Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Insuficiencia cardíaca
versión On-line ISSN 1852-3862
Insuf. card. v.3 n.2 Ciudad Autónoma de Buenos Aires abr./jun. 2008
REVISIÓN POR EXPERTOS
Cardiac transplantation for Chagas' disease
Fernando Bacal, MD PhD*, Prof. Edimar Alcides Bocchi, MD PhD**
* Cardiologist-Heart Failure and Transplant Unit. Heart Institute (InCor), University of São Paulo, Medical School. Brazil.
Editor-in-Chief of the Arquivos Brasileiros de Cardiologia. Brazil.
** Associate Professor, São Paulo University, Medical School.
Chief of the Heart Failure and Transplantation Clinics of the Heart Institute (InCor) University of São Paulo, Medical School. Brazil.
Correspondence: Fernando Bacal, MD, PhD
Av. Divino Salvador, 395, ap 201
Moema, São Paulo, Capital, Brasil
CEP: 04078-011
E-mail: fbacal@uol.com.br
Received: May 9/2008
Accepted: May 20/2008
Over the last 20 years the immunosuppression protocols in chagasic heart transplanted patients lived at least three different moments and we testified several changes and discoveries on Chagas' disease reactivation, mortality and neoplasias development. The primary phase was important especially because, until that time, Chagas' disease was an absolute contraindication for transplantation.
The second phase started when immunosuppression protocol adjustment was made, with lower dosage to avoid adverse effects, especially neoplasias.
Nowadays strategies to change the immunosuppression, especially replacement of mycophenolate mofetil by azathioprine were shown to be effective in reducing Chagas' disease reactivation.
Cardiac transplantation for Chagas' disease is a reality. Although chagasics have several different implications when submitted to the transplant comparing to others aethiologies, actually these difficulties are well known, so treatments and preventive strategies are also better established.
Keywords: Chagas' disease; Cardiac transplantation; Immunosuppression; Reactivation
Resumo
Transplante cardíaco para a doença de Chagas
Durante os últimos 20 anos o protocolo de imunossupressão em pacientes submetidos a transplante cardíaco por doença de Chagas vêm apresentando grandes mudanças, e testemunhamos descobertas na reativação da doença após transplante, na mortalidade e desenvolvimento de neoplasias. A primeira fase foi importante porque até aquele momento a doença de Chagas era uma contra-indicação absoluta para transplante.
A segunda fase começou quando ajustes no protocolo de imunossupressão foram feitos, especialmente com doses inferiores de ciclosporina, evitando efeitos adversos, principalmente neoplasias.
Hoje em dia, estratégias para mudar a imunossupressão, com a substituição do micofenolato mofetil pela azatioprina mostrou-se efetiva na redução da reativação de doença de Chagas.
O transplante cardíaco para a doença de Chagas é uma realidade. Embora o transplante nesta etiologia apresente peculiaridades próprias, estas dificuldades hoje são bem conhecidas, propiciando melhores tratamentos e estratégias preventivas.
Palavras chave: Doença de Chagas; Transplante cardíaco; Imunossupressão; Reativação
Introduction
Chagas' disease is a trypanosomiasis caused by a protozoan named Trypanosoma cruzi, which is endemic in Latin America from Mexico to Southern Argentina and Chile1. It is classically transmitted by contact with triatomine's feces. Blood derivatives and vertical transmission also can occur. The chronic form of the disease frequently compromises the digestive system and heart. The cardiac form is the most aggressive, attacking about 30% of infected patients2. This disease is responsible for up to 18% of the cases of refractory heart failure in Brazil3. The clinical manifestations of cardiac damage are variable. In the most benign cases there are alterations of cardiac rhythm with small changes in diastolic function, up to serious atrioventricular block, complex ventricular arrhythmias, sudden death and diffuse damage of ventricular contraction by chronic myocardial aggression, characterized as chagasic cardiomyopathy4.
Chagas' cardiomyopathy is considered the worst aethiology for heart failure when compared to others. It has been suggested that a more intense inflammatory activation, arrhythmias, and a high incidence of embolic events could be the responsibles for the excessive mortality. Mocelin et al. showed that inflammatory activation in Chagas' heart disease differs from idiopathic dilated cardiomyopathy and is associated with heart failure severity5.
