Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Odontológica Latinoamericana
versão On-line ISSN 1852-4834
Acta odontol. latinoam. vol.22 no.3 Buenos Aires dez. 2009
ARTÍCULOS ORIGINALES
Efficacy of 35% hydrogen peroxide on human enamel: in vitro evaluation in different tooth areas
Max José Pimenta Lima1, Danilo Barral Araújo2, Elisângela de Jesus Campos2, Roberto Paulo Correia de Araújo2
1 Dentistry School of Bahiana School of Medicine and Public Health, Brazil.
2 Institute of Health Sciences of Federal University of Bahia, Brazil.
CORRESPONDENCE Roberto Paulo Correia de Araujo Av. Reitor Miguel Calmon s/n, 4o. andar –sala 400 Vale do Canela CEP: 40110-902 - Salvador, Bahia, Brazil e-mail: rpcaraujo@hotmail.com
ABSTRACT
This study aimed to assess the efficacy of a 35% hydrogen peroxide – based gel without activation in vitro on three areas of the tooth surface. Vestibular faces of human premolar teeth were darkened, followed by two whitenings at 7-day intervals. The efficacy of whitening was determined in the cervical third, medium third and incisal third of the tooth surface with an Easyshade-Vita spectrophotometer based on the CIELab system. The L*, a*, b* parameters were determined for each third by the identification of high luminosity and hues tending to green and yellow; pigmentation luminosity was then reduced, and the parameters a* and b* became reddish and yellowish, respectively. Seven days after the first whitening, there were significant improvements in L* and a* values. Seven days after the second whitening, the three parameters returned to values close to the initial values; the b* parameter was most strongly correlated with whitening efficacy. DE values revealed a visually perceptible difference. There was a satisfactory removal of pigmentation after both whitenings, while the lack of uniformity among the tooth-surface thirds after the first session justified the performance of two whitening procedures. With regard to each third, DE indicated a visibly perceptible difference, although L*, a* and b* values showed no statistically significant differences.
Key words: Teeth whitening; Hydrogen peroxide; Darkening.
RESUMO
Eficacia do peroxido de hidrogenio a 35% sobre o esmalte dentario humano
Objetivo: Avaliar, in vitro, a eficacia do gel do peroxido de hidrogenio a 35%, sem ativacao, nos tres tercos dentarios. Faces vestibulares de dentes pre-molares humanos foram escurecidos, seguindo-se de duas sessoes de clareamento com intervalos de 7 dias. A eficacia do clareamento foi determinada no terco cervical, terco medio e terco incisal pelo espectrofotometro Easyshade-Vita, com base no sistema CIELab. Os parametros L *, a *, b * determinados em cada um terco revelaram alta luminosidade e tendencia aos tons verde e amarelo; apos a pigmentacao, a luminosidade foi reduzida e os parametros a* e b* revelaram as tonalidades avermelhada e amarelada. 7 dias apos o primeiro clareamento houve uma melhora significativa dos parametros L * e a*. 7 dias apos o segundo clareamento, os tres parametros retornaram aos valores proximos aqueles iniciais, sendo que o parametro b * foi o principal fator responsavel pela eficacia de clareamento. Os valores referentes ao parametro DE indicaram haver percepcao visual. A remocao da pigmentacao foi satisfatoria apos os dois clareamentos, sendo que a falta de uniformidade entre os tercos apos a primeira sessao justificou a realizacao de dois procedimentos de clareamento. Em relacao a cada terco, o parametro DE mostrou percepcao visual, embora os valores de L*, a* e b* nao demonstraram diferencas estatisticamente significativas.
Palavras chave: Clareamento dentario; Peroxido de hidrogenio; Escurecimento.
INTRODUCTION
Attention to aesthetic rehabilitation in dentistry has become a growing concern worldwide. However, having white, well-formed, well taken care of and well-aligned teeth requires more than attendance to aesthetic requirements to the extent that such conditions are important indicators of oral health. Exogenous dental staining is caused by ingestion of food and beverages containing coloring substances such as tea, coffee, cola-based sodas, mate, red wine, and beets. Exogenous dental staining is also associated with the deposition of dental plaque1. Tooth whitening is the recommended treatment for these enamel alterations2.
