Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Salud colectiva
Print version ISSN 1669-2381On-line version ISSN 1851-8265
Salud colect. vol.17 Lanús 2021 Epub Sep 27, 2021
http://dx.doi.org/10.18294/sc.2021.3583
ARTICLES
Benzodiazepine and Z-drug consumption in a national social security organization in Argentina: rational or excessive use?
1Physician. Full Professor, Faculty of Medicine, Universidad Nacional de la Plata, Consejo Nacional de Investigaciones Científicas y Técnicas. Instituto Obra Social de las Fuerzas Armadas y de Seguridad. Buenos Aires, Argentina. gmarin@med.unlp.edu.ar
2Pharmacist. Head of Drugs Unit, Instituto Obra Social de las Fuerzas Armadas y de Seguridad; Buenos Aires, Argentina. julieta.delmauro@iosfa.gob.ar
3Sociologist. Faculty of Medicine, Universidad Nacional de la Plata, Buenos Aires, Argentina. lupemmarin@gmail.com
4Physician. Pharmacology, Medical Federation of the Province of Buenos Aires. Associate Professor, Universidad Nacional Arturo Jauretche, Buenos Aires, Argentina. murtasun@femeba.org.ar
5Undergraduate Degree in Administration. Faculty of Medicine, Universidad Nacional de La Plata, Buenos Aires, Argentina. marin.gina@hotmail.com
6Physician. Assessor, Instituto Obra Social de las Fuerzas Armadas y de Seguridad. Buenos Aires, Argentina. dnucher@gmail.com
7Pharmacist. Head of Pharmacy, Instituto Obra Social de las Fuerzas Armadas y de Seguridad; Buenos Aires, Argentina. carlos.dacher@iosfa.gob.ar
8Dentist. President, Instituto Obra Social de las Fuerzas Armadas y de Seguridad. Buenos Aires, Argentina. dario.diazperez@iosfa.gob.ar
9Physician. Pharmacology, Medical Federation of the Province of Buenos Aires. Associate Professor, Universidad Nacional Arturo Jauretche, Buenos Aires, Argentina. macanaso@gmail.com
Benzodiazepines and “Z-drugs” (BZD/Z) are overprescribed in many countries. This study evaluates their consumption in a social security sector health insurance provider with national coverage in Argentina. With a descriptive and observational approach, outpatient dispensations of BZD/Zs were analyzed for people over 18 years old from April 2020 to March 2021, disaggregated by sex, age, active ingredient, and half-life. An annual prevalence of use of 11.6% was found among the 431,445 adult affiliates, with higher rates in women and in those over age 60. Overall consumption of BZD/Zs was 77.6 defined daily doses (DDD) per 1000 enrollee-days. The average user received 5.1 annual dispensations and the equivalent of 1.4 DDD for each day of the year. BZD/Zs with long half-life were the most used. We found high levels of BZD/Z consumption and for longer periods than recommended. It is necessary to improve the quality of consumption and reduce the negative impact of inappropriate use of these drugs among treated individuals.
KEYWORDS: Benzodiazepines; Drug Utilization; Pharmacoepidemiology; Social Security; Argentina
INTRODUCTION
Benzodiazepines are one of the most widely used pharmacological groups due to their anxiolytic, hypnotic, muscle relaxant, and anticonvulsant properties. Their use often lasts for longer periods than recommended, and that is why they continue to cause controversy globally.1 The so-called “Z-drugs”, such as zolpidem and zopiclone, which are closely associated with benzodiazepines, were developed as hypnotics that do not produce tolerance and physical dependence, but these problems were identified when their consumption increased.2
The prevailing way of life and the requirements of modern times lead individuals to undergo worryingly difficult and stressful situations, both in the workplace and at home.3 Those who commercialize drugs have made a determined effort to show that psychoactive substances are the solution to these problems. This is the reason why individuals and prescribing professionals link benzodiazepines and Z-drugs (BZD/Zs) to various uses such as sleep improvement, anxiety reduction, enhanced performance in daily activities, improved personal performance, or mitigation of unwanted social situations, to name but a few purported uses.3
The use of this type of drugs varies according to the studied population, and in the adult population across Argentina it ranges from 4.3% to 8.1%4,5; while in Brazil it accounts for 5.6%6; in Uruguay it accounts for 7.4%,7) and in Spain the rate is 11.4%.8
Other authors warn about the existence of an extraordinary diversity in consumption according to sex, age, profession or even the activity undertaken by the studied population.9 For instance, within the military forces of the Spanish Navy, the percentage of BZD/Z consumption is 1.8%9; among the nursing staff this percentage varies (from 10% to 32%) according to the country being studied10,11,12,13; and regarding other occupations and professions, variation is still higher.14
In all cases, indiscriminate use of BZD/Zs will result in chronic users, who are more likely to develop tolerance and dependence. This does not generally occur when they are prescribed for conditions such as anxiety or insomnia on a temporary basis, according to the guidelines of the World Health Organization.15,16 Suddenly quitting long-term treatments can lead to forms of anxiety, panic attacks, hyperventilation, moderate tremors, sleep disorders, muscle spasms, anorexia, weight loss, visual alteration, sweating, or dysphoria.17,18,19
For this reason, treatment duration should be the shortest possible, ideally not exceeding a period of four weeks when treating insomnia, and no more than three months when treating anxiety, including the period of tapering off up to drug discontinuation.20
Therefore, it is important for each place, institution or health financing agency to know the reality regarding the profile of BZD/Z consumption, not only to avoid an unnecessary use of these drugs, but also to prevent unwanted associated effects. It is within this framework that the authors decided to conduct this research work within a social security organization (employment-based health insurance provider) with national coverage in Argentina, with the aim of inquiring into outpatient BZD/Z use among affiliates aged 18 years and older, the amount and type of BZD/Zs dispensed during a period of 12 months, and duration of drug use.
