Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Salud colectiva
versão impressa ISSN 1669-2381versão On-line ISSN 1851-8265
Salud colect. vol.17 Lanús 2021 Epub 06-Mar-2021
http://dx.doi.org/10.18294/sc.2021.3246
ARTICLES
Reduction of social coverage for symptomatic slow-acting drugs for osteoarthritis: a disinvestment initiative in Argentina, 2015-2017
1Corresponding author. MD, Master’s in Epidemiology, Management, and Health Policies. Area of Pharmacology, FEMEBA, La Plata, Argentina. Instituto de Ciencias de la Salud, Universidad Nacional Arturo Jauretche, Florencio Varela, Argentina. martinurtasun@yahoo.com.ar
2Physician. Medication Management, INSSJP, Buenos Aires, Argentina. marianoblearg@gmail.com
3MD, Master’s in Pharmacoepidemiology. Area of Pharmacology, FEMEBA, La Plata, Argentina. Instituto de Ciencias de la Salud, Universidad Nacional Arturo Jauretche, Florencio Varela, Argentina. farmacol@femeba.org.ar
4Psychiatrist. INSSJP, Buenos Aires, Argentina. Instituto de Neurología Cognitiva, Buenos Aires, Argentina. julianbustin@gmail.com
5Doctor of Medicine. INSSJP, Buenos Aires, Argentina. kmastai@gmail.com
6Information Systems Engineer, Master’s in Administration. INSSJP, Buenos Aires, Argentina. regueiroalejandro@yahoo.com.ar
In April 2016, the National Institute of Social Services for Retirees and Pensioners discontinued its policy of 100% coverage for 159 drugs (the “social subsidy”), including symptomatic slow-acting drugs for osteoarthritis (SYSADOAs), due to insufficient evidence of significant clinical benefit. We evaluated the effect of this measure on the use of SYSADOAs as well as non-steroidal anti-inflammatory drugs (NSAIDs), which were unaffected by this policy change. We compared outpatient dispensations of SYSADOAs and NSAIDs from 2015 to 2017, measuring dispensed units, retail price, and out-of-pocket expenses for beneficiaries each month. After the change in coverage, there was a 61.6% total decrease in SYSADOA units dispensed, and a 63.4% decrease in the final sales price to the public, measured in constant values. Dispensation was not reoriented towards NSAIDs, which fell by 6.1%. The incidence of new treatments decreased (from 6.4 to 3.3 treatments per 1,000 beneficiaries per month), as did their continuity. Beneficiaries’ out-of-pocket spending on SYSADOAs increased by 75.8% (at constant values). Disinvestment in interventions with questionable therapeutic value is an important tool in working toward the sustainability of health systems.
KEYWORDS: Investments; Osteoarthritis; Drug Therapy; Glucosamine; Chondroitin Sulfates; Anti-Inflammatory Agents, Non-Steroidal; Health Services Coverage; Argentina
INTRODUCTION
Symptomatic slow-acting drugs for osteoarthritis (SYSADOAs) are a group of pharmaceuticals used to treat arthrosis that produce a clinical effect several weeks after treatment is initiated. This characteristic distinguishes them from paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs),1 which produce rapid analgesic effects. In Argentina, SYSADOAs on the market include chondroitin sulfate, diacerein, glucosamine, and avocado soybean unsaponifiables, along with their combinations.2
There is controversy over their real efficacy as analgesics and in modifying disease progression.1,3,4) Clinical trials and systematic reviews have found their analgesic effect to be mild to insignificant, and have also noted a difference in the preservation of articular cartilage volume in the knee, the clinical relevance of which is questionable.5,6,7,8,9,10
The uncertainty surrounding the efficacy of SYSADOAs is reflected in their uneven coverage by public health systems. They are covered in Spain, for example, but not in France, Denmark, or Sweden; in the United Kingdom and the United States they are considered dietary supplements, and are therefore not covered as medications.1,11 In Argentina, SYSADOAs are not included in the list of drugs covered by the Compulsory Medical Program,12 but 50% of their cost is covered for those insured by the National Institute of Social Services for Retirees and Pensioners (INSSJP, for Instituto Nacional de Servicios Sociales para Jubilados y Pensionados).2 This national public institution administers and finances the medical care for senior citizens and their dependents, as well as individuals of any age who receive disability benefits or who are veterans of war. More commonly known as PAMI (which stands for Programa de Atención Médica Integral), this institution enrolls approximately five million beneficiaries.13) The INSSJP covers outpatient medications through percentage discounts on retail price.14) Since numerous companies in Argentina market “similar drugs” - that is, drugs with an identical composition but a different manufacturer and price - patients’ out-of-pocket expenditure will depend on the chosen brand.15
For beneficiaries who due to socioeconomic limitations were unable to meet the costs of their outpatient medications - even with the established discounts - a social subsidy program was introduced in 2005 that expanded coverage to 100% of medication costs.16 The social subsidy program was extended over the next ten years, and by 2016 covered around 50% of dispensed medications.17
The social subsidy program was initially designed to cover all types of medications, including those with non-existent or questionable potential therapeutic value that are marketed in Argentina.18 Although new drug authorizations in the country require an analysis of their “efficacy and safety,” authorization is granted automatically when the drug in question has already been approved by the US Food and Drug Administration, the European Medicines Agency, or other reference agencies;19 in practice, this mechanism is used for the majority of new approvals.20 However, for a number of older medications that remain on the market, authorization is renewed every five years nearly automatically, even when they have been taken off the market in other countries due to adverse effects.21,22 A number of these products are fixed-dose combinations that are not substantiated by sufficient evidence in the scientific literature.23,24 Furthermore, for authorizations that are granted based on the decisions of reference agencies, current regulations do not indicate that the drug must be taken off the market if its authorization is revoked in its country of origin.19
A review conducted in partnership with the Institute for Clinical Effectiveness and Health Policy - an academic institution affiliated with the University of Buenos Aires - found that some of the medications covered by this mechanism were drugs “that lacked sufficient medical evidence to determine significant clinical benefits.”25 As a result, in 2016 the INSSJP adopted Resolution 439/2016, which discontinued 100% coverage for 159 monodrugs and fixed-dose combinations in all of their commercial preparations, although it preserved the original discounts for all listed drugs. As SYSADOAs were included in the list, once the measure went into effect they received the basic discount of 50% off retail price.25
