanoparticles a potential candidate for commercial vaccines. Also, nano adjuvants in vaccines protect the target antigen from degradation and increase absorption by immunological mediators of biological systems. This approach is malleable, having the ability to present the antigen in a repetitive manner leading to stable immunogenic properties.[11,12] This type of nanomedicine-based approach has already been used against SARS-CoV-1 and MERS and now with SARS-COV-2.[13]
Nanomedicine has been present in the modern world for decades, having its first product regulated by the Federal Drug Administration (FDA) in 1995.[14] There are many applications of nanomedicine for different uses. Here we highlight mainly the products and diagnostics, vaccines and treatments that are or can be used against COVID-19.
Methods
A literature review was carried out in studies published from February to November 2020 in PubMed, Scielo, and Google Scholar databases. According to the indexes of the various databases, search terms were used: “new coronavirus 2019”, “COVID-19”, “Nanotechnology against COVID-19”, “COVID-19 Vaccines“, “severe acute respiratory syndrome” without any language restrictions. Those who described a comparative overview of coronavirus, treatments, vaccines, diagnostics and productsnano-basedagainst COVID- 19 were eligible.
Results and Discussion
Products and diagnostics
During this fight against coronavirus 2019 (COVID-19), our main line of defense is our immune system; however, people who are immunocompromised or who have at least one underlying comorbidity are considered to be quite vulnerable and their only line of defense is disinfectants, facial masks, immune system stimulants and medications.[3](Fig. 1)
The nanotechnology field has grown a lot with these new technological advances, and several products based on nano or antiviral agents to block SARS-CoV-2 have been developed.[15,16] Antimicrobial and antiviral formulations based on nanotechnology can prevent the spread of the SARS-CoV-2 virus, and the development of highly sensitive biosensors and detection platforms can contribute to the detection and diagnosis of COVID-19.[3]
Viral disinfectants, produced using nano-effective antimicrobial and antiviral formulations, are suitable for disinfecting air and surfaces and are also effective in reinforcing personal protective equipment, such as face respirators. Metallic nanoparticles (silver, copper, titanium dioxide nanoparticles) have been proposed as alternatives due to their wide range of inherent antiviral activities, persistence, and ability to be effective at much lower doses.[4]
These nanomaterials have enormous potential as disinfectants against coronavirus, as they have intrinsic antiviral properties, such as the generation of reactive oxygen species (ROS) and photodynamic and photothermal capabilities. The adverse effects on human health and the environment of metallic nanomaterials can be avoided with the use of biodegradable (that is polymeric, lipid-based) nanomaterials.[3]
Preliminary tests showed that the silver nanocluster coating/silica composite in disposable FFP3 face masks (3M TM) had viricidal effects against SARS-CoV-2.[17] The NanoTechSurface developed by Italy is a durable and self-sterilizing formula composed of titanium dioxide and silver ions to disinfect surfaces. Graphene sheets with antibodies have the potential to quickly detect targeted viral proteins and are also used for the development of environmental sensors and filters, due to the low cost of graphene materials. Functionalized graphene has demonstrated a good capacity for viral capture that, combined with heat or light-mediated inactivation, can be used as a disinfectant.[18] Reusable and recyclable graphene surgical masks with excellent superhydrophobic, photothermal performances, and excellent self-cleaning properties are commercially available.[19]
Researchers in Egypt have developed a new device against SARS-CoV-2. This corresponds to a breathing filter mask design based on polylactic acid (PLA), a biodegradable and transparent polymer, cellulose acetate (CA), copper oxide nanoparticles (CuONPs), and graphene oxide (GO). The objective is to allow the polymeric network to prevent the entry of viral particles into the nasal cavity, while CuONPs and GO further inhibit the potential for viral transmission by inactivating the particles trapped in the membrane itself.[1]
Viral detection may be possible through the development of highly sensitive and accurate nanosensors that allow early diagnosis of COVID-19. Nanomaterials functionalized with nucleic acids or antibodies represent the main lines of detection based on nano, through colorimetric or antigen-binding assays, as well as light and photothermal platforms.[20]