As a dentistry procedure designed for dental rehabilitation, whitening procedures have spread widely due to their benefits with regard to enamel and dental crown preservation. The chemical whitening process consists of an oxidation- reduction reaction; the amount of pigment removed is proportional to the duration of enamel exposure to the whitening agent, within pre-established limits designed to ensure the maintenance of healthy tooth structure3. The darkening and whitening processes are only possible because of the relative permeability of the tooth structures. Thus, the greater the penetration of the whitening substance into the tooth, the greater is the quantity of pigmentation that can be removed, and therefore, the better are the aesthetic results obtained4. The dentifrices containing bleaching agents should be applied to human teeth under the supervision of a professional who can advise patients with regard to brushing technique and recommended duration of treatment application5. The extent of variation in tooth color can be determined through spectrophotometry. In this study, we used the CIELab system due to its previous use in major studies dealing with tooth color determination6- 9. The luminosity of any tooth area depends on the proportions of enamel and dentin structures10, 11. Staining of the tooth surface has multiple causes. The dental enamel is permeable to most substances of low molecular weight12. However, dental surface morphology can predispose this structure to pigment deposition1,3,13. Several studies show the effectiveness of a 35% hydrogen peroxide gel for tooth bleaching14,15,16,17.
Darkening of the teeth results in a reduction in the L * value, while the a * and b * values increase. Low levels of tooth luminosity are indicated via red and yellow colors in the image obtained through spectrophotometry 18. Many studies have emphasized that measurements of pigmentation are more effective in the cervical third of the tooth due to the thickness of enamel in this fragile area9,10,19,20. It can be asserted that parameter b* is the primary indicator of whitening efficacy. The whitening procedure effectively removes yellowish pigmentation in individuals that have more intense tooth pigmentation, as represented by the predominance of a yellow hue in the darkened teeth7,21. The ΔE values used by Dozic et al. (2005) are utilized as a reference in various studies10,11,22,23. Considering the increasing frequency of professionally performed whitening procedures, it is necessary to conduct scientific investigations in order to assess the efficacy of professional use of whitening agents for each tooth third. This study aims to elucidate the efficacy of a 35% hydrogen peroxide gel Whiteness HP (FGM Dental Products – Joinville, SC, Brazil) in bleaching darkened enamel, as determined by the L *, a *, b * and DE parameters.
METHODOLOGY
This study utilized 36 human dental samples donated by the UNIME tooth bank (upper and lower pre-molars). The teeth were kept in physiologic serum, and then cleaned and submitted to a removal of soft tissue residues. Using a carborundum disk linked to a slow rotation motor, the dental crowns were separated from the radicular portion of the tooth and then embedded in orthophitalic resin, to yield the study samples. The samples were separated into three groups of twelve units: an experimental group (ExG), which had its enamel submitted to experimental darkening and to professional whitening; a repetitive group (RG), which received no darkening or whitening treatment; and a darkening stability group (SG), which received only the experimental pigmentation treatment.
The degree of darkening or whitening was determined in cervical, medial and incisal dental thirds with an Easyshade - VitaR Spectrophotometer. A baseline evaluation of the samples was performed (L1) to record each specimen’s original color. GE and GExp groups underwent a darkening procedure during a nonstop 96 hour period and were kept in an incubator at 37oC. This pigmentation procedure consisted of immersing the samples in a mixture containing coffee, black tea, cola-based beverages, red wine and tobacco concentrated solutions in equal parts4. Once the darkening phase was completed, the samples were re-assessed (2nd reading – L2) to determine the coloring after darkening. After darkening, the samples in the experimental group (GExp) were exposed to the action of a whitening gel containing 35% hydrogen peroxide (Whiteness HP – FMG – Dentscare Ltd) for 15 minutes with no activation. This procedure was performed three times, as recommended by the manufacturer. The degree of whitening was determined from readings corresponding to cervical, medial and incisal dental thirds. Once this whitening technical procedure was completed, the samples were kept in a re-mineralizing solution (supersaturated solution with calcium phosphate and with potassium chloride in a buffer solution of TRIS - hydroxymethylaminomethane at pH 7.0) at 37oC in an incubator for seven days. Samples were submitted to three daily brushings (Oral B dental brush, attached to a dynamometer [0.2 kgF])5 with a fluoridated toothpaste (Colgate Maximum Strength Anticavity - Palmolive Ind. and Com. Ltd.). It was thus possible to prevent contact between the samples and any pigmenting or demineralizing substances, as recommended by the manufacturers. After seven days, a new reading (3rd reading – L3) was performed in the spectrophotometer and the whitening procedure was repeated. Once the second whitening procedure was finished, the samples were kept in a remineralizing solution in an incubator at 37oC for an additional seven days. Samples were then submitted to three brushings as previously described. After seven days, a final color assessment was performed (4th reading – L4) for each third. Before performing any experimental analysis, it was necessary to verify whether the methodology was reproducible (Reproducibility Group). Thus, the next step was to determine the color of each sample after 0, 24 and 48 h. During the time intervals, the specimens were kept in de-ionized water in an incubator at 37oC, thus assuring the control of both temperature and environmental pH.
