Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.22 no.2 Buenos Aires set. 2009
ARTÍCULOS ORIGINALES
Changes in saliva protein composition in patients with periodontal disease
Myriam A. Koss1, Cecilia. E. Castro2, Karina M. Salúm1, María E. López1
1 Department of Biochemistry,
2 Department of Periodontology, Faculty of Dentistry, National University of Tucumán, Argentina.
CORRESPONDENCE Prof. Myriam Adriana Koss Catedra de Quimica Biologica. Facultad de Odontologia. UNT Av. Benjamin Araoz 800 (4000) - San Miguel de Tucuman - Argentina Phone: 54-381-4107317 / Fax: 54-381-4227589 E-mail: myriam.koss@odontologia.unt.edu.ar
ABSTRACT
Periodontitis is a chronic inflammatory disease characterized by tissue destruction which is usually diagnosed through clinical and radiographic signs. The detection of changes in the chemical composition of saliva could be used to reflect gingivo- periodontal alterations. The aim of this study was to identify salivary parameters that could identify different stages of the periodontal disease. The study group included 118 adults, 89 of them with mild, moderate or severe chronic periodontitis. The remaining participants comprised the control group. Total saliva was analyzed for physical and chemical properties. Dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was used for protein detection and zymography for type IV collagenase identification. Salivary flow rate, pH and buffer capacity showed similar values in all groups. Proteins were augmented in severe periodontitis, as also shown by SDS-PAGE. Hydroxyproline rose significantly in all periodontal groups as secretory Immunoglobulin A significantly diminished compared with the control group. An increase in peroxidase was detected in moderate and severe periodontitis. All salivary samples contained 200-116-92 kDa gelatinases; minor bands at 66-31 kDa were also present in all periodontitis groups. Calcium levels showed significant differences between all periodontitis groups compared with the control group. Quantitative changes in the chemical composition of the saliva of patients with periodontal disease could be of significance in the diagnosis and progression of periodontal disease.
Key words: Whole saliva; Periodontal disease.
RESUMEN
Cambios en la composición proteica de la saliva en pacientes con enfermedad periodontal
La Periodontitis es una enfermedad inflamatoria crónica caracterizada por destrucción tisular que se diagnostica generalmente a través de signos clínicos y radiográficos. Sin embargo la detección de cambios en la composición química de la saliva podrían ser empleados para reflejar alteraciones gingivo-periodontales. El objetivo de este estudio fue encontrar parámetros salivales que pudieran identificar diferentes estadios de la enfermedad periodontal. El grupo de estudio incluyo 118 adultos, 89 de los cuales con enfermedad periodontal crónica leve, moderada o severa El resto constituyo el grupo control. Se analizaron las propiedades físicas y químicas de la saliva total. La separación de proteínas fue realizada empleando la electroforesis en geles de poliacrilamida conteniendo dodecil sulfato de sodio y la identificación de colagenasa tipo IV se realizo empleando la zimografia. Flujo salival, pH y capacidad bufferante mostraron valores similares en todos los grupos. Las proteínas estuvieron aumentadas en la periodontitis grave lo cual también se mostró por medio de la electroforesis. Hidroxiprolina aumento significativamente en todos los grupos de pacientes con enfermedad periodontal, mientras IgA secretoria se encontró significativamente disminuida respecto al grupo control. Un incremento en los valores de peroxidasa se detecto en las periodontitis moderada y grave. Todas las muestras contenían gelatinasas de 200-116-92 kDa; sin embargo todos los grupos de pacientes con enfermedad periodontal también presentaron bandas de peso molecular menor (66-31 kDa). Los niveles de calcio mostraron diferencias significativas entre todos los grupos de pacientes con periodontitis cuando se los comparo con el grupo control. Cambios cuantitativos en la composición química de la saliva de pacientes con enfermedad periodontal podrían tener significancia en el diagnostico y progresión de la enfermedad periodontal.
Palabras clave: Saliva total; Enfermedad periodontal.
INTRODUCTION
Saliva is described as a heterogeneous fluid constituted by proteins, glycoproteins, and electro lytes. It has been suggested that individual variations in salivary composition may influence oral health1.