METHODOLOGY
Design and study population
Drug use was analyzed with a descriptive and observational approach. The employment-based health insurance provider with national coverage, having enrollees across the 24 provinces in Argentina, provides services to a population amounting to 577,000 affiliates of different age groups, out of whom 20.1% are over 60 years old, performing duties in the armed forces and national law enforcement agencies, including active workers, their dependents, retired persons, and pensioners.
Data source and studied variables
The information was obtained from the database of outpatient dispensations, which includes pharmacies run by the institution itself and other third-party pharmacies. Each record states the dispensation date, patient’s identification details, number of containers, and composition of the drugs dispensed.
Outpatient dispensations were analyzed for the total number of affiliates over 18 years old that used drugs between April 1st, 2020 and March 31st, 2021. The analysis was focused on all benzodiazepines and benzodiazepines analogs with hypnotic action (eszopiclone, zolpidem, and zopiclone), in their presentations as monodrugs or as fixed dose combinations (FDCs).
Although a drug provision does not necessarily imply its effective use, in this work, for convenience purposes, the term “user” will refer to the person receiving a dispensation whereas the term “affiliates” will refer to the entire population covered by the employment-based health insurance.
In order to explore the indication for BZD/Z drugs, the diagnosis, if any, described on the prescription was recorded. To quantify the various drugs with a common unit, the defined daily dose (DDD) was used, which is the average maintenance dose per day for a drug, used for its main indication in adults. An international committee approved by the World Health Organization establishes the Anatomical Therapeutic Chemical (ATC) Classification System for drugs and their corresponding DDD.21
Under the ATC classification system, clonazepam is labeled as an anticonvulsant and the assigned DDD is 8 mg, according to this indication. However, the prevailing use of clonazepam among adults is as an anxiolytic, in which case the usual doses are substantially lower. To appropriately reflect the contribution of clonazepam to the total exposure of the population to BZD/Z drugs, we followed the literature that assigns an anxiolytic DDD of 1 mg to this drug.7,22,23,24 As for FDCs, the assigned DDDs depended on the BZD/Z component.
User’s sex and age were recorded as well as the active principle and its corresponding ATC code, the DDD value21) and half-life of the drug.1,25) BZD/Z drugs were classified as having short, medium or long half-life, depending on whether they were under 6 hours, from 6 to 24 hours or more than 24 hours, respectively.25
The total milligrams and total DDDs dispensed during the annual period per each drug were quantified and with this information the DDDs for each drug dispensed on a daily basis per 1,000 enrollees (DDD per 1,000 inhabitants per day = DID) were established, using the following formula26:
The annual prevalence of use for each sex and age group was calculated as well as the percentage of affiliates that received at least one package of BZD/Zs during the period under study.27) The average quantity of BZD/Zs dispensed throughout the year for each user was also defined, stated in packages and DDD per year, also disclosed as DDD/user/day.
As an approach to the duration and continuity of use, we explored the total number of months in which each user was dispensed at least one container of BZD/Zs and the maximum period of uninterrupted dispensation in consecutive months. Concomitant dispensation during the same month of other pharmaceuticals with potential drug interactions with BZD/Z drugs was recorded, in accordance with the cited reference text.28
With respect to statistical analysis, information was consolidated on a single database and later analyzed using statistical software “R” version 4.0. Data are described as average figures and their standard deviations or percentages, according to the type of variable.
All the data were encoded to hide given names, surname, number of affiliate, and any other detail that could reveal users’ identity. The protocol used in this study was authorized by the Ethics Committee of the Institution (D19-20).
RESULTS
During the study period (starting April 1st, 2020 and ending March 31st, 2021), the employment-based health insurance provider had a total of 431,445 adult affiliates over 18 years old, out of whom 48.3 % were female, the mean age being 46.5 (+/- 19.7) years.
During the 12 months of the study, we analyzed 4,833,768 dispensations, out of which 273,311(5.7%) accounted for BZD/Z drugs, directed at 50,049 different users (61.9% female; mean age 64.1 +/- 16.0 years).
The users received about 6.6 containers of BZD/Z drugs per year, containing 78.7 DDD per container on average, accounting for a total of 518 annual DDD per user, which amounts to 1.4 DDD per day.
The percentage of affiliates over 18 years old that received at least one dispensation of BZD/Z drugs during the year of study was 11.6 %. An increment of BZD/Z-drug consumption was observed as the age of the affiliates increased, being always higher in women (Figure 1).