An ANOVA Test was performed to verify if the differences between readings were statistically significant. We also performed a parametric analysis using Tukey’s test at a 5% significance level.
RESULTS
A reproducibility test verified that the readings ascribed to the parameters L*, a* and b* were repeated at 0, 24 and 48 hour time points. These data were confirmed by a variance analysis that indicated no statistically significant differences among the three parameters: L* (F=0.10/p=0.910), a* (F=0.87/p=0.426) and b* (F=0.81/p=0.454).
The degree of stability between both spectrophotometric readings was verified for the pigmentation produced: 1) E1 – after the darkening - L* (56.42±5.15); a* (10.94±1.63); b* (45.53±1.68); 2) E2 – fourteen days after the darkening - L* (56.00±4.47); a* (11.02±1.50); b* (46.20±1.86). No statistically significant differences (p>0.05) were found.
Colorimetric analysis L* Variable
Parameter L* data are presented in Table 1. In the three thirds, a reduction in L* was verified when the data corresponding to the first readings (L1 and L2) were compared. These differences were confirmed as statistically significant (p<0.05). Contrasting the first and third readings (L1 and L3) revealed a statistically significant (p<0.05) difference. However, when contrasting the first and last readings (L1 and L4), we did not observe statistically significant differences (p>0.05) for any third. However, when the values obtained for the second and third readings were compared (L2 and L3), we noticed statistically significant differences among the three thirds. However, a comparison between the second and the fourth readings (L2 and L4) for each third demonstrated statistically significant differences (p>0.05). Finally, a contrast between the third and fourth readings (L3 and L4) for each third demonstrated that the only difference not considered statistically significant (p>0.05) corresponded to the incisal third (63.31±6.00 and 69.55±4.83). With regard to the confidence interval analysis, the data corresponding to the third and fourth readings (L3 and L4) for the incisal third were considered borderline (0.64).
Table 1: Mean, standard deviation and confidence interval for L* values in cervical, medium and incisal thirds.
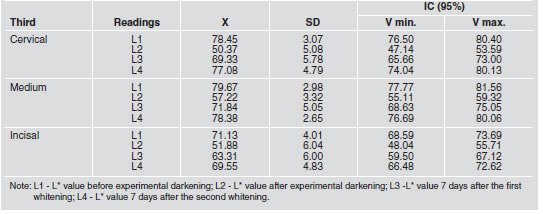
A comparison of L* values determined for the three thirds showed that there was no statistically significant difference (p>0.05) with regard to the specimen’s original color (L1) only among the medium and cervical thirds (78.45±3.07 and 79.67±2.98). This pattern was observed again seven days after the 2nd whitening procedure (77.08±4.79 and 78.38±2.65). Seven days after the first whitening procedure, a statistically significant difference (p<0.05) was verified only between the medium and incisal thirds (71.84±5.05 and 63.31±6.00). As for the L* parameter experimentally assigned to darkened specimens, a statistically significant difference (p<0.05) was observed only between the medium and cervical thirds (50.37 ± 5.08 and 57.22 ± 3.32).