Periodontal diseases and caries are plaque-associated dental diseases2. Periodontal diseases are chronic inflammatory entities characterized by infiltration of leukocytes, loss of connective tissue and alveolar bone resorption. Proteolytic enzymes released by the host cells are associated to tissue destruction. In this way, matrix metalloproteinases (MMPs) can degrade most of the extracellular matrix components3. The main source of type IV collagenase would be polymorphonuclear cells (PMN) that enter the oral cavity through the gingival sulcus4,5. Collagen breakdown in connective tissue can be measured by hydroxyproline (Hyp) determination6.However, no chemical determinations have been reported in total saliva of periodontal patients. Total proteins in saliva are approximately 3% of those present in plasma. When analyzed by Dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), visual inspection revealed more than 40 protein bands7. The major immunoglobulin type in saliva is secretory Immunoglobulin A (sIgA). Previous investigations determined that it increases in periodontal disease and suggested that sIgA plays an important role in oral mucosa defence8. Many biochemical systems such as salivary peroxidase are known to be involved in softtissue repair and bacterial attack9. The major components of the peroxidase system complex include different forms of lactoperoxidase secreted by the salivary glands, and mieloperoxidase produced by PMN in gingival crevicular fluid10. Mucins are also glycoproteins that can be isolated from total saliva and have shown to have antimicrobial activity. In this way, their alteration could also contribute to develop periodontal disease.2 Glucose has not been described in periodontitis.
The buffering action of saliva may help to prevent demineralization. Some investigations showed a positive correlation between high salivary calcium content and periodontitis11. Salivary flow rate, pH and buffer capacity were analyzed in relation to calculus formation. When total salivary flow increases, calcium concentration also increases12. Therefore, the currently accepted concept is that periodontitisaffected subjects have a high intraoral mineralization potential13.
Although salivary parameters in patients with periodontal disease have been described; no associations with the different states of periodontitis have been demonstrated yet. The aim of the present study was to identify a combination of salivary markers that exhibit statistically significant differences between patients with varying degrees of chronic periodontitis and periodontitis- free subjects. Having identified these parameters, they could be used as a tool for diagnosis and disease progression even in the early stages, given that chemical alterations should occur before macroscopic tissue changes are evident.
MATERIALS AND METHODS
Patient Selection
The study was performed on a group of patients with chronic periodontitis and a control group of periodontitis- free subjects. The control (C) group was composed of 29 subjects (14 males and 15 females) aged 45.9 ± 10.6 years. Patients were classified according to their clinical diagnosis, according to Lindhe (1998)14 in mild periodontitis (MiP) (n=29) (17 males and 12 females) aged 42.6 ± 0.6 years, moderate periodontitis (MoP) (n=34) (18 males and 16 females) aged 40.8 ± 10.5 years and severe periodontitis (SP) (n=26) (12 males and 14 females) aged 47.2 ± 11.2 years, as shown in Table 1.
Table 1: Characteristics Associated to Control Subjects and to the Clinical and Radiographic Diagnoses of Mild, Moderate and Severe Chronic Periodontitis Groups

Patient selection was based on preliminary screening. Inclusion criteria were as follows: 1) at least 2 sites with > 4 mm of attachment loss in each quadrant; 2) a minimum of 20 natural teeth excluding third molars; 3) no systemic medical affections that might impact directly on periodontal status; 4) no history of antibiotic use or periodontal treatment. Inclusion criteria 2-4 applied to the control group. Subjects were selected only according to the inclusion and the exclusion criteria from those who attended the Faculty of Dentistry, National University of Tucuman, for periodontal consultation. Written informed consents were obtained from all individuals prior to their participation.
Clinical Parameters
Clinical diagnoses were determined by a calibrated investigator and the following parameters at the sampled sites were considered: Gingival Index (GI)15, Plaque Index (PI)16, probing pocket depth (PD), bleeding on probing and attachment loss level. Bone resorption in chronic adult periodontitis was determined on the basis of clinical and radiographic criteria; periapical radiographs were taken using a standardized long-cone paralleling technique.
Saliva Collection
Patients were instructed not to eat or drink for 2 h prior to sample collection. Unstimulated whole saliva was collected at the initial visit, previous to the periodontal treatment, between 8-10 am for 5 min. Saliva accumulated in the antero-vestibular and sublingual region of the mouth was aspired avoiding contact with the mucosa17. Then saliva was placed in a glass tube on ice. Volume, pH, and buffer capacity were determined. Saliva was centrifuged at 10,000 rpm for 10 min at 4°C and immediately frozen at -20°C until chemical determinations were performed.