This measure is an example of what in the healthcare literature has become known as “disinvestment,” which refers to the partial or complete withdrawal of public financing for a determined product or service in health care based on an understanding that it provides little to no health benefits, and is therefore not an efficient use of resources. This, in turn, is meant to redirect funds toward measures with greater public health impact.26,27,28 “Passive disinvestment” refers to the spontaneous abandonment of practices that have fallen into disuse or that have been replaced by others, whereas “active disinvestment” occurs when changes are made in the coverage of interventions that have become obsolete or that were not properly evaluated before being put into practice.28
In this context, increased pharmaceutical spending is a central concern for public and private organizations that are charged with providing equitable and integral medical care within sustainable budgets. Reassessing the coverage of low-cost treatments is encouraged as a means to offset the constant incorporation of costly pharmaceuticals.26,28 A number of different disinvestment strategies exist: a partial or complete withdrawal of public funding (this is known as “delisting” in the specialized literature); subjecting certain drugs to usage guidelines; the selection of certain active ingredients in each therapeutic class; and encouraging the prescription of generic drugs.26,28
Disinvestment strategies have consequences for all stakeholders, including patients, prescribers, hospitals, manufacturers, and health care funders. These consequences may be clinical or economic in nature, or may involve satisfaction levels of different stakeholders. Assessing the outcomes of this type of intervention can contribute to an understanding of their consequences and inform future decisions.28,29
An analysis of pharmaceutical disinvestment initiatives carried out in the public health systems of Spain,31,32 France,29,32,33) Ireland,34 and a group of six OCED countries28 reveals some commonalities among them. First, a rapid decrease in the prescription and dispensation of medications whose coverage had been reduced, along with a subsequent decrease in public spending, coupled with increased out-of-pocket cost for patients who continued using these medications.30,31,32
A second common aspect has to do with the need to assess whether reduced coverage of certain drugs will reorient demand toward other drugs that continue to be covered, or if it eventually leads to increased demand for other healthcare services such as physician visits.34 In France, for example, the removal of financing for mucolytics led to an increase in the consumption of antitussives and bronchodilators.29 On the other hand, given the existence of different commercial preparations with identical chemical compositions, it is to be expected that individuals who continue to take these medications will tend to seek products with a lower price point. The final outcome may not always be what is expected: in a review of drug exclusion policies, Chambers et al. found that in one-fifth of cases, total costs actually increased.36
Other important factors to consider in comprehensive assessments of this type of initiative include health outcomes, economic outcomes, and the satisfaction of different stakeholders such as patients, service providers, and funders.28,33,36,37 Such an evaluation is difficult to implement, and published work on concrete experiences has shown the need for a balance between the ideal and the attainable.27
This study evaluated the impacts of the INSSJP discontinuing its social subsidy of 100% coverage of SYSADOAs on dispensations and costs, and explored the alternative pharmaceutical treatments employed in response to this measure.
METHODS
An observational retrospective study on the use of medications was carried out based on data from the administrative database on outpatient drug dispensations to INSSJP beneficiaries from 2015 to 2017. The change in coverage went into effect on April 7, 2016, which means that the study period includes a span of 15 months prior to the change and 20 months after.
Among the 159 active ingredients and fixed-dose combinations excluded from 100% coverage by Resolution 439/2016, SYSADOAs - found in subgroup M01A (“Anti-inflammatory and antirheumatic products, non-steroids”) of the World Health Organization’s Anatomical Therapeutic Chemical Classification System (ATC) - were identified and categorized as “group A drugs” for the purposes of this study. In order to explore possible shifts in prescribing in favor of drugs that did not have a reduction in coverage, trends in the dispensation of the remaining drugs in subgroup M01A, along with the analgesic paracetamol, were studied (these were designated “group B drugs”). A report on the commercial preparations available in Argentina of all selected drugs was obtained by searching the active ingredients in the commercial pharmaceutical database AlfaBeta used by the INSSJP to classify medications. Monthly data were obtained on the number of units dispensed, retail price, and price paid by beneficiaries for all drugs on lists A and B.
In order to evaluate trends in spending in a context of high inflation, and taking into account concerns over the reliability of national statistics published by the National Institute of Statistics and Census (INDEC) between 2007 and 2015,39 prices were adjusted to the value of the peso in January 2015 using the consumer price index of the Autonomous City of Buenos Aires.40
Changes in the average percentage of retail price covered by the INSSJP were calculated, which reflect the combination of beneficiaries that were able to access the social subsidy covering 100% of the retail price and those who obtained the standard discount of 50%.
Monthly usage prevalence proportions (hereinafter “prevalence of use”) of medications on each list were calculated as the proportion of the total number of beneficiaries who had been dispensed medications on the list over the course of the month.41 Changes in prevalence of use may be attributable to modifications in the incidence of new treatments, or to changes in their duration. In order to evaluate the contribution of the former, the incidence rate of new users was analyzed, defined as patients who had been dispensed a medication on list A or B in a given month but who had not been dispensed medications on that list in the previous six months. To assess continuation of treatment, drug dispensations in subsequent months were analyzed by calculating the proportion of patients that received a second and third dispensation after initiating a new treatment, as long as the patients were monitored for at least four months following the initial dispensation.