Variable a*
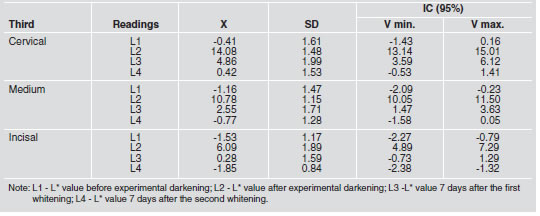
Parameter a* data are presented in Table 2. When comparing parameter a* readings among the three thirds, we found that there was no statistically significant difference (p<0.05) only between the first and fourth readings (L1 and L4), i.e., between a first reading of the specimen’s original color and a second reading seven days after the second whitening procedure (cervical: -0.41±1.61 and 0.42±1.53; medium: -1.16±1.47 and -0.77±1.28; incisal: -1.53±1.17 and -1.85±0.84). Comparing a* values assigned to the three thirds revealed no statistically significant difference (p>0.05) among the three thirds with regard to the specimen’s original color. No statistically significant difference (p>0.05) was found between the medium and cervical thirds for L3 and L4, i.e., seven days after the first whitening (4.86±1.99 and 2.55±1.71) and seven days after the second whitening (0.42±1.53 and -0.77±1.28). We observed no significant difference (p>0.05) between the medium and incisal thirds 7 days after the second whitening (-0.77±1.28 and -1.85±0.84). For the parameter a*, the comparison among experimentally darkened specimens, revealed statistically significant differences (p<0.05) among the readings obtained in the cervical, medium and incisal thirds.
Table 2: Mean, standard deviation and confidence interval for a* values in cervical, medium and incisal thirds.
Variable b*
Data corresponding to the parameter b* are presented in Table 3. Results corresponding to parameter b* indicated the lack of a significant difference (p>0.05) between L1 (original color) and L4 (color seven days after the second whitening procedure) for all thirds (cervical: 34.28±2.33 and 35.58±2.61; medium: 28.28±4.11 and 29.70±2.83; incisal: 22.29±3.73 and 21.72±2.90). This lack of any change in color was also observed when comparing the cervical and incisal thirds for L2 (after experimental darkening) and L3 (seven days after the first whitening procedure). In the medium third, the difference observed when comparing L2 and L3 was statistically significant (p<0.05) (44.67±1.93 and 38.36±2.86). A comparison of b* readings determined for the three thirds shows significant differences (p<0.05) with regard to the specimens’ original colors. The same trend was observed seven days after the first whitening procedure and seven days after the second whitening. As for b* values assigned to experimentally darkened specimens, only a comparison between the medium and cervical thirds (45.19±2.37 and 44.67±1.93) failed to yield a significant difference (p>0.05).
Table 3: Mean, standard deviation and confidence interval for b* values in cervical, medium and incisal thirds.

DE value
DE data are presented in Table 4. The DE value was determined after measurements of L*, a* and b* values. DE numerically expresses a color difference between spectrophotometric measurements of the same material. In this study, varying DE values resulted from the difference between the original dental specimen coloration and the pigmentation following two distinct whitening procedures. Table 4 presents these differences for the cervical, medium and incisal thirds. Color difference, expressed in visual perception units (DE), between the medium and cervical thirds did not reveal significant differences (p>0.05) for any of the comparisons performed. However, analyses between the cervical and incisal thirds yielded significant differences among DE1, DE4 and DE5 values. A comparison of DE values between the medium and incisal thirds yielded significant differences (p>0.05) only as related to DE1 and DE5 values.
Table 4: Mean, standard deviation for ΔE values in cervical, medium and incisal thirds.

DISCUSSION
A test of reproducibility is necessary for effective experimental protocol design. In this study we used the three time points that yielded highly reproducible values. With regard to the darkening stability test results (group GE), no significant difference was observed in L*, a* or b* parameters, from the first to the last day of the experiment. Thus, variations in L*, a* and b* resulted from the application of whitening chemical agents. Inter-specimen consistency was ensured by the maintenance of environment and temperature control, as specimens were stored in a remineralizing solution and kept at 37oC throughout the experiment. We used the CIELab system due to its widespread employment in major studies dealing with tooth color determination6- 9.