Physical and Chemical Determinations
pH was measured with a digital pHmeter (Broadley- James Corp. Irvine, California, USA) and buffer capacity was determined by the method described by Ericsson18 modified for small volumes. Determinations included total proteins by the colorimetric method of Lowry et al. (1951)19 as determined by other authors20, mucins by the method described by Mehl et al. (1949)21, peroxidase as described by Mansson-Rahemtulla et al. (1986)22 and Hyp as described by Jamall et al. (1981)23. sIgA was determined by radial immunodiffusion (Diffu Platte Lab, Argentina). Salivary calcium and phosphorus were determined by colorimetric methods (Wiener Lab, Argentina), and glucose was assessed by the glucose-oxidase method (Wiener Lab, Ar - gentina). SDS-PAGE was performed according to the method described by Schwartz et al. (1995)7 for salivary samples using a 12.5% resolving gel and a 4.0% stacking gel. Gels were fixed and stained in a solution of 0.1% Coomassie Brilliant Blue R-250. Collagenases were analyzed by zymography using the method described by Ingman et al. (1994)24. 10 μl samples of saliva were loaded in each lane of a 12% SDS-PAGE containing 1mg/ml gelatin as the substrate. Collagenase activity was identified on the gel as light bands on a dark background. SDSPAGE and zymographies were performed with at least 10 individual samples.
Statistical Analysis
The data were analyzed with a SPSS system. Differences among groups were analyzed by ANOVA One Way test. When the differences were significant, the Tukey–test was used.
RESULTS
None of the determinations showed statistical differences between sexes. Volume, pH and buffer capacity did not evidence statistically significant variations between periodontally-affected and periodontitis- free subjects (Table 2).
Table 2: Means ± SE of pH, Buffer Capacity and Volumen Total / minute in Total Saliva of Control and Chronic Periodontitis Groups
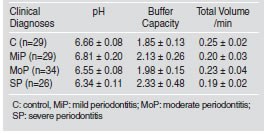
Results of the chemical determinations are shown in Fig 1. Total proteins were significantly higher in the SP group than in the MiP, MoP and C groups (p<0.001) (Fig. 1A). Mucins were augmented in the MiP and MoP groups compared with SP patients (p<0.05). However, differences with the C group were not significant (Fig. 1B). Peroxidase was augmented in MoP and SP compared with the MiP and C groups (p<0.05) (Fig. 1C). Hyp was significantly higher in all periodontitis groups than in the C group (p<0.001) (Fig. 1D). The sIgA level was statistically diminished in all periodontitis groups compared with the C group, and no significant differences were detected between periodontitis groups (Fig. 1E).
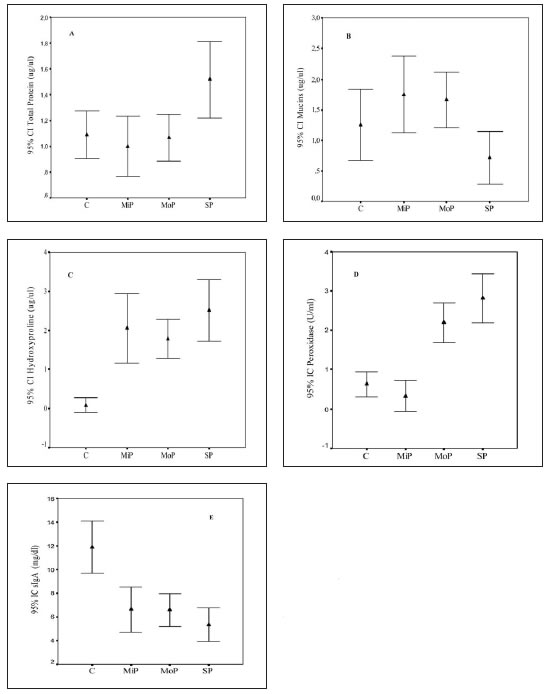
Fig. 1: Means and standard error of salivary chemical determinations of the control (C) and mild (MiP), moderate (MoP) and severe (SP) chronic periodontitis groups. A. Total proteins (mg/ml); B. Mucins (mg/ml); C. Peroxidase (U/ml); D. Hydroxyproline (mg/ml); E. sIgA (mg/dl).