For the purpose of identifying the overall effect of this measure, monthly averages of aforementioned variables were calculated for three time periods: the period prior to the studied intervention, from January 2015 to March 2016; a transition period, comprising the first four months after the measure went into effect; and the period after its implementation was completed, from August 2016 to December 2017.
In order to more precisely characterize usage patterns of group A drugs, prevalence of use was analyzed by sex and by place of residence (defined according to the 38 local offices of the INSSJP), and results from one of the months before the measure was implemented (July 2015) were compared with one of the months after it was in effect (July 2017). We also explored whether group A drugs were initially indicated by the patient’s general practitioner or by a specialist, using administrative data available through the INSSJP data server.
Lastly, we assessed whether or not the removal of the 100% subsidy to group A drugs led to changes in brands selection in favor of more inexpensive products with the same composition. To that end, the most widely used drug from group A (glucosamine + meloxicam) was taken as a reference, and dispensations of its different commercial preparations in July 2015 were compared with those of July 2017.
This study made use of publicly available administrative data, which were processed in such a way that the identity of individuals participating would be impossible to determine, and it was therefore not necessary to seek approval from a Research Ethics Committee or to obtain informed consent, consistent with the treatment of unidentifiable data outlined in Article 11.3.e of Law 25,326 on the Protection of Personal Data.
RESULTS
The list of group A drugs that were excluded from 100% coverage includes eight SYSADOAs - both as monodrugs and as fixed-dose combinations - while the list of group B drugs includes fifteen products found in subgroup M01A of the ATC classification (not affected by the measure), as well as paracetamol (Table 1)
Table 1 Drugs listed in subgroup M01A of the ATC classification* by coverage, following Resolution 439/2016. Argentina, 2016.
| Excluded from the social subsidy (Group A Drugs) | Included in the social subsidy (Group B Drugs) |
|---|---|
| chondroitin sulfate, chondroitin sulfate + glucosamine, diacerein diacerein + glucosamine, diacerein + meloxicam, glucosamine, glucosamine + meloxicam, avocado-soybean unsaponifiables | celecoxib, lysine clonixinate , dexketoprofen, diclofenac diclofenac+asoc,dipyrone, etoricoxib, flurbiprofen, ibuprofen, indomethacin, ketoprofen, meloxicam, naproxen, paracetamol, piroxicam |
Source: Own elaboration based on data from the National Institute of Social Services for Retirees and Pensioners.
*Subgroup “Anti-inflammatory and antirheumatic products, non-steroids” of the Anatomical Therapeutic Chemical Classification System of the World Health Organization.38
Dispensations before and after the measure
Prior to the implementation of the measure, 364,000 units of group A drugs were dispensed on average per month. This figure fell to 139,000 once the measure went into effect, a reduction of 61.6%. Dispensations of group B drugs did not increase proportionally, but in fact decreased slightly after the measure went into effect, from 504,000 to 473,000 units per month, a 6.1% reduction. This decrease affected all products on the list of group A drugs similarly (Table 2).
Table 2 Average monthly dispensations of group A drugs before and after their exclusion from the social subsidy. Argentina, 2015-2017.
| Drugs | Units per month prior to measure (01/2015 to 03/2016) | Units per month after measure (08/2016 to 12/2017) | Percent change |
|---|---|---|---|
| glucosamine + meloxicam | 180.936 | 71.546 | -60.5 |
| chondroitin sulfate + glucosamine | 58.974 | 15.686 | -73.4 |
| unsaponifiables | 50.467 | 25.302 | -49.9 |
| glucosamine | 34.583 | 9.577 | -72.3 |
| diacerein | 24.728 | 10.510 | -57.5 |
| diacerein + glucosamine | 8.722 | 4.132 | -52.6 |
| diacerein + meloxicam | 4.937 | 2.616 | -47.0 |
| chondroitin sulfate | 214 | 96 | -55.0 |
| Total | 363.561 | 139.466 | -61.6 |
Source: Own elaboration based on data from the National Institute of Social Services for Retirees and Pensioners.
The number of prevalent users prior to the measure’s implementation was 328,400 for group A drugs and 382,400 for group B drugs, 73,900 of whom consumed medications on both lists. Monthly prevalence of use prior to implementation among all beneficiaries was 6.7% for group A drugs and 7.8% for group B drugs, which dropped to 2.7% and 7.7% respectively after the measure went into effect, a 60% reduction in users of group A drugs and 0.4% in users of group B drugs (Figure 1).