Reactivation of the Trypanosoma cruzi infection after transplantation persists a real problem in the clinical follow-up, however an acceptable survival was demonstrated in many studies conducted on different organs and tissues6-10. With those results Chagas' disease is no longer a contraindication for transplantation, and the criteria for patient selection for transplantation among Chagas' disease patients are similar to those used for nonchagasics, except for chagasic megaesophagus or megacolon, which can be considered contraindications11.
Chagas' disease diagnosis
The diagnostic criteria for chronic Chagas' disease were a combination of epidemiological data, positive serological tests for anti-T cruzi antibodies (complement fixation and indirect immunofluorescence) and a compatible clinical syndrome (in heart disease it is also important to exclude other cause for the cardiomyopathy). Complement-fixation tests (Machado-Guerrero) are considered positive when the reaction is observed in a serum dilution equal to or greater than 1:8 and the indirect immunofluorescence equal to or greater than 1:32 in at least two different assays.
Immunosuppression in chagasic heart transplanted patients
Over the last 20 years the immunosuppression protocols in chagasic heart transplanted patients lived at least three different moments.
During this period we testified several changes and discoveries about Chagas' disease reactivation, mortality and neoplasias. We will analyze the treatment of chagasic patients, divided in three different periods (Phases 1, 2 and 3).
Phase 1
That was an important phase especially because until that time Chagas' disease was an absolute contra-indication for transplantation. During that first period we accumulated experience and several good results which proved the viability of this surgery for this aethiology. Bocchi et al. published a review where they showed that, compared to different aethiologies, chagasics had the best survival rate after cardiac transplant12 (Figure 1). The survival probability of patients submitted to heart transplantation for chagasic aethiology at 1, 6 months, 1, 2, 4, 6, 8, 10, and 12 years was 83%, 76%, 71%, 62%, 57%, 55%, 55%, 46%, and 46%. The survival rate of patients who underwent transplants for the treatment of Chagas' heart disease was better in comparison with the total group survival (p:0.03). Survival of chagasics was significantly better in comparison with idiopathic and ischemic cardiomyopathy (p:0.027). Survival of chagasics was better than patients with ischemic cardiomyopathy in both short- and long- term follow-up. Reactivation of the T cruzi infection was a rare cause of death and neoplasia was more frequent in chagasics.
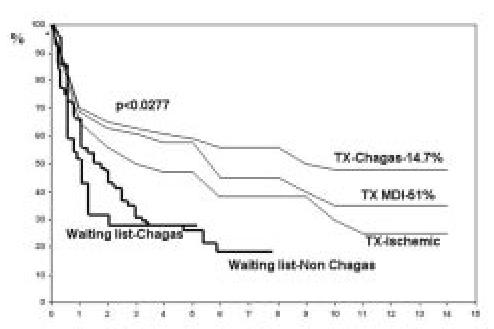
Figure 1. Comparison of survival of chagasic and non-chagasic patients submitted to cardiac transplantation. Adapted from Bocchi et al. Ann Thorac Surg 2001;71:1833-8.
The main reason explaining the better long-term survival rate after heart transplantation for Chagas' heart disease could be the low incidence of graft coronary artery disease, the patients were younger than other aethiologies and consequently with less co-morbidities. Possible factors associated with this low incidence of graft coronary artery disease should be better investigated. On the other hand the high incidence of malignant neoplasias was probably caused by multiple factors such as reactivation of T cruzi infection, benznidazole treatment, and immunological disorders associated with T cruzi infection9.
Phase 2
The main objective of this second phase was the immunosuppression protocol adjustment with lower dosage to avoid adverse effects, especially neoplasias.
When they compared these two phases, it was found a significant difference between the number of Chagas' reactivation (2.3 per patient in the first group and 0.25 in the second group) and survival, which had better results in the group with low immunosuppression dosage (p<0.05). There was no difference about number of rejection episodes between these two protocols. Malignant diseases developed during follow-up in 6 patients, 5 belonging to the earliest group (three lymphomas, two Kaposi's sarcoma and one squamous carcinoma).
