INTRODUCTION
In the 1990s, Cuba developed a network of 14 biofactories for the production of in vitro plants of different species with a view to producing seeds of high genetic and phytosanitary quality for the country's agricultural and forestry development plans (Pérez, 19981). The sugar cane biofactory at the Territorial Station for Sugar Cane Research (ETICA Centro Villa Clara), specialised in the country for this crop with a view to supporting the seed production programme for this plant species, belongs to the aforementioned network.
The growing demand for food is a goal to be reached in order to achieve the necessary food security and is one of the main challenges for biofactories (business organisations dedicated to the mass production of in vitro plants by biotechnological methods) because they provide seeds of different species (Portillo, 20072; Rani and Kumar, 20173). The annual market for in vitro plants is estimated in millions with different destinations, such as: the production of human food, animal feed, to tackle climate change, energy plants, among others. (Argenbio, 20184).
There are countless variables that influence increases in sugar production, with sugarcane production being a basic link and in particular the seed and its quality, which has a significant impact on agricultural and industrial yields and where the biofactory plays a vital role (Argenbio, 20184). This is why the development of seed and sugar production is one of the main priorities of the Cuban economy, endorsed in the main documents for the country's development (Nova, 20155).
In the current scenario, business organisations reinforce their organisational culture through an orientation towards quality and the control of processes, products and services, generating behaviours and attitudes that favour the fulfilment of productive and economic goals (ISO, 20156). Organisations dedicated to the mass production of in vitro plants using biotechnological methods are also facing this problem, which requires the development of processes to adapt to the new circumstances, under conditions of high productivity, quality and efficiency.
With the creation of the first Cuban biofactories, the quality control system was defined and implemented. It was based on the definition of the critical points of the processes involved in in vitro propagation via organogenesis, the end product of which is the in vitro plant. Twenty-nine control points were defined and in each case the procedures for their execution, the control techniques and methods, the frequency of controls, among other aspects, were established. This system was also used to design and apply work procedures (34) for each of the operations carried out in the biofactories.
However, this quality control system and the operating procedures applied in the biofactories have a number of shortcomings, such as: a large amount of information from the controls that are not related to each other; difficulties in determining the causes of production losses; the operating procedures associated with the quality control system have lost some of their usefulness, among others. Precisely these problems constitute the necessary arguments for the definition of technical solutions to the aforementioned system in search of a greater simplification and at the same time concentration of the controls on the variables of greatest interest in the production of in vitro plants.
The objective of the present work is the design and implementation of improvements in the quality control system in the in vitro production of sugar cane in the biofactory of the ETICA Centro Villa Clara.
MATERIALS AND METHODS
For the development of the present work, the following was taken into account:
The sugar cane biofactory has three areas: preparation of culture media, in vitro production and ex vitro acclimatisation. It has four specialists, distributed in the management of the biofactory, quality control, in vitro production and acclimatisation respectively. There are 27 workers: 6 operators of laminar flow cabinets (CFL), one CFL assistant, 8 workers in the preparation of culture media and 6 workers in the acclimatisation area (umbraculum).
In vitro propagation technology via organogenesis for the production of sugar cane plants. This is combined with somatic embryogenesis in Phase I: In vitro establishment by modifying a group of processes with a high degree of technical detail, such as:
Phase 0. Selection of elite plants. A Donor Plant Bank is available under semi-controlled conditions, which is in line with what is recognised in the scientific literature (Chandra et al., 20137) and allows for the availability of the cultivars of interest in a juvenile and disease-free state, with adequate agro-technology and phytosanitary protection, constituting the indispensable material base for the in vitro establishment processes.
Phase I. In vitro establishment. A modification is made to what has been established in existing protocols up to now. The initial explant is leaf discs, which are placed in a specific culture medium that allows the regeneration of sugar cane plants in vitro. For this purpose, there is a Donor Bank under semi-controlled conditions of different sugar cane cultivars of interest for mass production and a Germplasm Bank that provides services to the biofactory.