Researchers at the Korea Institute of Basic Sciences developed a field-effect transistor (FET) -based biosensor device to detect SARS-CoV-2 in clinical samples. The sensor was produced by coating FET graphene sheets with a specific antibody against the SARS-CoV-2 spike protein. Sensor performance was determined using antigen proteins, cultured viruses, and nasopharyngeal smear specimens from COVID-19 patients. This device has an extraordinary ability to distinguish the SARS-CoV-2 antigen protein from those of the MERS-CoV.[21]
Vaccines
Data from November12nd show that there are 19 vaccines, from 212, against COVID-19, being developed and that describe in their production method nanomedicine, such as lipid nanoparticles, liposomes, or viral particles (DRAFT landscape of COVID-19).[21] Four of them are already under clinical evaluation, three in phase 3 (one from Moderna / NIAID, other from BioNTech / Fosun Pharma / Pfizer and Novavax), and the one from Imperial College London in phase 1, the latter has not yet released its results so far (Table 1). All of these in the clinical phase use lipid nanoparticles encapsulating RNA encoding structures of the new coronavirus.[22] (Fig. 2)
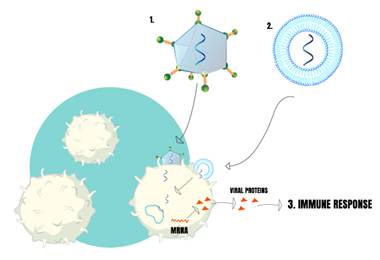
Figure 2: Vaccines using nanotechnology. 1) Nanometric Virus carring “particles” of SARS-COV-2 (RNA or other subunits). 2) Nanoparticles carrying “particles” of SARS-COV-2. These particles inside of virus or nanoparticles are reponsible for stimulating factors (like viral proteins from SARS-COV-2) that causes imune response in several individues.
The potential vaccine in phase 3, RNA-1273, manufactured by ModernaTXInc, is an mRNA-based vaccine encapsulated by lipid nanoparticles, which is capable of encoding the Spike (S) protein of the SARS-CoV-2 virus in the host cell. Since this RNA is encapsulated by lipid nanoparticles, the vaccine can be injected intramuscularly into patients. So, when injected, it takes the mRNA to host immune cells encoding the spike protein, so these cells make copies of the protein as if the cells have been infected by the coronavirus and then other immune cells can interact with these spike proteins and trigger the cascade effect of the immune system, leaving the individual protected against possible infection by the coronavirus. This technology took just 42 days to develop and induced anti-SARS-CoV-2 immune responses in all participants, with no limiting safety concerns identified in the trial.[23]
The other potential vaccine with phase 3 nanotechnology is BNT162b1, manufactured by BioNTech / Fosun Pharma / Pfizer. This is a nucleoside-modified mRNA vaccine, also formulated with lipid nanoparticles that encode the trimerized receptor (RBD) binding domain of the SARS-CoV-2 virus spike glycoprotein. The first results of BNT162b1 were published by Nature. Different doses were administered, with an acceptable tolerability and safety profile, where, according to the researchers themselves, they conclude: “The clinical findings were encouraging and strongly support the accelerated clinical development for the rapid production of a vaccine against SARS-CoV-2 to prevent COVID- 19”.[24]
Finally, the third potential vaccine against COVID-19 in phase 3 using nanotechnology is produced by Novovax (NVX-CoV2373). This vaccine utilize immunogenic virus-like nanoparticles based on recombinant expression of the S-protein. The results from phase 1-2 trial were detailed in the New England Journal of Medicine. The NVX-CoV2373 showed good results. being safe, and triggered immune responses that exceeded levels in Covid-19 convalescent serum.[25]
Treatments
Several nanobiotechnological platforms were able to combat human viruses, and preclinical studies, such as herpes, hepatitis B, HIV, in addition to some respiratory viruses.[26,27] These platforms can be used to develop therapies and diagnoses against SARS-CoV-2 or other future pandemics.[13,28,29]
When analyzing the panorama of the therapeutic development of COVID-19 with other diseases, we can see that, in both cases, the most important thing is to align the correct drug with the most promising nanocarrier. [30] Thus, we can infer that the challenges for the development of therapies against COVID-19, as well as for other viral diseases or even cancer, for example, are very similar.[31-32]
However, in general aspects, the encounter of the virus with the susceptible host is what makes viral infection possible. We can divide four veryimportant points of the relationship between viruses and host cells for the maintenance of the infectious process. First - the adsorption and penetration of the viral agent in the host; second - transcription, translation (synthesis) and maturation of the viable progeny; third - the release of the host's progeny; and fourth - the resistance of the viral agent to the adversities of the environment.[33]
According to that, we can also observe that therapeutic drugs based on NP can inhibit the effects of viral infections in three main ways (I) blocking receptor binding and entering the cell, (II) inhibiting viral infection, and (III) viral inactivation.