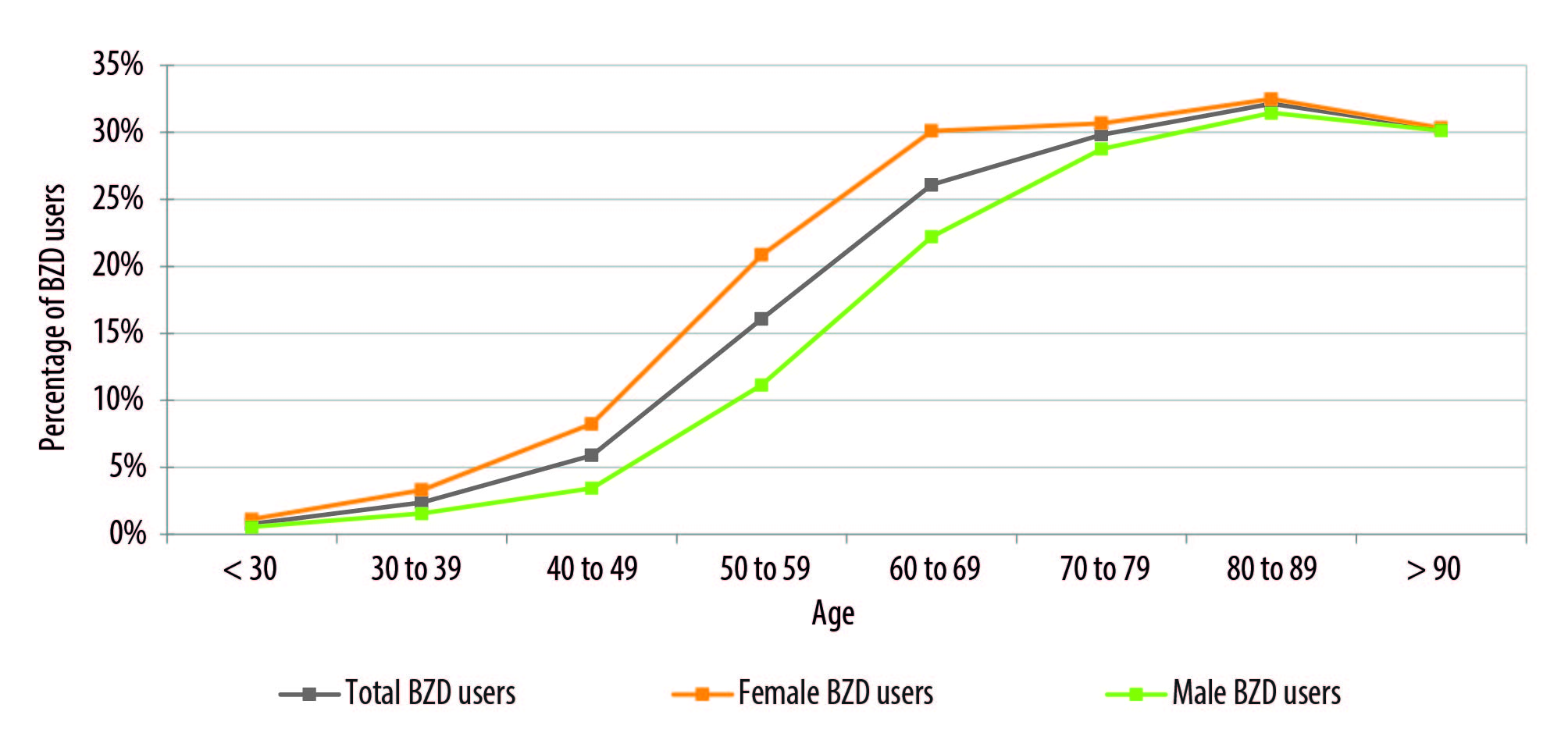
Source: Own elaboration based on data provided by Instituto Obra Social de las Fuerzas Armadas y de Seguridad. BZD= Benzodiazepines
Figure 1 Annual prevalence of benzodiazepines and Z-drug use, according to age group and sex, in a social security organization (employment-based health insurance provider). Argentina, from April 1st, 2020 to March 31st, 2021.
The total average dispensation of BZD/Z drugs measured in DID was 77.6 daily DDD per 1,000 enrollees. The main BZD/Zs dispensed during the period were clonazepam with a DID of 36.8 and alprazolam with a DID of 27.6 (Table 1). They were followed, according to the quantities dispensed, by lorazepam (DID 5.4), zolpidem (DID 3.8), diazepam (DID 1.8), and bromazepam (DID 0.9). The least commonly dispensed BZD/Z drugs were eszopiclone and zopiclone, both with a DID lower than 0.1 (Table 1).
Table 1 Dispensation of benzodiazepines and Z-drugs by a social security organization (employment-based health insurance provider). Argentina, from April 1st, 2020 to March 31st, 2021.
| Active ingredient | Half-life | DDD (ATC) | Total Milligrams | DID (DDD/1,000 enrollees /day) | Porcentaje acumulado de DHD dispensadas |
|---|---|---|---|---|---|
| Clonazepam* | Long | 1 | 5,801,502 | 36.84 | 47.38 |
| Alprazolam | Medium | 1 | 4,350,442 | 27.63 | 82.92 |
| Lorazepam | Medium | 2.5 | 2,124,982 | 5.40 | 89.86 |
| Zolpidem | Short | 10 | 5,917,576 | 3.76 | 94.69 |
| Diazepam | Long | 10 | 2,891,394 | 1.84 | 97.05 |
| Bromazepam | Medium | 10 | 1,426,952 | 0.91 | 98.22 |
| Clobazam | Long | 20 | 2,249,820 | 0.71 | 99.14 |
| Flunitrazepam | Medium | 1 | 42,021 | 0.27 | 99.48 |
| Midazolam | Short | 15 | 486,025 | 0.21 | 99.75 |
| Clordiazepóxido | Medium | 30 | 485,025 | 0.10 | 99.88 |
| Zopiclona | Short | 10 | 131,152 | 0.08 | 99.98 |
| Eszopiclona | Short | 10 | 18,748 | 0.01 | 100.00 |
Source: Own elaboration based on data provided by Instituto Obra Social de las Fuerzas Armadas y de Seguridad.DDD= defined daily doses; ATC= Anatomical Therapeutic Chemical Classification System; DID= defined daily dosis per 1,000 inhabitants per day.*Regarding the DDD of 1 mg for clonazepam, we followed the works by Speranza et al,7) Quaglia Planas et al,22 Zorzanelli et al,23 Kurko et al.24
The main FDCs were bromazepam + trimebutine, bromazepam + domperidone + simethicone, chlordiazepoxide + sulpiride, alprazolam + domperidone, alprazolam + sulpiride.