SDS-PAGE of salivary samples from periodontal patients showed a number of protein bands that increased with the degree of the periodontal disease compared to C subjects (Fig. 2 A-B). These proteins were especially localized in the upper third of the gel. Saliva of all groups, including C, contained 200-116-92 kDa gelatinases. Other gelatinolytic bands were also observed in the region of 66-31 kDa in all periodontitis groups (Fig. 3). Calcium levels showed significant differences between all periodontitis groups compared with C (p<0.001) (Fig. 4A); differences were also detected between SP and MiP and MoP (p<0.001). Phosphorus levels were significantly higher in the SP and MoP groups compared with the C group (p<0.05) (Fig. 4B). Glucose showed no differences between all periodontitis and C groups (data not shown).
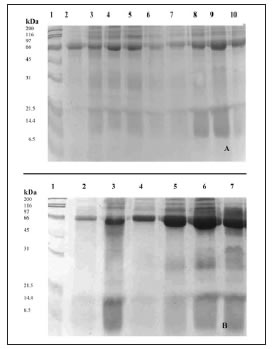
Fig. 2: SDS-PAGE of saliva A. Control group (C) (lines 2-10) and B mild (MiP) (lines 2-4), moderate (MoP) (lines 5-6) and severe (SP) (lines 7) chronic periodontitis groups. Molecular size markers (lines 1 A and B) are included.
Fig. 3: SDS–PAGE of the gelatinolytic activity of saliva of the control (C) (lines 2-3) and mild (MiP) (lines 4-5), moderate (MoP) (lines 6-8) and severe (SP) (lines 9-10) chronic periondontitis groups. Molecular size markers (line 1) are included.

Fig. 4: Means and standard error of salivary ions of the control (C) and mild (MiP), moderate (MoP) and severe (SP) chronic periodontitis groups. A. Calcium (mg/dl) B. Phosphorus (mg/dl).
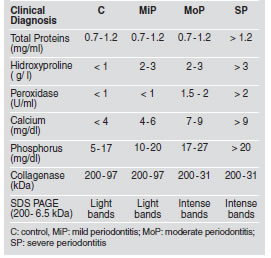
Table 3 expresses the range of values of the salivary chemical parameters that were statistically altered in relation to the different stages of the perio dontal disease. The healthy state could be distinguished from the different states of the disease by hydroxiproline, sIgA, calcium, and the high molecular weight bands of collagenases. The determination of the enzyme peroxidase, calcium and phosphorus could distinguish MiP from MoP and SP, while SP could be distinguished from MoP by the high values of total proteins, calcium and the low levels of sIgA.
Table 3: Concentration Ranges for Total Proteins, Hidroxyproline, Peroxidase, Calcium and Phosphorus in Total Saliva of Control and Chronic Periodontitis Groups
DISCUSSION
For many years, assessment of salivary flow rate and composition has been used to understand variations in oral diseases. Saliva has become a topic of increasing interest as compared with other biological fluids such as blood and urine. Within this context saliva analysis would constitute a contributory aid in clinical diagnosis of active periodontitis, assessment of risk factors and evaluation of disease progression. In this study the chemical composition of total saliva of patients with chronic periodontal disease was analyzed. Patients were classified according to their clinical symptoms, according to Lindhe (1998)14 in three groups: MiP, MoP and SP. In this study, gelatinase activity increased in all periodontal patients, as evidenced by the corresponding bands of type IV collagenase in the 31-66 kDa region in keeping with Ingman et al. (1994)24. Bands were also detected in the area of high molecular weight, both in healthy individuals and in periodontal patients. They could correspond to polymerized or complex enzymes bonded by disulfide bridges present in saliva25. The 92-109 kDa gelatinase bands could be derived from neutrophils, which are the main cellular source of a 92 kDa gelatinase or MMP-9, that has also been shown to be synthesized by epithelial and bone cells26, and gingival granulation- tissue fibroblasts27. The bands of 69-80 kDa are forms represented by gelatinase or MMP-2 originated from fibroblasts, macrophages or bone cells26. The low molecular weight forms could result from the action of proteolytic enzymes or of multiple stages of an autocatalytic cascade28.