(I) Blocking the binding to the receptor and entering the cell, as shown in the work by Huang et al.[34] (2019), where the AuNRs nanoparticle blocked the entry of the MERS virus.[26]AgNPs showed efficient antiviral activity against RSV (herpes simplex virus) infection by directly inactivating the virus before entering host cells.[35] Silica nanoparticles (SiNPs) can act as efficient eliminators of the human immunodeficiency virus (HIV) and the respiratory syncytial virus (RSV).[36]
(II) The inhibition of viral infection, as shown in the study by Lin and coauthors (2017), Se @ ZNV (selenium nanoparticles with the antiviral zanamivir) revealed good biological activity to contain the proliferation of the influenza virus H1N1.[37] According to Silva and coauthors[38], (2016), SiO NPs were able to inhibit HIV infection, showing that the use of these functionalized silica particles presented a promising approach for the control of HIV infection and viral control.[38]
(III) Viral inactivation, as presented in the study by Ghaffari and coauthors[30](2019), the PEGylated ZnO-NPs nanoparticles had an antiviral activity with inhibitory properties against the H1N1 influenza virus.[39] Kong and coauthors[40] (2019) observed that nanodisks inhibited the infection of the influenza virus H1N1, even suggesting nanodisks as therapeutic agents against enveloped viruses.[41](Fig. 3)
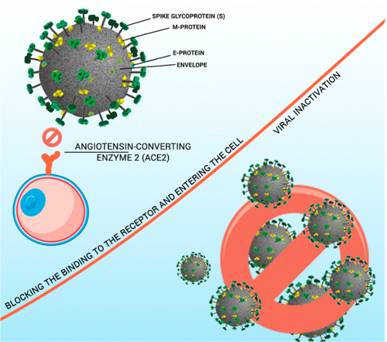
Figure 3: Nanoparticles inhibiting the effects of viral infections in three main ways. (I) blocking receptor binding and entering the cell, (II) inhibiting proliferation, and (III) viral inactivation.
Despite the treatment options currently proposed, the number of serious cases and deaths of patients infected with SARS-CoV-2 is still high. Much of the side effects of antivirals are caused by their accumulation in off-target organs. Nanoparticles can optimize drug delivery to target infection sites and with controlled release properties.[42] Therefore, we must also focus on alternative approaches, such as nanotechnology, to achieve an effective treatment for this disease and to minimize the side effects of the compounds.
Conclusion
There are several options for products, treatments, and vaccines nano-based against COVID-19. The great hope is placed on vaccines and three of them, in phase 3, use nanotechnology. Nanomedicine has already demonstrated its ability to protect, diagnose, and treat other viral diseases or infections; therefore, it may also have a great capacity to fight COVID-19. One of the biggest challenges is ensuring the safe use of these nanomaterials for the entire world population.
Acknowledgements
The authors gratefully acknowledge financial support from: the Brazilian National Council for Technological and Scientific Development (CNPq), CoordinatingAgency forAdvanced Training of GraduatePersonnel (CAPES),FoundationforResearch Supportof theFederal District(FAPDF) an the Dean of Research and Post-Graduation of the University of Brasília (DPP-UnB).