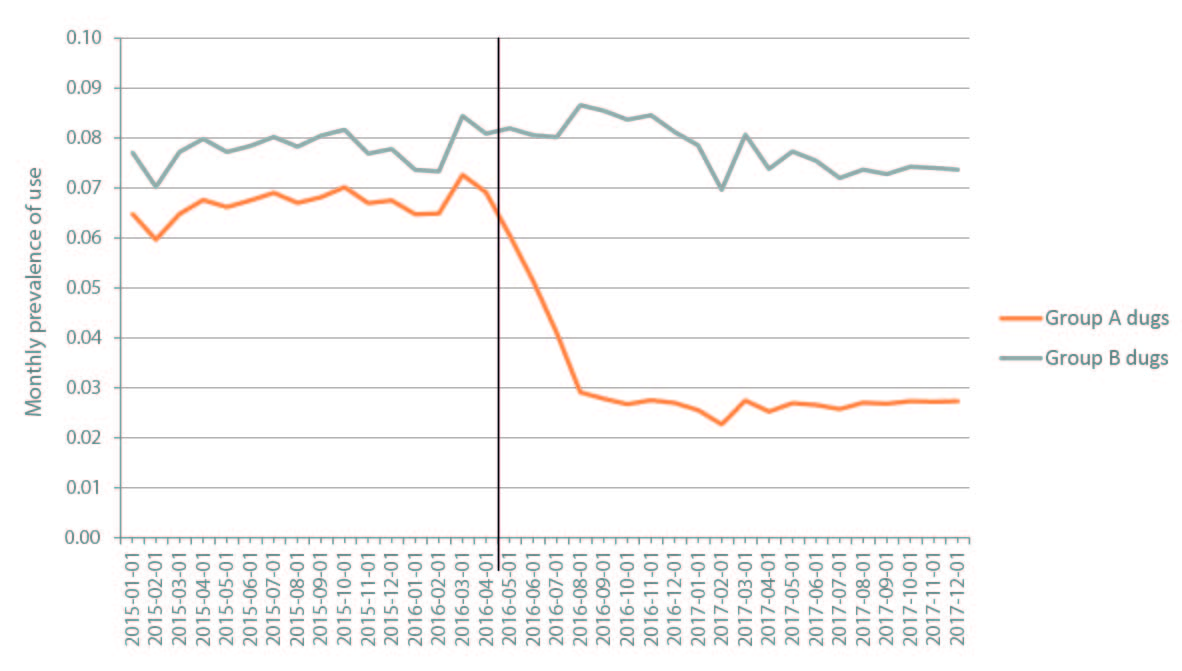
Source: Own elaboration based on data from the National Institute of Social Services for Retirees and Pensioners.
Figure 1 Monthly prevalence of use of excluded arthrosis medications (group A drugs) and those not excluded from the social subsidy (group B drugs). Argentina, 2015-2017.
Total spending on group A drugs dispensed showed a similar tendency, in a general context of high inflation, which reached 125% over the course of the three years included in the study period. Average monthly sales to the public of group A drugs totaled $145.3 million pesos per month prior to the measure, and quickly fell to $87.1 million four months after its implementation, representing a decline of 40.0% in nominal values and 63.4% in constant values (January 2015 pesos) (Figure 2). Group B drugs, on the other hand, totaled an average of $74.5 million pesos per month prior to the measure, and grew to $126.2 million after it went into effect, which represents an increase of 3.6% in constant values (Figure 2).

Source: Own elaboration based on data from the National Institute of Social Services for Retirees and Pensioners
Figure 2 Total monthly sales of excluded arthrosis medications (group A drugs) and those not excluded from the social subsidy (group B drugs) dispensed per month, in constant January 2015 pesos. Argentina, 2015-2017.
The average discount to retail price of group A drugs included in coverage before the measure’s implementation was 89.6%, reflecting the fact that 79.2% of dispensations were fully covered by the social subsidy (100% discount). With the exclusion of group A drugs from this subsidy, the average discount fell to 50% after the measure went into effect. As a result, beneficiaries’ total out-of-pocket spending on group A drugs increased from $15 million per month before the measure’s implementation to $43.5 million per month after it went into effect, a 189% increase. Out-of-pocket spending on group B drugs increased from $20.5 million per month to $35.9 million, an 84.7% increase. In constant January 2015 pesos, the increase in out-of-pocket spending was 75.8% for group A drugs and 11.9% for group B drugs (Figure 3).

Source: Own elaboration based on data from the National Institute of Social Services for Retirees and Pensioners.
Figure 3 Beneficiaries’ out-of-pocket spending on excluded arthrosis medications (group A drugs) and those not excluded from the social subsidy (group B drugs), in constant January 2015 pesos. Argentina, 2015-2017.
Initial prescription and continuity of treatment
Over the course of the 36-month study period, group A drugs were dispensed to 1,196,000 different individuals, roughly 1 out of every 4 INSSJP beneficiaries. Prior to the measure’s implementation, the monthly incidence rate of new treatments with group A drugs was 6 per 1000 beneficiaries, which fell to 3 per 1000 after it was effect.
Despite being medications associated with long-term use, continuity of use was not very high prior to the measure’s implementation: only 69% of patients that initiated treatment with a group A drug received a second dispensation, and only 54% received a third. After the measure went into effect, these figures decreased to 50% and 33% respectively.
The 61.6% reduction in dispensations of group A drugs following the measure’s implementation can therefore be attributed to a combination of two factors: a one-half reduction in the number of new treatments initiated, and a substantial decrease in the continuity of treatment for those previously initiated.
Record keeping on the specialties of prescribing physicians was in the process of being implemented during the study period, so complete data were not available: data on 40% of prescribing physicians were available in January of 2015, and reached 75% as of July 2017. Taking into account these limitations, we were able to determine that at least 80% of new treatments with group A drugs were initiated by general practitioners, and only 20% were initiated by specialists.
Dispensations by sex and by place of residence
Monthly prevalence of use was twice as high among women as among men, both before the measure’s implementation (8.9% and 4.4% respectively in July 2015) and after it was in effect (3.2% and 1.6% respectively in July 2017).
There was great deal of variation in prevalence of use among INSSJP local offices before and after the measure went into effect, and it was possible to observe regional trends. In July 2015, the lowest figure was reported in the Autonomous City of Buenos Aires (3.6%), and the highest corresponded to the provinces of Catamarca, La Rioja, and Tucuman (12.8% each), a relation of 3.5 to 1. In July 2017, the lowest prevalence of use was once again registered in the Autonomous City of Buenos Aires (1.6%), and the highest was found in La Rioja (4.7%) (Table 3).