The first results obtained for parameter L*, corresponding to the initial readings (L1 [original specimen color]) showed that all thirds had high luminosity values; the medium third showed the highest L* value, followed by the cervical and incisal thirds. Comparisons among the three thirds verified that the L* values were similar between the medium and cervical thirds (p>0.05), indicating equivalent luminosities. However, the relationship between the incisal third and the medium and cervical thirds revealed statistically significant differences. This finding is justified by the fact that the incisal third has a decreased dentine layer and a higher amount of enamel. Light passing through this third goes straight into the inner part of the oral cavity, causing less light absorption in this area of the specimens and consequently less luminosity. Thus, the luminosity of any tooth area depends on the relative proportions of enamel and dentin structures10,11. Parameter a* variations (red - green), as well as parameter b* variations (yellow-blue) are relevant to tooth color determination to the extent that, when values are close to zero, they indicate neutral colors (grey and white); when these values are elevated significantly, they represent saturated and intense hues6,8. Analysis of parameters a* and b* shows that the highest values were found in the cervical third, followed by the medium and incisal thirds. According to these results, natural tooth coloration tends to have green and yellow hues. The parameter a* values recorded were rather uniform across the three thirds (p>0.05). Parameter b* values indicated a different degree of whitening in each third of the tooth. This variation resulted from the differing amounts of dentin in each region of the tooth. Staining of the tooth surface has multiple potential sources and dental enamel is relatively permeable to most low molecular weight substances12. Furthermore, dental surface morphology can predispose this structure to pigment deposition facilitated by superficial roughness, intrinsic porousness, and the presence of cracks, fissures, wrinkles and depressions1,3,13. In this context, our study sought to assess the efficacy of a 35% hydrogen peroxide gel14-17.
Experimentally darkening the specimens resulted in drastically reduced L* values, while a* and b* parameters increased significantly in all thirds. This confirms the specimens’ low luminosity, suggested by acquired in vitro hues close to red and yellow18. All the spectrophotometric readings obtained from the darkened specimens differed significantly from those of the original specimens (L1). Pigmentation was most noticeable in the cervical third because of the thickness of the enamel in this area9,10,19,20.
Comparisons among the darkened thirds show that L*, a* and b* parameters exhibit distinct patterns in each third. Parameter L* differed significantly between the medium and cervical thirds. Parameter a* varied significantly among all thirds, as did parameter b*, except for the case of medium and cervical thirds that did not differ significantly. In this study, the readings performed seven days after the first whitening procedure (L3) showed the efficacy of 35% hydrogen peroxide on the darkened specimens. Among the parameters analyzed, those responsible for the whitening response were L* and a*. These findings allow us to assert that a large increase in luminosity is associated with a decrease in the red hue of each third. Similarly, Gerlach, Gibb and Sagel7 correlate improved L* and b* parameter response with whitening efficacy. Although the protocol utilized by Gerlach, Gibb and Sagel (2002)7 differs in a number of aspects from the protocol adopted in this study, the conclusions of both are similar. In both studies, L* and b* parameters are considered to play a major role in whitening effects after fourteen days. A comparison between specimen readings obtained at baseline and after the first whitening procedure showed that although the whitening was effective, it was not sufficient to return L*, a* and b* parameter readings to initial values. It must be taken into account, however, that the intensity of experimental darkening was extremely stereotyped: the L*, a* and b* values obtained from this in vitro procedure are rarely detected in vivo for human teeth subjected to chemical whitening alternatives. A comparison of the three thirds points to the existence of significant differences between the medium and incisal thirds for parameter L*, seven days after the first whitening procedure. As for parameter a*, no statistically significant differences were found between the medium and cervical thirds, while statistically significant differences were detected for parameter b* and all comparisons performed among the three thirds. These results confirm that the first whitening procedure was not sufficient for complete pigmentation removal. Seven days after the second whitening procedure, parameters L*, a* and b* were very similar to baseline values. Such results indicate a need to perform a second whitening procedure, at least seven days after the first, with the aim of maximizing the reduction of impregnated pigmentation. The goal is to reduce pigmentation enough so that the naked eye cannot perceive the color difference between the original specimens and those that have undergone two consecutive whitening processes. This study aimed to assess the efficacy of the whitening gel under analysis; to this end, the adoption of a 96 hr darkening procedure for in vitro specimens was justified. Had this protocol not been used, whitening could probably have been accomplished with one procedure. It can be asserted that parameter b* is most closely correlated with whitening efficacy after the second whitening procedure, at which point the yellowish pigmentation is effectively removed. These findings demonstrate the importance of prescribing a second whitening procedure for individuals with more intense tooth pigmentation (as represented by the yellow hue of darkened teeth)7,21.