Phase II. In vitro multiplication. This phase contemplates the development of subcultures of the in vitro material, favouring the generation of a high number of in vitro plants (1-10,000), using specific culture media. The handling and conditions for this phase are set out in detail in micropropagation technology via organogenesis using semi-solid media (Perez, 19881; Naik, 20018; Georgiev, 20149; Kavita and Saxena, 2016(10). In multiplication subcultures, Temporary Immersion Systems with plastic flasks (usually commercially available flasks for drinking water) are used.
Phase III. In vitro rooting. This is carried out by means of the Temporary Immersion Systems using specific culture media. At the end of this process, these plastic culture flasks are cut for the extraction of the in vitro rooted plants.
Phase IV. Ex vitro acclimatisation. They have defined the processes to be carried out, highlighting: substrate preparation, sowing and replanting, agricultural and phytosanitary care, among others, as recognised in the scientific literature on this subject (Aragón et al., 201011; Singh et al., 201112).
Quality control system in place.
It includes 29 control points, which are carried out by a specialist in this activity (MINAG, 199613).
The quality control system in force is complemented by the work procedures of each operation of the biofactory under study (MINAG, 1998 14).
For the development of the work, different methods and technical tools were applied:
For the design of improvements to the quality control system and to the operating procedures.
Diagnosis of the results obtained through information analysis.
Proposed improvements to the system and operating procedures.
Quality performance analysis tools.
Cause and effect diagram.
Decision tree for the analysis of microbiological contamination.
Results of the implementation of the proposed improvements.
RESULTS AND DISCUSSION
For the design of improvements in the quality control system and operating procedures
Diagnosis of the results obtained through information analysis.
Quality control is a regulatory process through which the actual quality can be measured, compared with standards, differences can be detected and acted upon. Quality control in in vitro plant production must therefore concentrate the process control function on the critical points that define the quality of the product, which confirms the trend in this area that more controls do not mean more quality (ISO, 20156).
Through the application of a checklist between managers and specialists of the biofactory under study, a diagnosis of the quality control system in force was carried out. As a result of this tool, a group of inadequacies were detected, such as:
The application was partial and incomplete, due to the high number of controls established and the personnel available, which meant that the main processes were not regulated, the detection of deviations and the definition of solutions were excessively delayed.
A large amount of information obtained in the established controls was not related to each other in order to determine the probable causes of production losses.
It was found that more than 50.0 % of the controls in the current system applied an inspection method and with a frequency that did not provide valid and accurate information, which could not be related to production losses.
The controls defined did not fit the organisational structure of the biofactory, which made it difficult to define responsibilities for the causes of production losses and solutions.
There was a strong tendency for losses and their solutions to be the responsibility of quality control personnel.
The operating procedures were not taken into account by the quality control system to pinpoint the causes of losses and solutions, so that the two tools are not integrated.
For the determination of these shortcomings, it was taken into account that quality control in in vitro plant production must consider factors specific to in vitro propagation technologies, such as: the execution of biological processes generates a different response in plants with a significant level of uncertainty, regardless of having standardised processes and operations.
In addition to the above, there is the presence of predominantly manual processes and therefore with a decisive weight of people in the production results and the quality of in vitro plants, these being decisive variables in the achievement of production goals and quality, which coincides with what was reported by Orellana and Suarez, 201715; Romero, 201916.
There are countless procedures for the design and application of a quality control system (Juran, 199617; Feigenbaum, 200018; Globalgap, 201219), but the one proposed by Martin, 200720, constitutes a specific adaptation for the production of in vitro plants.
It is important to note that the total quality of in vitro sugarcane plants is composed of the quality of the final product (acclimatised plants, ready for planting in the field) and the quality of the production processes involved (Martin, 200720). These qualities were measured taking into account:
a) Quality of the final product: This includes the genetic, phytosanitary and morphological quality of the acclimatised in vitro sugar cane plants, validated by the assessment of customer satisfaction.
b) Quality of the processes: It includes the determination of the total production losses, which are broken down by:
Losses of in vitro material due to microbial contamination (fungi and bacteria).
Losses of in vitro material due to mortality.
Losses of in vitro material outside quality specifications.
Losses of production of culture media.