Table 3 Prevalence of use of group A drugs before and after the measure went into effect, by local INSSJP office corresponding to beneficiary’s place of residence. Argentina, 2015 to 2017.
| Local INSSJP office | 07/2015 (%) | 07/2017 (%) | Decrease(%) |
|---|---|---|---|
| La Rioja | 12.8 | 4.7 | 63.3 |
| Catamarca | 12.8 | 4.2 | 67.2 |
| Tucumán | 12.8 | 3.7 | 71.1 |
| San Juan | 12.3 | 2.5 | 79.7 |
| Mendoza | 11.3 | 3.6 | 68.1 |
| Salta | 11.2 | 3.6 | 67.9 |
| Río Cuarto | 10.0 | 3.2 | 68.0 |
| Entre Ríos | 10.0 | 3.7 | 63.0 |
| San Luis | 9.8 | 2.4 | 75.5 |
| Jujuy | 9.4 | 3.6 | 61.7 |
| Córdoba | 8.5 | 2.9 | 65.9 |
| Formosa | 8.5 | 2.3 | 72.9 |
| Concordia | 8.5 | 3.2 | 62.4 |
| Santiago del Estero | 8.5 | 2.8 | 67.1 |
| Rio Negro | 8.4 | 3.1 | 63.1 |
| Chaco | 8.2 | 3.2 | 61.0 |
| Rosario | 7.7 | 2.9 | 62.3 |
| Mar del Plata | 7.4 | 2.2 | 70.3 |
| National Total | 7.2 | 2.6 | 63.9 |
| Neuquén | 7.1 | 2.4 | 66.2 |
| Bahía Blanca | 7.1 | 2.7 | 62.0 |
| Misiones | 7.1 | 2.5 | 64.8 |
| Santa Fe | 6.7 | 3.0 | 55.2 |
| Morón | 6.5 | 2.6 | 60.0 |
| Corrientes | 6.5 | 2.7 | 58.5 |
| Quilmes | 6.3 | 2.6 | 58.7 |
| Junín | 6.0 | 2.1 | 65.0 |
| Chivilcoy | 6.0 | 2.4 | 60.0 |
| Luján | 5.9 | 2.3 | 61.0 |
| San Justo | 5.7 | 2.3 | 59.6 |
| Lanús | 5.6 | 2.2 | 60.7 |
| La Pampa | 5.5 | 2.7 | 50.9 |
| San Martin | 4.9 | 2.1 | 57.1 |
| La Plata | 4.9 | 1.8 | 63.3 |
| Azul | 4.9 | 2.3 | 53.1 |
| Chubut | 4.6 | 2.7 | 41.3 |
| Tierra del Fuego | 4.4 | 1.9 | 56.8 |
| Santa Cruz | 4.2 | 2.6 | 38.1 |
| Ciudad Autónoma de Buenos Aires | 3.6 | 1.6 | 55.6 |
Source: Own elaboration based on data from the National Institute of Social Services for Retirees and Pensioners.
Choice of commercial product
The combination of glucosamine and meloxicam was the most commonly prescribed SYSADOA before the measure went into effect, accounting for 49.8% of dispensed units. Available brands were evaluated by price point, taking as a reference the presentation of 30 packets of effervescent powder containing 1500 mg of glucosamine and 15 mg of meloxicam. In July 2015, there were 13 different brands on the market, with an average retail price of $390 pesos (range of $274-$451); the average retail price of units effectively dispensed was $417 - 92% of the maximum price - given that the most expensive brand was also the most frequently purchased (43% of units sold). In July 2017, there were 15 different products, with an average retail price of $689 pesos (range: $279-$808); no change was observed in the available brands, and the average price of dispensed units was $745, once again 92% of the maximum price.
The case of meloxicam
The nonsteroidal anti-inflammatory drug meloxicam is marketed in fixed-dose combinations with glucosamine and with diacerein - both group A drugs - and also as a monodrug, included the list of group B drugs. Prior to the analyzed measure’s implementation, these presentations accounted for 79% and 21% respectively of dispensed units of medications containing meloxicam. After the measure went into effect, and despite a 60% drop in dispensations of SYSADOAs combined with meloxicam, no compensatory proportional increase was observed in meloxicam monotherapy, even though it continued to receive 100% coverage.
DISCUSSION
The exclusion of SYSADOAs from the list of medications eligible for 100% coverage by the INSSJP led to a 61.6% reduction in the number of units dispensed per month - even though these medications continued to receive 50% coverage - along with a 63.4% fall in the final sales price to the public, measured in constant January 2015 values. Nonetheless, beneficiaries’ out-of-pocket expenditure increased by 75.8% in constant values due to the reduction in coverage, despite a significant decrease in the number of units dispensed. All of these outcomes emerged in the four-month period following the measure’s implementation, and remained stable over the next 16 months.
It is worth noting that excluding the combination of glucosamine and meloxicam from 100% coverage - which accounted for half of all SYSADOA units dispensed before the measure’s implementation - did not lead to a shift in prescribing toward meloxicam monotherapy, which retained 100% coverage. This could reflect a lack of information on the part of both physicians and patients regarding the scope of the new regulations, or perhaps a certain “organizational inertia” which delays adaptation to changes in institutional regulations. There was also no evidence to suggest that this led to increased dispensation of lower-priced products, even though this would have generated significant savings to beneficiaries.