In the present study, Hyp values significantly differed between the periodontitis and the C group. Considering that the ages of all groups were similar, tissue destruction and our results of Hyp and collagenase determination evidence that collagen would be the main protein that increases with periodontal damage. However, these results on total basal saliva contrast with data from other studies on stimulated saliva, which revealed no differences in the protein content of patients with periodontitis compared to healthy subjects29. Akalin et al. (1992)6 found that Hyp levels in gingival tissue and in GCF from periodontally healthy subjects in different age groups were higher in the younger group than in the older one. However, there are no references in the literature that salivary Hyp variations were detected in different stages of periodontitis. Total proteins level in saliva of patients with severe forms of periodontitis was significantly higher than in the C group. Values for controls, around 1.0 mg/ml, were similar to those reported by other authors20,30, and those around 1.6 mg/ml in subjects with periodontal disease29 were similar to those in this study for the SP group. This rise is partly due to the contribution of proteins from GCF, since its increase toin whole saliva varies according to the degree of gingival inflammation15. In previous studies we demonstrated that GCF of patients with periodontitis has a high protein content31. It was also demonstrated that plasmatic protein concentration in dental pellicle samples increased 2-10 times from healthy to inflamed conditions3. The rise of proteins was also evident through SDS-PAGE of salivary samples from periodontal patients. SDS-PAGE for C was coincident with those reported by Schwartz et al. (1995)7.
Regarding salivary proteins with antimicrobial activity, significant differences compared to the C group were found in our study for peroxidase of patients with MoP and SP, but not for sIgA. One of the most important functions of salivary peroxidase is the control of oral bacteria that form dental plaque and of the imbalance in the buccal ecology10. A high level of peroxidase activity in saliva is described for periodontitis patients concomitantly with an increase in the gingival index22. As for the other determinations, no associations with the different stages of periodontitis were demonstrated before. Periopathogenic bacteria give rise to the degranulation of leucocytes resulting in the release of myeloperoxidase32. Its activity is increased in gingival fluid during inflammation and this increase may be reflected in whole saliva33. These authors reported similar values of peroxidase activity (0.65±0.43 U/ml) for subjects with periodontitis to those in our study for MiP. The authors recommended caution in the interpretation, since these values represent a single-point measurement and they do not necessarily reflect the status of the disease process. We also found even higher salivary values of peroxidase activity in MoP and SP. In the present study, sIgA levels evidenced the loss of the healthy state; other authors33, 34 reported variations (8±4 mg/dl) that are similar to those in this study for subjects with MiP, MoP and SP.
From our results it seems that in the SP the immune control could be much less efficient than in the MiP and MoP. The increase in mucins was not particular to a specific stage of periodontitis. In relation to the antibacterial function of saliva it is suggested that specifically MG2 is likely to be an important component of the innate immune system. The increase of MG2 could be due to the immune imbalance that characterizes the periodontal disease35. These proteins can form homo and heterotropic complexes with other proteins such as sIgA36, which favor bacteria agglutination2. High calcium concentration of saliva seems to be a characteristic feature of periodontitis affected subjects, since people with a high content of intraoral calcium could be susceptible to develop calculus and periodontal disease, regardless of the periodontal treatment37. Our results and those of other groups29,38 are similar for the C (4.96±1.04 mg/dl) and for the MiP and MoP groups (7.17±1.55 mg/dl). However, higher values were recorded for the SP saliva. In this study, significant differences in phosphorus levels between the SP and the C groups were obtained, unlike other authors29,38 who found no significant differences between subjects with (10.55±2.16 mg/dl) and without (9.24±1.05 mg/dl) periodontitis. Our results would evidence that in the SP, as occurred with plasmatic proteins, calcium and phosphorus could originate in blood as a consequence of advanced tissue destruction.
Regarding pH, salivary volume per minute and buffer capacity, no differences were observed between groups. Results obtained in our study for salivary flow rate and pH in C subjects and periodontal subjects were in agreement with other authors13,33. However, the SP group showed less salivary flow rate than the C group, although the difference was not significant29,37. Regarding the buffer capacity in healthy subjects and in subjects with periodontitis we found lower values than those reported by Grahn et al. (1998)33 and Sewon et al. (1990)29, perhaps due to the use of a different method of determination. Although many studies analyze the composition of saliva in relation to the periodontal health state, the issue of the different degrees of the periodontal affection has not been addressed to date. The present study provides evidence for the association between some chemical parameters and the presence of periodontal disease, regardless of the status of the disease. The present data reveal that certain parameters vary with the progression of the affection. The periodontal disease state could be distinguished from the healthy state through Hyp, sIgA determinations and the light molecular weight bands of collagenases. The determination of the enzyme peroxidase, total proteins, calcium and phosphorus could be used as progression markers of the disease since their values rose from the MiP to the SP. Chemical components may not only be identified but also quantified in order to understand the biological properties of saliva in the pathobiology of oral diseases. The same factors should be investigated after treatment in further studies. Although this is not an epidemiological study, and the daily dental practice is not usually associated to the biochemical investigation, there are many reasons to think that Dentistry and Periodontology could take advantage of this knowledge and apply it to follow the patients with periodontal disease.