Taking into account the half-life of the BZD/Z drugs dispensed, 50.7% of the total DDD accounted for a long half-life, 44.2% for a medium half-life and only 5.1% for a short half-life (Table 1).
Regarding the estimated duration and continuity of treatment, each user received on average 5.1 ± 3.1 dispensations throughout the year, with a maximum average period of consecutive dispensations of 3.0 ± 2.4 months.
The main reasons for prescription were anxiety (88.4%) and insomnia (11.6%). Nevertheless, diagnoses of these clinical conditions could be registered only in 24.2% of the patients.
We observed potential drug interactions of BZD/Z drugs, including the concomitant use with antacids in 1.3% of the cases, with antidepressants (1.1%), with estrogens/ progestogens (0.9%), with antipsychotics 0.8%, and with opioid derivatives (0.1%).
DISCUSSION
In an employment-based health insurance provider with national coverage in Argentina, the total dispensation of BZD/Z drugs, evaluated with a series of different parameters, proved to be really high. The most general indicator is population exposure measured as DID, which accounted for 77.6 DDD dispensed on a daily basis per 1,000 affiliates over 18 years old. This quantity proved to be somewhat lower than the 112.4 DID registered in the study of an Argentine employment-based health insurance provider with provincial coverage,29) and than the 82.9 DID consumed in a municipality near Rosario.22) When compared to other studies conducted in other countries, the value found was just lower than the values registered in European countries with higher consumption such as Spain or Portugal (89.3 and 96.0 DID, respectively, in 2012)30,31,32) and higher than the findings reported in several studies conducted in Australia, Costa Rica, Honduras, Denmark, among others.33,34,35,36,37,38) Out of 26 countries of the Organization for Economic Cooperation and Development (OECD), ranked according to their consumption combining sedatives and hypnotics, the employment-based health insurance provider under review would be placed in the highest fifth position.39
It should be noted that, given the predominance of consumers among individuals over 60 years old, the age composition of the study population determines the aggregate results and may limit comparability among research studies. In this sense, the 20.1% of individuals over 60 years old registered with the employment-based health insurance provider being studied is somewhat lower than the 23.1% in North America and the 25.7% in Europe.40 If the population distribution was leveled with the distribution in these regions, the values presented in this study could be somewhat higher.
Thanks to the information about users, we could calculate the prevalence of BZD/Z consumption, where 11.6 % is a value that is practically identical to the value reported in Spain.30) When the analysis was performed based on sex and age, we could confirm that the use of BZD/Z drugs was higher in women than in men, and that consumption increased with age, the highest peak being among octogenarian individuals, in line with the findings reported by other authors that studied this type of consumption in different countries.41,42,43
As to intensity of consumption, an average of 1.4 DDD per user and per day clearly surpass the values of 1.1 reported in the cited Argentine research study and the 0.8 reported in a study conducted in Spain.29,30 The general panorama is then a population with a high frequency and intensity of use of these psychotropic drugs.
With respect to the selection of the drugs used, the profile of consumption observed in this work was, mainly, BZD/Z drugs of a long half-life such as clonazepam, followed by drugs of a medium half-life such as alprazolam, which coincides with the observations described in other works carried out in Argentina, Brazil, and Uruguay.7,22,23,29,44 This preference for long-acting pharmaceuticals is contrary to the recommendations on the use of BZD/Zs in older adults, who are most of the users in this study. Beers’s criteria45) and the recommendations to review potentially inappropriate medications (PIM) included in the Screening Tool of Older Persons Prescriptions (STOPP) - Screening Tool to Alert Doctors to Right Treatment (START)46) highlight that they imply a higher risk of prolonged sedation, confusion, balance disorder, and falls.
Potential drug interactions detected here were relatively rare. The most common drug interactions include the combination of BZD/Z use with antacids, female hormones, and antidepressants, which is an aspect already observed by other researchers.28 The combined administration of these drugs can produce changes in their plasmatic concentration modifying their effects.
