INTRODUCTION
The global threat of drug-resistant tuberculosis (DR-TB) has promoted the research of new treat ment regimens, new drugs, and repurposed drugs1 (not originally sold for TB, such as fluoroquino lones, linezolid and clofazimine) together with the traditionally called “second-line drugs” for the purpose of improving the efficacy of treatment of these forms of the disease.
The objective of this review is to briefly analyze current treatment regimens according to interna tional rules, and to describe dosages in adults and children, mechanisms of action, adverse reactions, and use in cases of renal and liver failure, preg nancy and tuberculous meningitis, of available drugs to treat drug-resistant TB. Thorough review on DR-TB found in2.
There are different DR-TB categories3-5: mono resistant TB, caused by strains of Mycobacterium tuberculosis (Mtb) and resistant to only one drug, the most concerning being monoresistance to isoniazid (Hr) and rifampicin (Rr); multidrug-resistant TB (MDR-TB), which shows resistance to at least isoniazid (H) and rifampicin (R); pre-extensively drug resistant TB (pre-XDR-TB), which is MDR and also shows resistance to one of the antituberculous fluoroquinolones (levofloxacin or moxifloxacin); and at last, extensively resistant (XDR-TB), which apart from being pre-XDR-TB, is also resistant to at least bedaquiline or linezolid (group A of the World Health Organization, WHO).
In 2018, the WHO published a new classifica tion of drugs to be used for DR-TB, updated in 20206 (Table 1) and based on the meta-analysis of individual MDR-TB patients data published by Ahmad et al7.

Table 1 WHO classification of drugs for the treatment of MDR-TB and their inclusion in a treatment regimen6
Treatment regimens of Hr-TB, Rr-TB and MDR-TB
The treatment recommended by the international guidelines for Hr-TB is a 6-month regimen with four drugs, without initial phase: levofloxacin, pyrazinamide, rifampicin and ethambutol. Treat ment duration is determined by the need to com plete 6 months of levofloxacin6,8.
Rr-TB is a category that arose from the advent of the rapid molecular method for diagnosis called Xpert Mtb RIF, which detects in Mtb, with almost 100% specificity, the presence of the five most common mutations of the RpoB gene that explain resistance to R9. Given the fact that approximately 80% of the Rr strains show additional resistance to H10 and that a first-line drug for the treatment of TB has been lost, Rr-TB should be treated as MDR-TB6,8.
According to new recommendations, MDR-TB can be treated with a 100% oral regimen including the three drugs of the WHO Group A (bedaquiline, linezolid, fluoroquinolone), together with one or two drugs from Group B (cycloserine or clofazi mine). Bedaquiline is administered the first 6 months ( see Table 2), and the other three or four drugs are given throughout the whole treatment, which lasts 18 months (it may be shortened in mild cases). Group C drugs would be left as replacement of Groups A and B if they can’t be used due to resistance or adverse reactions6,8.
Alternatives to isoniazid and rifampicin for the treatment of DR-TB
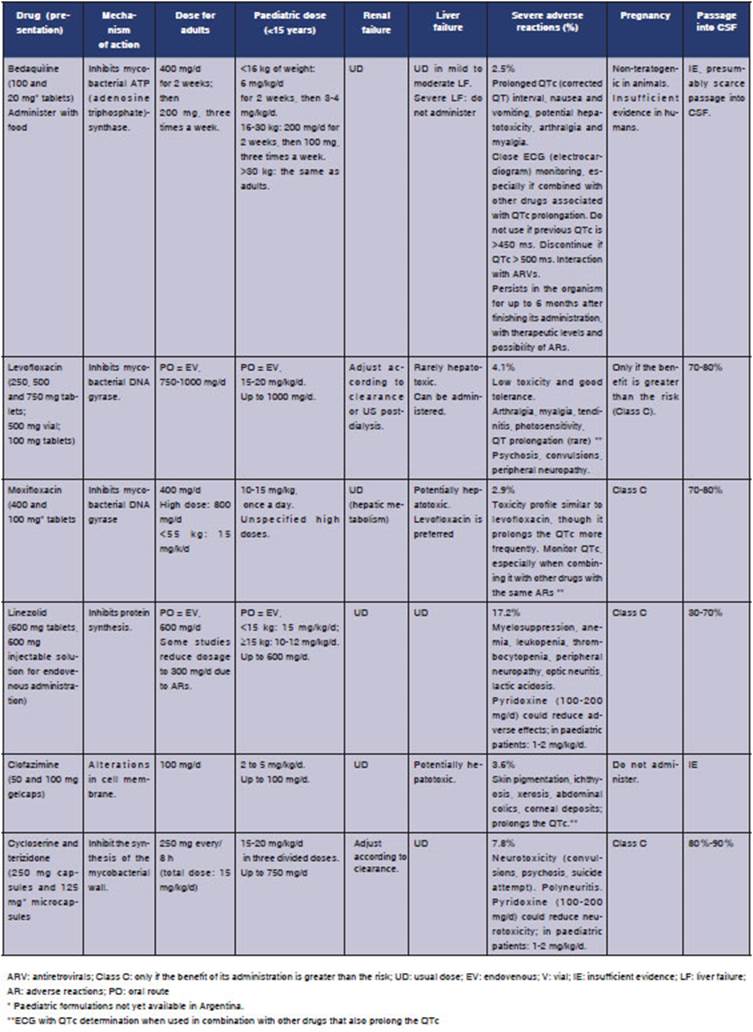
Tables 2and3 show every drug, mechanisms of action, dosage in adults and children, most common adverse reactions, use in renal and liver failure and pregnancy and passage into CSF (ce rebrospinal fluid), a fundamental element in the treatment of tuberculous meningitis.
Treatment of XDR-TB11-13
As seen in its definition, it implies resistance to at least H, R, one fluoroquinolone (levo or moxi floxacin) and bedaquiline or linezolid. From that minimum base of the definition (which already cre ates a complicated situation but still leaves other therapeutic options), resistance can be extended practically to all anti-TB drugs. Also, the antibio gram of multi-treated patients doesn’t correlate very well with the clinical picture, as in MDR-TB, and it is more common to find differences between the phenotypic and genotypic methods. So, it is important to ask the patients detailed questions regarding their previous treatments and clinical and bacteriological responses. To sum up, the design of a regimen for XDR-TB is individualized, and no guidelines can be provided as in other forms of DR-TB. Regimens are indicated with drugs that show persistent antibiogram sensitivity plus those that weren’t previously used, trying to get a minimum number of potentially effective drugs (3 or 4). In an effort to improve the diagnosis of these patients, the bedaquiline-delamanid combi nation has been used, as well as bedaquiline alone, for one year of treatment. New regimens such as BPaL (bedaquiline, pretomanid and linezolid) will provide evidence on the efficacy of the new drug, pretomanid, under these circumstances14. The prognosis of these patients is worse than in other forms of DR-TB.
CONCLUSIONS
In this brief review of the practical pharmacologi cal aspects of drugs for the treatment of DR-TB in adults and children, we show drugs (such as bedaquiline, delamanid and pretomanid) that have been specifically studied as antituberculosis drugs, something that hadn’t occurred since the discovery of rifampicin, half a century ago. This is an auspicious fact, together with the evidence showing the activity of drugs that allow a 100% oral treatment in children and adults. There is availability of regimens based on published evidence for the treatment of monoresistant and multi-drug resistant TB. Unfortunately, XDR-TB, the most severe mycobacterial resistance situation, is still a complex problem in terms of therapeutic and prognostic aspects.