Production losses in greenhouses: due to deaths, presence of mutants, with disease symptoms, out of specifications.
As a result of the development of this procedure, the improvements in the quality control system (by work area) in the sugar cane biofactory are designed, including the main variables of each proposed control point.
The operating procedures that accompany the improvements to the quality control system indicated were reworked, through recognised methodological procedures (Sorol, 200721), by means of a process of integration, simplification and updating of their contents, in correspondence with the technological and productive process existing in the biofactory under study.
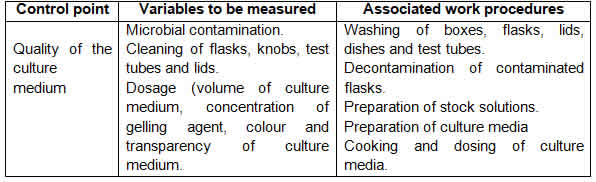
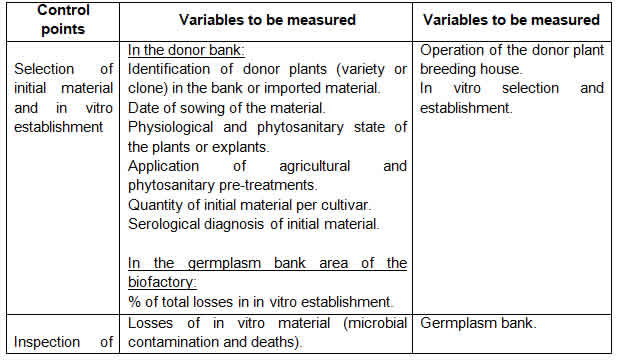
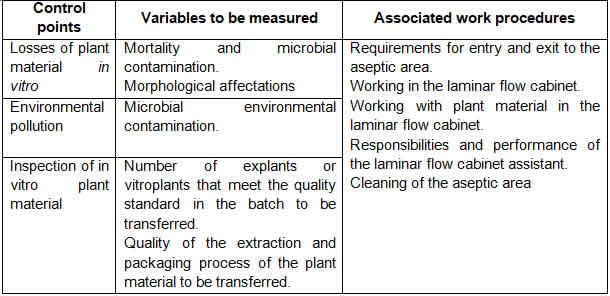
Tables 1, 2, 3 and 4 show the improved quality control system by work area and the improved operating procedures of the biofactory under study.
The main characteristics of the proposed improvements of the quality control system and the operating procedures are:
Simplification of the quality control points of the system, with a 69% reduction compared to the current system and its actual integration with operating procedures;
Integration of the quality control system with operating procedures strengthens the concept that the responsibility for the quality of production lies with the workers who generate it, while the quality control function focuses on verifying, alerting and participating in the state of quality regulation in the production process and final products;
The system concentrates on the quality control points of the product and the indispensable points of the production process of in vitro sugar cane plants, which, together with the compliance with the operating procedures, provides the innovative character of this improvement proposal.
2. Quality performance analysis tools
2.1 Cause and Effect Diagram
Figure 1 shows the Cause and Effect Diagram (Ishikawa, 1988 22) of all the variables influencing the percentage of microbial contamination (data with the greatest influence on the efficiency of in vitro production) detected in the sugar cane biofactory, which serves as a reference for the determination of the probable causes and the proposed solutions for their reduction and/or elimination.

Figure 1: Cause and Effect Diagram for the percentage of microbial contamination in the sugar cane biofactory. Source: Own elaboration.Decision Tree
It is applied to determine the probable causes of the contamination percentage from the statistical information provided by the quality control system. This technique is accompanied by a system of numerical codes, different situations in the presence of the contamination percentage, which are prefixed lines of investigation of the probable causes before each coded situation.
Figure 2 shows this technique, which is complemented by Table 5 with the lines of investigation in some variants of behavior of the contamination percentage.

Figure 2: Decision Tree Technique applied for the percentage of microbial contamination in the sugar cane biofactory of ETICA Centro Villa Clara.
Source: Own elaboration.

Table 5: Examples of lines of investigation of probable causes of the percentage of contamination in coded variants of the Decision Tree. Source: Own elaboration.