In analyzing the factors that determine consumer demand of BZD/Z drugs within a population, it can be postulated that the different prevalence rates of clinical conditions explain the different patterns of consumption of sedatives and hypnotics among countries, but existing evidence does not go in that direction. When comparing data of prevalence of anxiety disorders of the initiative Global Burden of Disease (GBD) with the data on consumption of sedatives and hypnotics by the Organization for Economic Cooperation and Development (OECD) mentioned above, the prevalence rate of anxiety conditions for the year 2017 ranged from 3.7% to 9.0% (a ratio of 2.5 between extreme values) while the consumption of BZD/Zs varied between 1.9 DID and 110.8 DID (a quotient of 58.3)39,47) If we take into account countries such as Portugal, which has maximum values of prevalence of anxiety and consumption of BZD/Zs, the correlation between pathology and treatment would be confirmed. However, countries like Spain and Italy, with practically identical levels of anxiety (6.0 and 6.1% respectively), have diametrically opposed levels of consumption of BZD/Zs (88.0 and 2.9 DID, respectively).39,47) According to the data reported by the Global Burden Disease (GBD) study, there is prevalence of anxiety disorders of 5.5% for Argentina, while values of 77.6 DID found in our study, along with the findings in other works,(22.29) place us in a high-consumption pattern, similar to the situation in Spain.
It seems then that differences in the prescription of BZD/Z drugs have to do, to a considerable extent, with prescriptive habits inherent in each society. It has been argued that among the determinants we could identify the training of professionals, the health system organization, legal regulations on psychotropic drugs, systems of medication coverage, and the degree of medicalization of non-pathological discomforts and of the social problems.48)
This set of factors will result in a definite incidence of new treatments with BZD/Z drugs that, according to the literature consulted, can range from 1.2% to 7.5% of the adult population every year, varying according to country, sex, and age of patients. 49,50,51,52 Once the drug treatment is commenced, and due to its potential to generate dependence, a percentage of the patients under treatment will become chronic users of BZD/Z drugs, a fraction that is estimated to be 20% in a careful study with a 10-year follow-up.53) In this way, the increasing prevalence of chronic BZD/Z users is shaped year after year, which in our study shows a fast growth between 40 and 60 years old, then reaching a plateau in the last decades of an individual’s lifetime (Figure 1).
From a qualitative approach, a study conducted in Belgium on the motivations of primary care professionals to initiate a treatment with benzodiazepines showed that, although they perceive themselves as cautious, the pressure to offer an answer to a patient’s psychosocial problem prevails. Among the reasons given, they mention lack of alternative proposals and lack of time during consultation, arguing that benzodiazepines are “the lesser evil,” without perceiving that the addictive nature of these drugs is a problem in itself. It should be noted that demands from patients were not identified in this study as a factor for prescription.54) In another US study, doctors highlighted efficacy and speed of action of benzodiazepines for treating anxiety, with great satisfaction from the patient. The use in older adults was not considered to be problematical since there was no increase in the dose or drug craving suggesting addiction. The professionals tended to be skeptical about the risks of chronic use and little optimistic as to the chances of achieving a reduction or suspension of drug use by their patients.55
The appropriate way of using BZD/Zs continues to be controversial at an international level, showing largely different prescriptive practices. On the one hand, adverse effects deriving from a long-term use, psychological and physical dependence, and absence of research studies backing up efficacy from the continued use of these pharmaceuticals over years or for decades are highlighted.1,56,57 It can be argued that the example of countries with low level of BZD/Z use demonstrates that a substantial part of the chronic use in countries like Argentina proves to be unnecessary. On the other hand, some authors endorse the need to give an answer to problems having a difficult solution, in which the use of psychotropic drugs would constitute the lesser evil.54 Other authors highlight that, despite decades of research in the field, a lot of key aspects of the discussion still require more conclusive evidence.58 The search of efficacious non-pharmacologic alternatives for treating anxiety and insomnia proves to be crucial in this sense.59
Limitations of this study
The duration and continuity of treatments could only be approached with indirect indicators, like number of annual dispensations per user and the maximum period of monthly consecutive dispensations. These show a minimum value for these variables, assuming that each dispensation covers up only a month of treatment. However, considering that dispensations account, on average, for 78.7 DDD per container, one would expect that each of them would cover - for many users - treatment for over a month. It is known that the use of BZDs over four months is a factor of high risk for developing adverse effects, especially among older adults.60,61
The studied period coincided with the emergence of COVID-19 pandemic in Argentina. It is interesting to consider the possible role of economic and social crises in the consumer demand of BZD/Z drugs, taking into account research studies that have explored whether an increase in emotional stress may result in a higher consumption of psychotropic drugs.62,63 However, a slant in the opposite direction is possible, since restrictions on citizens mobility and suspension of non-essential activities - including habitual medical care - may have hindered access to medication. As it was not possible to make a comparison with consumption data from previous years, the present work is not sufficient to determine the direction of the net effect.
Another limitation of this study was impossibility to register the reason for prescription of BZD/Z drugs in all cases, since a diagnosis is not always required or registered on the medication database of dispensations. Based on available data, it was observed that anxiety was the main reason for use, as opposed to other research studies conducted in Argentina in which indication for insomnia prevailed.44
Based on the results obtained in this investigation, institutional actions were implemented for the purpose of giving rationality to BZD/Z prescription, such as educational interventions directed at the professionals of the employment-based health insurance provider, which included auditing prolonged treatments, training prescribing professionals to limit terms of dispensation of BZD/Z drugs to the period suggested by the WHO, and proper communication by being in contact with the affiliates and health-care professionals to warn about potential drug interactions and expected adverse effects deriving from the use of this therapeutic group of drugs.
CONCLUSION
Consumption of BZD/Z drugs by the population of affiliates of the studied employment-based health insurance provider with national coverage in Argentina proved to be high, which does not conform to the good international practices of rational use of this group of psychotropic drugs. Implementation of corrective actions based on the results obtained in this study may not only control this situation, but also improve the quality of consumption, reducing the negative impact of inappropriate use of these drugs among treated individuals.