These findings allowed to conclude that the reduction of cyclosporine dose was associated to a lower rate of Chagas' disease reactivation, neoplasias, and improved survival (Figure 2).
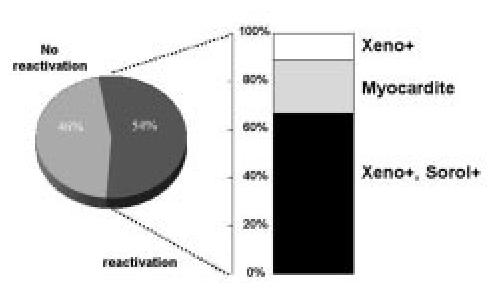
Figure 2. Chagas disease reactivation rate in a sub-group of patients with low doses of cyclosporine.
Especially about neoplasias, it is important to remember that immunosuppressive agents have direct correlation with greater tumor incidence in this population. The neoplasias incidence is about four times greater among the immunosuppressed than the normal population. Cyclosporine has an inhibitory action on T cells, through interleukin-2, and alters elements controlling B-cells proliferation, resulting from viral infections or during the episodes of reactivation of latent virus. In this way, it predisposes to lymphoma appearance13.
Phase 3
As the Chagas' disease became a usual indication for transplantation, the T cruzi reactivation monitoring turn necessary and was defined as a routine.
The Trypanosoma cruzi infection is specifically investigated at the time of routine endomyocardial biopsies and in the blood if there is clinical or laboratorial suspicious of Chagas' disease reactivation. In our experience, we routinely investigate reactivation in blood each 4 months (hemoculture, xenodiagnosis and QBC -quantitative buffy coat-). Recent articles have shown that polymerase chain reaction could be a sensitive and specific method for Chagas' reactivation diagnosis14,15. The diagnosis of Chagas' disease reactivation is considered when the parasite is detected in blood or tissues with inflammatory process surrounding it, in association with symptoms or signs attributable to infection by T cruzi. Chagasic myocarditis is diagnosed by the demonstration of the parasite surrounded by a myocardial inflammatory infiltrate according to the Dallas' criteria16. Parasitemia is diagnosed when T- cruzi is detected in blood samples. Episodes of parasitemia, myocarditis or Chagas' disease reactivation are already treated with benznidazole in a 5 mg/kg/day dosage for 60 days. Allopurinol at a daily dose of 600-900 mg is another option for the Chagas' disease reactivation17 as Nifurtimox 8-10 mg/Kg2.
Since 2001 we included mycophenolate mofetil in our immunosuppression protocol as a replacement of azathioprine.
After this change we noticed that the number of episodes of Chagas' disease reactivation increased. A retrospective analysis proved this clinical observation18. The use of mycophenolate mofetil did not change the number of rejections or survival curve when compared to the protocol with azathioprine, however increased the number of Chagas' disease reactivation (p<0.0001, hazard ratio 6.06). These findings were determinant leading us to prescribe azathioprine again only for chagasic heart transplanted patients, in the place of mycophenolate mofetil.
Another recent study from Bestetti et al. also revealed a high incidence of Chagas' reactivation with mycophenolate mofetil based immunosuppression19 (Figure 3).
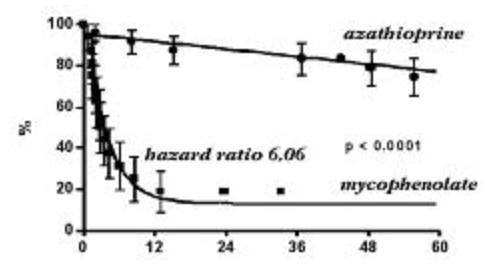
Figure 3. Free reactivation curve, comparing two different immunosuppression protocols. Adapted from Bacal et al. Am J Transplant 2005;5:2017-2021.
Conclusion
Nowadays cardiac transplantation for Chagas' disease is a reality.