Combinations of an SYSADOA with an NSAID - which in this study accounted for roughly 50% of dispensations - are not very rational, as they combine a rapid acting analgesic (meant to be used for the shortest time possible) with slow-acting drugs that produce beneficial effects only after months of treatment. Although it could be argued that such a combination might be advantageous in early stages of treatment if it is replaced with SYSADOA monotherapy after only a few weeks, in practice it often becomes a chronic treatment for arthrosis, implying prolonged exposure to the adverse effects of NSAIDs. In light of these and other considerations, the combination of glucosamine and meloxicam was taken off the market in 2020 by the National Administration of Drugs, Foods, and Medical Devices.42
As previously mentioned, it is necessary to determine whether disinvestment initiatives reorient demand toward other drugs not affected by these measures. In this study we did not observe an increase in dispensations of alternative treatments (NSAIDs and paracetamol) due to the implementation of this measure, despite the fact that they continued to receive 100% coverage. It should be noted that the information system utilized in this study does not collect data on over-the-counter dispensations, which may have seen changes.
This study did not analyze health outcomes. Nonetheless, taking into account the questionable efficacy of SYSADOAs, significant consequences of their reduced dispensation are not to be expected. It should be noted that nearly half of all SYSADOA dispensations were in combination with meloxicam, which exposes an at-risk elderly population to the prolonged use of an NSAID, and therefore it can be expected that the result obtained reduces the risk of its adverse digestive and cardiovascular effects.43,44
For the payer, the economic outcome was a substantial reduction in spending on SYSADOAs. On the other hand, increased out-of-pocket expenditure on the part of beneficiaries was an undesired effect of the measure, given that it implied the allocation of scarce resources to drugs with low potential therapeutic value.45 There was no evidence to suggest that patients and providers activated existing strategies to limit out-of-pocket spending: on one hand, reorienting medical treatment toward products that retained 100% coverage; and on the other, for beneficiaries that continued treatment with SYSADOAs, choosing lower-priced brands. These strategies could have been outlined in communications to patients and prescribers prior to the measure’s implementation, including a description of its scope, justification, and available alternatives; additionally, a more gradual rollout would have facilitated adjustment to the measure. Although no formal assessment of patient and provider satisfaction with the measure was conducted, the increase in out-of-pocket spending on the part of beneficiaries and the lack of adjustment to the new measures on the part of prescribers presumably indicate low levels of satisfaction.
The measure analyzed in this article has also produced consequences in the pharmaceutical market, as the INSSJP is the largest buyer of medications in Argentina. It is estimated that the institution accounts for 35 to 40% of total pharmaceutical sales.15 It is for this reason that the institution’s medication policy exerts an influence on the overall state of market supply and price setting for society at large. Given that SYSADOAs are drugs primarily used by senior citizens, and considering that 76% of the population over age 65 in Argentina receives healthcare coverage through the INSSJP,17 the observed 61.6% reduction in dispensations that resulted from the measure limiting their coverage has wider implications for this market segment. Generalizing this effect, we contend that the adoption of a medication policy by the INSSJP focused on covering drugs with demonstrated efficacy would have the effect of improving the rationality of the entire pharmaceutical market in Argentina.
Some of the limitations of this study that should be mentioned include the absence of a control group, given that the measure reducing coverage described here was simultaneously applied to the entire population of beneficiaries. Second, analysis of shifts in demand toward other medications was limited to NSAIDs, even though other drug classes - such as corticosteroids or opioid analgesics - could have been affected. Furthermore, as we have previously pointed out, this study did not examine the satisfaction of beneficiaries or providers, nor did it look at health outcomes.
Disinvestment strategies represent a challenge for healthcare financing systems, in an effort to combine the best possible allocation of budgetary resources with improvements in health outcomes and in patient and provider satisfaction. These interventions face resistance from all involved stakeholders, and it is therefore necessary to anticipate their effects on the different aspects discussed here, with an eye toward attaining a balance between the ideal and the attainable, or what MacKean et al. refer to as “the art of the possible.”27,28 In order to increase the probability of success, this should be a transparent process and measures should be properly justified and consensus with key actors should be sought.28,46
REFERENCIAS BIBLIOGRÁFICAS
1. Información Farmacoterapéutica. Tratamiento de la artrosis. INFAC [Internet]. 2018;26(1):1-7 [citado 11 dic 2019]. Disponible en: Disponible en: https://tinyurl.com/2v3678e5 . [ Links ]