ACKNOWLEDGEMENTS
This study was partially supported by a grant from CIUNT (Consejo Nacional de Investigaciones Cientificas y Tecnicas of Universidad Nacional de Tucuman).
1. FDI Working Group 10, CORE. Saliva: its role in health and disease. Int Dent J 1992;42:291-304. [ Links ]
2. Giuliana G, Dangelo M. Saliva e malattia parodontale. Estratto da Minerva Stomatol 1996;45:101-111. [ Links ]
3. Rudiger S, Charlen A, Meurman JH, Kari K, Olsson J. Dental biofilms at healthy and inflamed gingival margins. J Clin Periodontol 2002;29:524-530. [ Links ]
4. Castro CE, Koss MA, Lopez ME. Biochemical markers of the periodontal ligament. Med Oral 2003;8:322-328. [ Links ]
5. Overall, CM. Sodek, J Mc Culloch, CAG, Birek, P. Evidence for polymorphonuclear leukocyte collagenase and 92- kilodalton gelatinase in gingival crevicular fluid. Infect Immun 1991;59:4687-4692. [ Links ]
6. Akalin FA, Sengun D, Renda N, Eratalay K, Caglayan G. Hydroxyproline and total protein levels in gingival fluid in periodontally healthy human subjects. J Nihon Univ Sch Dent 1992;34:172-177. [ Links ]
7. Schwartz SS, Zhu WX, Sreebny LM. Sodium dodecyl sulphate- polyarylamide gel electrophoresis of human whole saliva. Arch Oral Biol 1995;40:949-958. [ Links ]
8. Barr-Agholme M, Dahllof G, Modeer T, Erik-Engstrom P, Engstrom GN. Periodontal condition and salivary immunoglobulins in individual with Down syndrome. J Periodontol 1998;69:1119-1123. [ Links ]
9. Mac Culloch CAG. Host enzymes in gingival crevicular fluid as diagnostic indicators of periodontitis. J Clin Periodontol 1994; 21: 497-506. [ Links ]
10. Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. The antioxidant capacity of saliva. J Clin Periodontol 2002;29:189-194. [ Links ]
11. Sewon L, Laine M, Karjalainen S, Doroguinskaia A, Lehtonen- Veroma M. Salivary calcium reflects skeletal bone density of heavy smokers. Arch Oral Biol 2004;49:355-358. [ Links ]
12. Dawes C, Mac Pherson LM. The distribution of saliva and sucrose around the mouth during the use of chewing gum and the implications for the site specificity of caries and calculus deposition. J Dent Res 1993;72:852-857. [ Links ]
13. Pattanaporn K, Navia JM. The relationship of dental calculus to caries, gingivitis, and selected salivary factors in 11-to 13-year-old children in Chiang Mai, Thailand. J Periodontol 1998;69:955-961. [ Links ]
14. Lindhe J. Examination of patients with periodontal disease. In: Clinical periodontology and implant dentistry. 3 th Edition.: pp 383-395;1998. Munksgaard Copenhagen. [ Links ]
15. Loe H, Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol Scand 1963;21:533-551. [ Links ]
16. Silness J, Loe H. Periodontal disease in pregnancy. II Correlation between oral hygiene and periodontal conditions. Acta Odontol Scand 1964;22:121-135. [ Links ]
17. Lopez ME, Colloca ME, Paez RG, Schallmach JN, Koss MA, Chervonagura A. Salivary characteristics of diabetic children. Braz Dent J 2002;14:26-31. [ Links ]
18. Ericsson Y. Clinical investigation of the salivary buffering action. Acta Odontol Scand 1959;17:131-165. [ Links ]
19. Lowry OH. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265-275. [ Links ]
20. Karjalainen S, Karjalainen M, Forrester D. Physiologic variation of sucrase activity and microbial counts in human saliva. Scand Dent Res 1992;100:111-116. [ Links ]