Although chagasics have several different implications when submitted to the transplant comparing to others aethiologies, actually these difficulties are well known, so treatments and preventive strategies are also better established. The most important care is with the immunosuppressive dosages, which must be different and lower than used to others aethiologies.
1. Laranja FC, Dias E, Nóbrega G, et al. Chagas's disease: a clinical epidemiologic and pathologic study. Circulation 1956;14:1035-1060. [ Links ]
2. Prata A. Clinical and epidemiological aspects of Chagas' disease. Lancet Infect Dis 2001;1:92-100. [ Links ]
3. Bocchi EA. Situação atual das indicações e resultados do tratamento cirúrgico da insuficiência cardíaca. Arq Bras Cardiol 1994;63:523-530. [ Links ]
4. Feitosa MF, Kriger H. An appraisal of the epidemiology of Trypanosoma cruzi serology in Brazil. Mem Inst Oswaldo Cruz 1991;86:159-167. [ Links ]
5. Mocelin AO, Issa VS, Bacal F et al. The influence of aethiology on inflammatory and neurohumoral activation in patients with severe heart failure: A prospective study comparing Chagas' heart disease and idiopathic dilated cardiomyopathy. Eur J Heart Failure 2005;7:869-873. [ Links ]
6. Riarte A, Luna C, Sabatiello R, et al. Chagas' disease in patients with kidney transplants: 7 years of experience 1989-1996. Clin Infec Dis 1999;29;3:561-567. [ Links ]
7. Altclas J, Sinagra A, Dictar M et al. Chagas' disease in bone marrow transplantation: an approach to preemptive therapy. Bone Marrow Transplant 2005;36;2:123-129. [ Links ]
8. Bocchi EA, Bellotti G, Mocelin A et al. Heart transplantation for chronic Chagas' heart disease. Ann Thorac Surg 1996;61:1727-33. [ Links ]
9. Bocchi EA, Bellotti G, Uip DE et al. Long-term follow-up after heart transplantation in Chagas' disease. Transplantation Proc 1993;25:1329- 1330. [ Links ]
10. Carvalho VB, Sousa EFL, Vila JHA et al. Heart transplantation in Chagas' disease. Circulation 1996;94:1815-1817. [ Links ]
11.Vagelos R, Fowler MB. Selection of patients for cardiac transplantation. Cardiol Clin 1990;8:23-28. [ Links ]
12. Bocchi EA, Fiorelli AI, on behalf of the First Guidelines Group for Heart Transplantation of the Brazilian Society of Cardiology. The paradox of survival results after heart transplantation for cardiomyopathy caused by Trypanosoma cruzi. Ann Thorac Surg 2001;71:1833-1838. [ Links ]
13. Sheil AG. Cancer after transplantation. World J Surg 1986;10:389-396. [ Links ]
14. Maldonado C, Albano S, Vettorazzi L, et al. Using polymerase chain reaction in early diagnosis of re-activated Trypanosoma cruzi infection after heart transplantation. J Heart Lung Transplant 2004;12:1345-1348. [ Links ]
15. Diez M, Favaloro L, Bertolotti A et al. Usefulness of PCR strategies for early diagnosis of Chagas' disease reactivation and treatment follow-up in heart transplantation. Am J Transplant 2007;7:1633-1640. [ Links ]
16. Aretz HT. Myocarditis: the Dallas criteria. Human Pathol 1987;18:619-624. [ Links ]
17. Almeida DR, Carvalho AC, Branco JN, et al. Chagas' disease reactivation after heart transplantation: efficacy of allopurinol treatment. J Heart Lung Transplant 1996;10:988-992. [ Links ]
18. Bacal F, Silva CP, Bocchi EA et al. Mychophenolate mofetil increased Chagas' disease reactivation in heart transplanted patients: comparison between two different protocols. Am J Transplant 2005;5:2017-2021. [ Links ]
19. Bestetti RB, Souza TR, Lima MF, et al. Effects of a mycophenolate mofetil-based immunosuppressive regimen in Chagas' heart transplant recipients. Transplantation 2007;84;3:441-442. [ Links ]