2. Alfabeta.net. Manual Farmacéutico Online. [Internet]. 2018 [citado 3 nov 2018]. Disponible en: Disponible en: http://www.alfabeta.net [ Links ]
3. Calvo Pita C. Fármacos sintomáticos de acción lenta y administración oral para la artrosis: dudosa eficacia en el control sintomático y nula actividad condroprotectora. El Comprimido [ Internet ]. 2020;(18):1-7 [citado 10 may 2020]. Disponible en: Disponible en: https://tinyurl.com/3z2thf4v . [ Links ]
4. Gutiérrez-Ibarluzea I, Ibargoyen-Roteta N, Benguria-Arrate G, Rada D, Mateos M, Regidor I, Domingo C, González R, Galnares-Cordero L. Sysadoas: Condroprotectores en el tratamiento de la artrosis [ Internet ]. San Sebastián: Eusko Jaurlaritzaren Argitalpen Zerbitzu Nagusia, Servicio Central de Publicaciones del Gobierno Vasco; 2014 [citado 10 ene 2019]. Disponible en: Disponible en: https://tinyurl.com/x2ncs639 . [ Links ]
5. Fidelix TS, Macedo CR, Maxwell LJ, Fernandes Moça Trevisani V. Diacerein for osteoarthritis. Cochrane Database of Systematic Reviews. 2014;(2):CD005117. doi: 10.1002/14651858.CD005117.pub3. [ Links ]
6. Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database of Systematic Reviews. 2015;(1):CD005614. doi: 10.1002/14651858.CD005614.pub2. [ Links ]
7. Hochberg MC, Martel-Pelletier J, Monfort J, Möller I, Castillo JR, Arden N, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Annals of the Rheumatic Diseases. 2016;75(1):37-44. doi: 10.1136/annrheumdis-2014-206792. [ Links ]
8. Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, et al. Glucosamine and chondroitin for knee ostearthritis: a double-blind randomized placebo-controlled clinical trial evaluating single and combination regimens. Annals of the Rheumatic Diseases. 2015;74(5):851-858. doi: 10.1136/annrheumdis-2013-203954. [ Links ]
9. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: A six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis & Rheumatology. 2017;69(1):77-85. doi: 10.1002/art.39819. [ Links ]
10. Reginster JY, Dudler J, Blicharski T, Pavelka K. Pharmaceutical-grade chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: The ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Annals of the Rheumatic Diseases. 2017;76(9):1537-1543. doi: 10.1136/annrheumdis-2016-210860. [ Links ]
11. Haute Autorité de Santé. Art 50, Zondar, Chondrosulf, Piasclédine, Dolenio, Flexea, Osaflexan, Structoflex et Voltaflex: service médical rendu insuffisant dans le traitement symptomatique de l’arthrose [ Internet ] HAS, 2013 [citado 20 jun 2020]. Disponible en: Disponible en: https://tinyurl.com/36u2jy73 . [ Links ]
12. Ministerio de Salud. Resolución 310/2004 [ Internet ]. 2004 [citado 10 mar 2020]. Disponible en: Disponible en: https://tinyurl.com/2nwddmf3 . [ Links ]
13. Instituto Nacional de Servicios Sociales para Jubilados y Pensionados. Historia [ Internet ]. 2019 [citado 4 feb 2019]. Disponible en: Disponible en: https://www.pami.org.ar/historia . [ Links ]
14. Instituto Nacional de Servicios Sociales para Jubilados y Pensionados. Listado de precios de medicamentos para afiliados [ Internet ]. 2019 [citado 4 feb 2019]. Disponible en: Disponible en: https://tinyurl.com/a4absch8 . [ Links ]
15. Bisang R, Luzuriaga JP, San Martín M. El mercado de los medicamentos en Argentina. Fundación CECE [ Internet ]. 2017 [citado 10 jun 2020]. Disponible en: Disponible en: https://tinyurl.com/2d2jykz7 . [ Links ]
16. Instituto Nacional de Servicios Sociales para Jubilados y Pensionados. Resolución 337/2005. Boletín del Instituto [ Internet ]. 2005;1(96):1-19 [citado 10 may 2020]. Disponible en: Disponible en: https://tinyurl.com/r3nd8yut . [ Links ]
17. Instituto Nacional de Servicios Sociales para Jubilados y Pensionados. El Modelo prestacional y los desafíos del PAMI [ Internet ]. Consejo Profesional de Ciencias Económicas de la Ciudad Autónoma de Buenos Aires; 2017 [citado 20 jun 2020]. Disponible en: Disponible en: https://tinyurl.com/9y9upyjh . [ Links ]
18. Cermignani EC, Cañás M. Medicamentos esenciales en la Atención Primaria de la Salud. En: Roa R, Torres R. (eds). Atención Primaria de la Salud. En prensa, 2020. [ Links ]
19. Argentina, Poder Ejecutivo Nacional. Decreto 150/92: Normas para el registro, elaboración, fraccionamiento, prescripción, expendio, comercialización, exportación e importación de medicamentos [ Internet ]. 1992 [citado 15 dic 2020]. https://tinyurl.com/f7hdskxz . [ Links ]
20. Cañás M, Buschiazzo HO, Urtasun MA. Valor terapéutico y precio de los nuevos fármacos comercializados en Argentina: ¿valen lo que cuestan? Salud Colectiva. 2019;15:e1962. doi: 10.18294/sc.2019.1962. [ Links ]
21. Cañás M, Carlson S, Petinelli A, Raimondi M, et al. Medicamentos de riesgo inaceptable comercializados en 7 países de América latina. Researchgate. doi: 10.13140/RG.2.2.11872.48645. [ Links ]
22. Trionfetti M, Mordujovich-Buschiazzo P, Cañás M, Marín G, Buschiazzo HO, Marín L, et al. Medicamentos presentes en el mercado farmacéutico argentino y retirados de otros mercados internacionales por efectos adversos graves. En: Resúmenes de la XXVII Reunión de Gapurmed. La Plata: Comisión de Investigaciones Científicas de la Provincia de Buenos Aires; 2019. [ Links ]
23. Giulietti M, Carlson S, Cañás M, BuschiazzoH, M. de Buschiazzo PM. Medicamentos en combinaciones a dosis fijas en el mercado argentino durante el año 2006. 1ra etapa: AINES. Póster XIV Reunión del DURG-La, Foro-Taller Internacional: “Acceso Universal a los Medicamentos Antiretrovirales: Encuentro Universitario de Farmacoterapéutica”. Santo Domingo, República Dominicana 26 al 29 de Septiembre de 2007. [ Links ]