21. Mehl JW, Golden F, Winzler RJ. Mucoproteins of human plasma; electrophoretic demonstration of mucoproteins in serum at pH 4.5. Proc Soc Exp Biol Med 1949;72,110-114. [ Links ]
22. Mansson-Rahemtulla B, Baldone DC, Pruit KM, Rahemtulla F. Specific assays for peroxidase in human saliva. Arch Oral Biol 1986;31:661-668. [ Links ]
23. Jamall IS. A simple method to determine nanogram levels of 4-hydroxiproline in biological tissues. Analyt Biochem 1981;112:70-75. [ Links ]
24. Ingman T, Sorsa T, Lindy O, Koski H, Konttinen YT. Multiple forms of gelatinases/type IV collagenases in saliva and gingival fluid of periodontitis patients. J Clin Periodontol 1994;21:26-31. [ Links ]
25. Kivela-Rajamaki M, Maisi P, Srinivas R, Tervahartiala T, Teronen O, Husa V, Salo T. Sorsa T. Levels and molecular forms of MMP-7 (matrilysin-1) and MMP-8 (collagenase- 2) in diseased human peri-implant sulcular fluid. J Periodont Res 2003;38:583-590. [ Links ]
26. Lorenzo JA, Pilbeam CC, Kalinowski JF, Hibbs MS. Production of both 92- and 73- kDa gelatinases by bone cells. Matrix 1992;12:282-290. [ Links ]
27. Makela M, Salo T, Uitto VJ, Larjava H. Matrix Metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. J Dent Res 1994;73:1397-1406. [ Links ]
28. Grant G.A, Goldberg GI, Willhelm S.M, He C, Eisen AZ. Activation of extracellular MMPs by proteases and organomercurials. Matrix 1992;1:217-223. [ Links ]
29. Sewon L, Makela M A study of possible correlation of high salivary calcium levels with periodontal and dental conditions in young adults. Arch Oral Biol 1990; 35 suppl. 211S-212S. [ Links ]
30. Lenander-Lumikari M, Johansson I, Viljja P, Samaranayake LPN. Newer saliva collection methods and saliva composition: a study of two SalivetteR kits. Oral Dis 1995; 1:86-91. [ Links ]
31. Koss MA, Lopez ME, Castro CE, Loi JA, Chervonagura A Gingival fluid and saliva in serious and complicated periodontitis. J Dent Res 2000;79:1015. [ Links ]
32. Jentsch H, Sievert Y, Gocke R. Lactoferrin and other markers from gingival crevicular fluid and saliva before and after periodontal treatment. J Clin Periodontol 2004; 31:511-514. [ Links ]
33. Grahn E, Tenovuo J, Lehtonen OP, Eerola E, Vilja P. Antimicrobial systems of human whole saliva in relation to dental caries, cariogenic bacteria, and gingival inflammation in young adults. Acta Odontol Scand 1988;46:67-74. [ Links ]
34. Jalil RA, Ashley FP, Wilson RF, Wagaiyu EG. Concentrations of thiocyanate, hipothiocyanate, free and total lysozyme, lactoferrin and secretory Ig A in resting and stimulated whole saliva of children aged 12-14 years and the relationship with plaque accumulation and gingivitis. J Periodont Res 1993;28:130-136. [ Links ]
35. Soares RV, Siqueira CC, Bruno LS, Oppenheim FG, Offner GD. MG2 and lactoferrin form a heterotypic complex in salivary secretion. J Dent Res 2003;82:471-475. [ Links ]
36. Biesbrock AR, Reddy MS, Levine MJ. Interaction of a salivary mucin-secretory inmunoglobulin A complex with mucosal pathogens. Infect Immun 1991;59:492-497. [ Links ]
37. Sewon LA, Karjalainen S, Sainio M, Seppa O. Calcium and other salivary factors in periodontitis-affected subjects prior to treatment. J Clin Periodontol 1995;22:267-270. [ Links ]
38. Sewon LA, Karjalainen SM, Soderling E, Lapinleimu H, Simell O. Associations between salivary calcium and oral health. J Clin Periodontol 1998;25:915-919. [ Links ]