Results of the implementation of the proposed improvements
The application of the improvements in the quality control system in the sugar cane biofactory of the ETICA Centro Villa Clara led to a group of very favourable results in the period under evaluation, which are summarised below:
100% customer satisfaction was achieved (certified sugar cane seed banks in the country), receiving no non-conformities for the acclimatised in vitro plants in terms of genetics, phytosanitary quality and compliance with the quality specifications of the same.
2. 99.3% of conformities are achieved in the quality of the culture media produced (more than 1000 litres in the period), which is less than 1.0 % of admissible losses in the process.
3. 95% of in vitro establishment is obtained (more than 10 in the period) of leaf discs, less than 10% of admissible losses for this process and generating on average more than 12 initial explants per disc, very satisfactory rates compared to those recorded in the scientific literature.
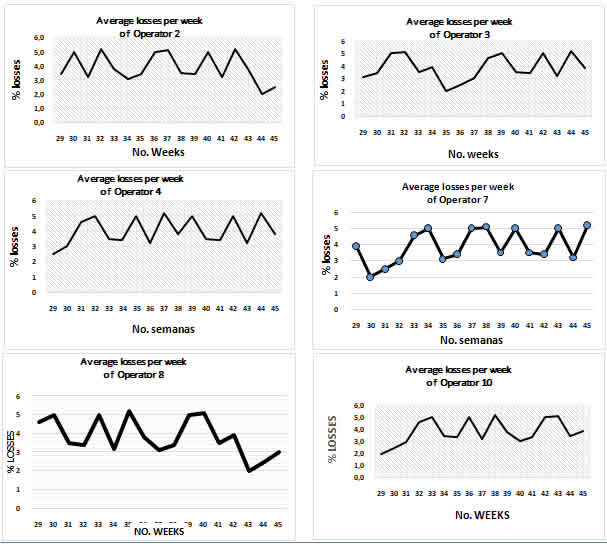
The losses due to microbial contamination in the period have an excellent behaviour with an average of 3.8%, lower than the permissible rate of 5.0% (Figure 3). This general behaviour is also reflected in the results of the percentages of losses per week in the same period of microbial contamination obtained by the operators of laminar flow cabinets (CFL) in the period under evaluation, as shown in figure 4, which confirms that this process is under control.

Figure 3: Behaviour of the average percentage of losses per week of microbial contamination in the period evaluated in the sugar cane biofactory of the ETICA Centro Villa Clara.
The survival rate of the in vitro sugar cane plants in the acclimatisation process was 93.0%, which is higher than the admissible rate of 90.0%, which also shows a very favourable result.
More than 50 corrective actions were carried out in the different processes that are developed for the in vitro production of sugar cane, such as: asepsis in the hands of the operators of the laminar flow cabins, control of the relative humidity in the rooting chamber, the quality of the substrate used in the acclimatisation of in vitro sugar cane plants, among others.
CONCLUSIONS
The improvements in the quality control system in the sugar cane biofactory of the ETICA Centro Villa Clara, constitute an innovation to the current system, conceiving 9 controls where the attention is concentrated on the key critical points related to the quality of the product and the processes, an adequate integration of the primary information is achieved that facilitates the determination of the probable causes of the non-conformities and of the improved operating procedures.
The improved operating procedures and the proposed quality control system are harmoniously integrated since the former express the good practices in each process guaranteeing the quality assurance of the same, while the latter determines the deviations and non-conformities present both in the processes and in the final product.
The Cause and Effect Diagram applied to losses due to microbial contamination is a valuable tool as it allows the identification of all the variables that influence it, which, together with the Decision Tree technique and the defined code system, allows a quick and precise analysis of the causes of non-conformities.
The Decision Tree and the defined code system are based on the detailed analysis of the primary data collected by the quality control system, which allows time, human and material resources to be concentrated on the definition of technical solutions to the presence of non-conformities in products and processes.
The work carried out made it possible for the ETICA Centro Villa Clara sugar cane biofactory to achieve 100 % compliance with the planned production plan, with very favourable quality indices both in the marketed product and in the processes.