REFERENCES
1. Ashton C. Benzodiazepines: How they work & how to withdraw - The Ashton manual. Newcastle: Institute of Neuroscience; 2002. [ Links ]
2. O’Donnell JM, Bies RR, Shelton RC. Tratamiento farmacológico de trastornos de depresión y ansiedad. En: Brunton LL, Hilal-Dandan R, Knollmann BC, editores. Goodman & Gilman: Bases Farmacológicas de la Terapéutica. 13a ed. México: McGraw-Hill; 2019. [ Links ]
3. Lopez Vantour A, Aroche Arzuaga A, Bestard Romero J, Ocaña Fontela N. Uso y abuso de las benzodiazepinas. MEDISAN. 2010;14(4):556-560. [ Links ]
4. Touze G, Pawlowicz, MP, Rossi D, Goltzman P, Cymerman P. Consumo de drogas en Argentina. En: Drogas en América Latina: Estado del arte en estudios de toxicomanía en Argentina, Brasil, Colombia, Chile y Ecuador. Santiago de Chile: Ediciones Universidad Católica Silva Henríquez; 2008. [ Links ]
5. Brasesco MV, Legisa A, Pighin R, Tufro F. Consumo de psicofármacos y género en la Ciudad Autónoma de Buenos Aires. Buenos Aires: Observatorio de Drogas Dirección General de Políticas Sociales en Adicciones Gobierno de la Ciudad Autónoma de Buenos Aires; 2010. [ Links ]
6. Duarte P, Formigoni ML. O uso de substâncias psicoativas no Brasil: módulo 1. 11a ed. Brasília: Secretaria Nacional de Políticas sobre Drogas, SUPERA; 2017. [ Links ]
7. Speranza N, Domínguez V, Pagano E, Artagaveytia P, Olmos I, Toledo M, Tamosiunas G. Consumo de benzodiazepinas en la población uruguaya: un posible problema de salud pública. Revista Médica del Uruguay. 2015;31(2):112-119. [ Links ]
8. Agencia Española de Medicamentos y Productos Sanitarios. Utilización de medicamentos ansiolíticos e hipnóticos en España durante el periodo 2000-2012: Informe de utilización de medicamentos U/HAY/V1/17012014 [Internet]. 2014 [citado 10 mar 2021] Disponible en: Disponible en: https://tinyurl.com/52u2zbdc . [ Links ]
9. González Gómez C. Prevalencia de consumo de benzodiacepinas en un grupo de población militar. Sanidad Militar. 2017;73(3):184-186. [ Links ]
10. Caballero Aranda I, Sevilla Lerena MP. Abuso de fármacos en medio sanitario: programas de tratamiento. Medicina y Seguridad del Trabajo. 2014;60(235):434-454. [ Links ]
11. Griffith J. Substance abuse disorders in nurses. Nursing Forum. 1999;34(4):19-28. [ Links ]
12. Trinkoff A, Zhou Q, Storr C, Soeken K. Workplace access, negative proscriptions, job stain, and substance use in registered nurses. Nursing Research. 2000;49(2):83-90. [ Links ]
13. Diaz CL. El uso de drogas en el personal de enfermería. Ciencia y Enfermería. 2011;17(2):37-45. [ Links ]
14. Observatorio Español sobre Drogas. Encuesta 2007-2008 sobre consumo de sustancias psicoactivas en el ámbito laboral en España [Internet]. Madrid: Ministerio de Sanidad, Política Social e Igualdad; 2014 [citado 10 mar 2021]. Disponible en: Disponible en: https://tinyurl.com/wjyx2b2w . [ Links ]
15. Organización Mundial de la Salud. Tratamiento farmacológico de los trastornos mentales en la atención primaria de salud. Washington DC: OPS; 2010. Disponible en: https://tinyurl.com/2cj8s7m8. [ Links ]
16. Medical & Health Care Products Regulatory Agency. Benzodiazepines: Prescribing points [Internet]. London: MHRA; 2015 [citado 10 mar 2021]. Disponible en: Disponible en: https://tinyurl.com/9a2tb6z8 . [ Links ]
17. O’Brien CP. Benzodiazepine use, abuse and dependence. Journal of Clinical Psychiatry. 2005;66(Suppl 2):28-33. [ Links ]
18. Smith AJ, Tett SE. Improving the use of benzodiazepines: Is it possible? A non-systematic review of interventions tried in the last 20 years. BMC Health Services Research. 2010;10:321. [ Links ]
19. Minaya O, Ugalde O, Fresán A. Uso inapropiado de fármacos de prescripción: dependencia a benzodiazepinas en adultos mayores. Salud Mental. 2009;32:405-411. [ Links ]
20. Velert Vila J, Moreno Royo L, Velert Vila MM, Salar Ibáñez L. Se puede mejorar el uso de las benzodiazepinas desde la farmacia. Pharmaceutical Care España. 2012;14:94-101. [ Links ]
21. World Health Organization, WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2021 [Internet]. 2020 [citado 5 ago 2021]. Disponible en: Disponible en: https://tinyurl.com/3fjytmjt . [ Links ]
22. Quaglia Planas NB, Paciaroni J, Elías MM, Leiva M. Consumo de benzodiazepinas en una comuna de la región metropolitana de Rosario, provincia de Santa Fe, Argentina. Atención Primaria. 2009;41:520-521. [ Links ]