24. Cañás M, Wirtz V, Ibáñez SE, Vargas A, Melgarejo S, Valsecia M. Estudio de utilización de medicamentos de combinaciones a dosis fijas (CDF): disponibilidad comercial y riesgo beneficio en 4 países latinoamericanos. En: Resúmenes XX Reunión anual GAPURMED 2011, San Luis, Argentina. [ Links ]
25. Instituto Nacional de Servicios Sociales para Jubilados y Pensionados. Resolución 439/2016. Boletín del Instituto. 2016;12(2516):8-11. [ Links ]
26. Repullo JR. Taxonomía práctica de la «desinversión sanitaria» en lo que no añade valor, para hacer sostenible el Sistema Nacional de Salud. Revista de Calidad Asistencial. 2012;27(3):130-138. doi: 10.1016/j.cali.2012.02.010. [ Links ]
27. MacKean G, Noseworthy T, Elshaug AG, Leggett L, Littlejohns P, Berezanski J, et al. Health technology reassessment: the art of the possible. International Journal of Technology Assessment in Health Care. 2013;29(4):418-423. doi: 10.1017/S0266462313000494. [ Links ]
28. Parkinson B, Sermet C, Clement F, Crausaz S, Godman B, Garner S, et al. Disinvestment and value-based purchasing strategies for pharmaceuticals: an international review. Pharmacoeconomics. 2015;33(9):905-924. doi: 10.1007/s40273-015-0293-8. [ Links ]
29. Pichetti S, Sorasith C, Sermet C. Analysis of the impact of removing mucolytics and expectorants from the list of reimbursable drugs on prescription rates: A time-series analysis for France 1998-2010. Health Policy. 2011;102(2-3):159-169. doi: 10.1016/j.healthpol.2011.07.001. [ Links ]
30. Antoñanzas Villar F, Rodríguez-Ibeas R, Juárez-Castelló CA, Lorente Antoñanzas MR. Impacto del Real Decreto-Ley 16/2012 sobre el copago farmacéutico en el número de recetas y en el gasto farmacéutico. Revista Española de Salud Pública. 2014;88(2):233-249. doi: 10.4321/S1135-57272014000200006. [ Links ]
31. Compairé Bergua I, Compairé Bergua A, Arner Navarro JA, García Lerma D, Gazo AR. Análisis de las consecuencias de la desfinanciación de medicamentos del 1 de septiembre de 2012. Farmacéuticos Comunitarios 2014;6(2):5-10. [ Links ]
32. Pichetti S, Sermet C. Le déremboursement des médicaments en France entre 2002 et 2011: éléments d’évaluation. Questions d’économie de la santé. 2011;(167):1-7. [ Links ]
33. Lasio L. Delisting of pharmaceuticals from insurance coverage: effects on consumption, pricing and expenditures in France [ Internet ]. 2016. [citado 9 nov 2019]. Disponible en: Disponible en: https://tinyurl.com/4zwxz6bf . [ Links ]
34. Kenneally M, Walshe V. Pharmaceutical cost-containment policies and sustainability: recent Irish experience. Value in Health. 2012;15:389-393. doi:10.1016/j.jval.2011.10.007. [ Links ]
35. Gür Ali O, Topaler B. How removing prescription drugs from reimbursement lists increases the pharmaceutical expenditures for alternatives. European Journal of Health Economics. 2011;12:553-562. doi 10.1007/s10198-010-0270-2. [ Links ]
36. Chambers JD, Rane PB, Neumann PJ. The impact of formulary drug exclusion policies on patients and healthcare costs. American Journal of Managed Care. 2016;22(8):524-531. [ Links ]
37. Park Y, Raza S, George A, Agrawal R, Ko J. The effect of formulary restrictions on patient and payer outcomes: a systematic literature review. Journal of Managed Care & Specialty Pharmacy. 2017;23(8):893-901. doi: 10.18553/jmcp.2017.23.8.893. [ Links ]
38. WHO Collaborating Centre for Drug Statistics Methodology. ATC index with DDDs [ Internet ]. 2020 [citado 20 feb 2020]. Disponible en: Disponible en: https://tinyurl.com/ys5zn5yr . [ Links ]
39. Cavallo A, Bertolotto M. Serie completa de inflación de Argentina desde 1943 a 2016 [ Internet ]. 2016 [citado 20 feb 2020]. Disponible en: Disponible en: http://dx.doi.org/10.2139/ssrn.2787276 . [ Links ]
40. Dirección General de Estadística y Censos. Índice de precios al consumidor [ Internet ]. 2019 [citado 10 ene 2019]. Disponible en: Disponible en: https://tinyurl.com/wes75mcu . [ Links ]
41. Hallas J, Støvring H, Pottegård A. Individual-level drug utilization analyses. En: Elseviers M, Wettermark B, Almarsdóttir AB, (eds). Drug Utilization Research: Methods and Applications. New York: John Wiley & Sons; 2016. p. 68-76. [ Links ]
42. Administración Nacional de Medicamentos, Alimentos y Tecnología Médica. Disposición 528/2020. Boletín Oficial de la República Argentina [ Internet ]. 7 feb 2020 [citado 10 mar 2020]. Disponible en: Disponible en: https://tinyurl.com/23v857wj . [ Links ]
43. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. New England Journal of Medicine. 1999;340(24):1888-1899. doi: 10.1056/NEJM199906173402407. [ Links ]
44. Prozzi GR, Cañás M, Urtasun MA, Buschiazzo HO, Dorati CM, Mordujovich-Buschiazzo P. Riesgo Cardiovascular de los antiinflamatorios no esteroideos. Medicina (Buenos Aires). 2018;78(5):349-355. [ Links ]
45. Capellà D, Laporte JR. Métodos aplicados en estudios descriptivos de utilización de medicamentos. En: Laporte JR, Tognoni G. Principios de epidemiología del medicamento. Barcelona: Salvat; 1983. [ Links ]
46. Pace J, Laba TL, Nisingizwe MP, Lipworth W. Formulating an ethics of pharmaceutical disinvestment. Journal of Bioethical Inquiry. 2020;17(1):75-86. doi: 10.1007/s11673-020-09964-z. [ Links ]
Received: October 22, 2020; corrected: December 17, 2020; Accepted: December 26, 2020; pub: March 06, 2021











 texto em
texto em 