23. Zorzanelli RT, Giordani F, Guaraldo L, Matos GC, Brito Junior AG, Oliveira MG, Souza RM, Mota RQM, Rozenfeld S. Consumption of the benzodiazepine clonazepam (Rivotril®) in Rio de Janeiro State, Brazil, 2009-2013: an ecological study. Ciência & Saúde Coletiva. 2019;24(8):3129-3140. [ Links ]
24. Kurko T, Saastamoinen LK, Tuulio-Henriksson A, Taiminen T, Tiihonen J, Airaksinen M, Hietala J. Trends in the long-term use of benzodiazepine anxiolytics and hypnotics: A national register study for 2006 to 2014. Pharmacoepidemiology and Drug Safety. 2018;27(6):674-682. [ Links ]
25. Mihic SJ, Mayfield J, Harris RA. Hypnotics and sedatives. En: Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman & Gilman’s: The pharmacological basis of therapeutics. 13e ed. New York: McGraw-Hill Medical; 2018. p. 339-353. [ Links ]
26. Laporte JR, Tognoni G. Principios de epidemiología del medicamento. Barcelona: Masson-Salvat; 1983. [ Links ]
27. Hallas J, Støvring H, Pottegård A. Individual-level drug utilization analyses. En: Elseviers M, Wettermark B, Almarsdóttir AB, eds. Drug utilization research: Methods and Applications. New Jersey: John Wiley & Sons; 2016. p. 68-76. [ Links ]
28. Moody DE. Drug interactions with Benzodiazepines. En: Mozayani A, Raymon LP, eds. Handbook of Drug Interactions: a Clinical and Forensic Guide. New York: Humana Press; 2004. [ Links ]
29. Cañás M, Marin GH, Urtasun M. Estudio de utilización de benzodiazepinas (BZD) en un seguro de salud provincial (IOMA) con 2 millones de beneficiarios, a partir de los datos de dispensa. XXVIII Reunión de Grupo Argentino para el Uso Racional de los Medicamentos (GAPURMED), “Situación actual de los Medicamentos: entre el conocimiento científico y las políticas sanitarias”, Santa Fe - Argentina; 2019. doi: 10.13140/RG.2.2.24788.55683. [ Links ]
30. Agencia Española de Medicamentos y Productos Sanitarios. Utilización de medicamentos ansiolíticos e hipnóticos en España durante el período 2000-2012 [Internet]. 2014 [citado 10 mar 2021]. Disponible en: Disponible en: https://tinyurl.com/m486ed2u . [ Links ]
31. Rayón P, Montero D, Santamaría B, Madurga M, De Abajo FJ. Benzodiazepine consumption in Spain. European Journal of Clinical Pharmacology. 1997;52:321-323. [ Links ]
32. Furtado C. Psicofármacos: evolução do consumo em Portugal Continental (2000 - 2012) [Internet]. Lisboa: Infarmed, Autoridade Nacional do medicamento e produtos de saúde; 2012 [citado 10 mar 2021]. Disponible en: Disponible en: https://tinyurl.com/9j5zdkrm . [ Links ]
33. Estrela M, Herdeiro MT, Lopes Ferreira P, Roque F. The use of antidepressants, anxiolytics, sedatives and hypnotics in Europe: Focusing on mental health care in Portugal and prescribing in older patients. International Journal Environmental Research and Public Health. 2020;17(22):8612. [ Links ]
34. Islam MM, Conigrave KM, Day CA, Nguyen Y, Haber PS. Twenty-year trends in benzodiazepine dispensing in the Australian population. Internal Medicine Journal. 2014;44(1):57-64. [ Links ]
35. Brandt J, Alessi-Severini S, Singer A, Leong C. Novel measures of Benzodiazepine and Z-Drug utilisation trends in a Canadian Provincial Adult Population (2001-2016). Journal of Population Therapeutics and Clinical Pharmacology. 2019;26(1):22-38. [ Links ]
36. Jiménez M, Claret M. Estudio de utilización de benzodiazepinas en el área de salud de cartago (CCSS), durante el período de febrero 2007 a enero 2008. Revista Médica de la Universidad de Costa Rica. 2009;3(1):43-55. [ Links ]
37. Huerta C, Abbing‐Karahagopian V, Requena G. Exposure to benzodiazepines (anxiolytics, hypnotics and related drugs) in seven European electronic healthcare databases: a cross‐national descriptive study from the PROTECT‐EU Project. Pharmacoepidemiology and Drug Safety. 2016;25(Suppl. 1):56-65. [ Links ]
38. Pollmann AS, Murphy AL, Bergman JC, Gardner DM. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacology & Toxicology. 2015;16:19. [ Links ]
39. Organisation for Economic Co-operation and Development. Pharmaceutical market [Internet]. 2020 [citado 26 jun 2020]. Disponible en: Disponible en: https://tinyurl.com/4jj5vchz . [ Links ]
40. United Nations, Department of Economic and Social Affairs. World population Prospects 2019 [Internet]. 2019 [citado 26 jun 2020]. Disponible en: Disponible en: https://tinyurl.com/sz3pxa4e . [ Links ]
41. Bejarano Romero F, Lluís Piñol Moreso J, Mora Gilabert N, Claver Luquec P, Brull López N, Basora Gallisa J. Elevado consumo de benzodiazepinas en mujeres ancianas asignadas a centros de salud urbanos de atención primaria. Atención Primaria. 2008;40(12):617-621. [ Links ]
42. Matud Aznar MP, García Pérez L, Bethencourt Pérez JM, Rodríguez-Wangüemert C. Género y uso de medicamentos ansiolíticos e hipnóticos en España. Journal of Feminist, Gender and Women Studies. 2017;(5):23-31. [ Links ]
43. Torres-Bondia F, de Batlle J, Galván L, Buti M, Barbé F, Piñol-Ripoll G. Trends in the consumption rates of benzodiazepines and benzodiazepine-related drugs in the health region of Lleida from 2002 to 2015. BMC Public Health. 2020;20(1):818. [ Links ]
44. Bertoldo P. Perfil del consumo de benzodiazepinas en oficinas de farmacia. Revista Cubana de Farmacia, 2019;51(4):22-32. [ Links ]
45. American Geriatrics Society. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society. 2019;67(4):674-94. [ Links ]
46. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): Consensus validation. International Journal of Clinical Pharmacology and Therapeutics. 2008;46(2):72-83. [ Links ]
47. Global Burden of Disease Collaborative Network. Findings from the Global Burden of Disease Study 2017 [Internet]. Seattle: IHME; 2018 [citado 20 jun 2020]. Disponible en: Disponible en: https://tinyurl.com/389bp93 . [ Links ]
48. Potočnjak I, Likić R, Degoricija V, Nham E, Wettermark B. The benzodiazepine nation of Croatia: an observational, comparative study of psychotropic drug utilization between Croatia and Sweden 2014-2015. Expert Review of Pharmacoeconomics & Outcomes Research. 2018;18(6):641-646. [ Links ]
49. Alessi-Severini S, Bolton JM, Enns MW, et al. Use of benzodiazepines and related drugs in Manitoba: a population-based study. CMAJ Open. 2014;2(4):E208-E216. [ Links ]
50. Brett J, Karanges EA, Daniels B, Buckley NA, Schneider C, Nassir A, et al. Psychotropic medication use in Australia, 2007 to 2015: changes in annual incidence, prevalence and treatment exposure. Australian and New Zealand Journal of Psychiatry. 2017;51(10):990-999. [ Links ]
51. Agence Nationale de Sécurité du Médicament et des Produits de Santé. Etat des lieux de la consommation des benzodiazépines en France [Internet]. 2017 [citado 26 oct 2020]. Disponible en: Disponible en: https://tinyurl.com/p39aps2z . [ Links ]
52. Brett J, Maust DT, Bouck Z, Ignacio RV, Mecredy G, Kerr EA, Bhatia S, Elshaug AG, Pearson SA. Benzodiazepine Use in Older Adults in the United States, Ontario, and Australia from 2010 to 2016. Journal of the American Geriatrics Society. 2018;66(6):1180-1185. [ Links ]
53. Schonmann Y, Goren O, Bareket R, Comaneshter D, Cohen AD, Vinker S. Chronic hypnotic use at 10 years-does the brand matter? European Journal of Clinical Pharmacology. 2018;74(12):1623-1631. [ Links ]
54. Anthierens S, Habraken H, Petrovic M, Christiaens T. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scandinavian Journal of Primary Health Care. 2007;25(4):214-219. [ Links ]
55. Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. Journal of General Internal Medicine. 2007;22(3):303-307. [ Links ]
56. Lader M. Benzodiazepines revisited--will we ever learn? Addiction. 2011;106(12):2086-2109. [ Links ]
57. Lader M. Benzodiazepine harm: how can it be reduced? British Journal of Clinical Pharmacology. 2014;77(2):295-301. [ Links ]
58. Silberman E, Balon R, Starcevic V, Shader R, Cosci F, Fava GA, Nardi AE, Salzman C, Sonino N. Benzodiazepines: it’s time to return to the evidence. British Journal of Psychiatry. 2021;218(3):125-127. [ Links ]
59. Markota M, Rummans TA, Bostwick JM, Lapid MI. Benzodiazepine use in older adults: Dangers, management, and alternative therapies. Mayo Clinic Proceedings. 2016;91(11):1632-1639. [ Links ]
60. Cañás M, Urtasun M. Benzodiazepinas: Uso crónico y deprescripción. Folia Doc [Internet]. 2020;XXIII(2) [citado 10 mar 2020]. Disponible en: Disponible en: https://tinyurl.com/4mw4vezz . [ Links ]
61. García MAF, Olry de Labry Lima A, Ferrer Lopez I. et al. Analysis of changes in trends in the consumption rates of benzodiazepines and benzodiazepine-related drugs. Journal of Pharmaceutical Policy and Practice. 2018;11:1. [ Links ]
62. Nicieza-Garcia ML, Alonso-Lorenzo JC, Suarez-Gil P, Rilla-Villar N. Efecto de la crisis económica sobre el consumo de psicofármacos en Asturias. Gaceta Sanitaria. 2016;30(6):464-467. [ Links ]
63. Colell E. Prevalencia de consumo de hipnosedantes en población ocupada y factores de estrés laboral asociados. Gaceta Sanitario. 2014;28(5):369-375. [ Links ]
Received: May 03, 2021; corrected: August 23, 2021; Accepted: September 09, 2021; pub: September 27, 2021











 text in
text in 