In this study the ΔE values obtained by Dozic et al. (2005) were utilized as reference. In the Dozic et al. investigation, the color difference among treated teeth was clinically perceptible, above 3.0 visual perception units10,11,22,23. Color variation between readings obtained before the darkening and after the second procedure (ΔE3) demonstrated the continued existence of a color difference (cervical: 6.04, medium: 5.22, incisal: 5.11). It must be noted that the ΔE values obtained are considered as visually perceptible. This finding reinforces the importance of deducing ΔE, since residual color differences can only be detected through this constant when L*, a* and b* values for similar groups indicate color homogeneity within the same group.
Our results compel us to assert that L*, a* and b* parameters, despite their standardization, do not accurately reflect uniformity in coloration among the three thirds and, in some cases, even in a particular third of the tooth surface. These inferences justify the importance of performing an analysis of L*, a* and b* parameters both independently (to determine the coloring trend of each tooth) and in an integrated way (to determine ΔE values in comparative studies).
CONCLUSION
In the experimental conditions of this study, and according to the results obtained regarding the efficacy of a 35% hydrogen peroxide gel in whitening human dental enamel, it can be asserted that:
satisfactory removal of darkening pigmentation is achieved after two chemical application procedures with a seven-day interval; after the first application procedure, there is an increase in luminosity (L* parameter) and an elimination of red pigmentation, although some greenish pigmentation still remains (a* parameter); after the second application procedure, a reduction in luminosity (L* parameter) as well as a severe reduction in yellow pigmentation (b* parameter) are observed; the degree of luminosity decreases from the medium to the cervical third and again from the cervical to the incisal third; the reduction in yellow and red coloration decreases from the medium to the cervical to the incisal third; Although DE unities corresponding to cervical, medium and incisal thirds of both original and treated enamel samples present numeric values corresponding to possible visual perception variations in L*, a* and b* values, they were not considered statistically significant.
1. Watts A, Addy M. Tooth discolouration and staining: a review of the literature. Br Dent J 2001;190:309-316. [ Links ]
2. Jones HA, Dias-Arnold MA,Vargas AM, Cobb SD. Colorimetric assessement of layser and home bleaching techiniques. J Esthet Dent 1999;2:87-94. [ Links ]
3. Baratieri LN et al. Odontologia Restauradora: Fundamentos e Possibilidades. Sao Paulo: Santos Livraria Editora, 2001. [ Links ]
4. Gomes LO. Avaliacao de alteracoes cromaticas do esmalte bovino submetido a procedimento de clareamento dental apos descolagem de braquetes ortodonticos. 2005, 108p. Dissertacao (Mestrado em Odontologia). Faculdade de Odontologia – UFBA.
5. Neves A et al. Microstrutural analysis of demineralized primary enamel after in vitro toothbrushing. Pesqui Odontol Bras 2002;16:137-143. [ Links ]
6. Fraser B, Murphy C, Bunting F. Real World Color Management. USA: Peach Press, 2003. [ Links ]
7. Gerlach RW, Barker, ML, Sagel PA. Objective and subjective whitening response of two self-directed bleaching systems. Am J Dent 2000;21:22-28. [ Links ]
8. Joiner A. Tooth colour: a review of the literature. Journal of Dentistry 2004;32:3-12. [ Links ]
9. Kleber CJ, Moore MH, Nelson BJ. Laboratory assessment of tooth whitening by sodium bicarbonate dentifrices. J Clin Dent 1998;9:72-75. [ Links ]
10. Dozic A, Kleverlaan CJ, Aartman IHA, Feilzer AJ. Relation in color of three regions of vital human incisors. Dent. Mater. 2004;20:832-838. [ Links ]
11. Dozic A, Kleverlaan CJ, Aartman IHA, Feilzer AJ. Relation in color among maxillary incisors and canines. Dent Mater 2005;21:187-191. [ Links ]
12. Paiva JG, Antoniazzi JH. Endodontia: Bases para a pratica clinica. 2nd ed. Sao Paulo: Artes Medicas, 1988. [ Links ]
13. Pontefract H, Courtney M, Newcombe RG, Addy M. Development of methods to enhance extrinsic tooth discoloration for comparison of toothpaste. 1. Studies in vitro. J Clin Periodontol 2004;3:1-6. [ Links ]
14. Baratieri LN et al. Clareamento dental. 3rd ed. Sao Paulo: Santos, 1995. [ Links ]
15. Haywood VB, Heymann OH. Nightguard vital bleaching: how safe is it? Quintessence Int. 1991;22:515-523. [ Links ]
16. Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil 1996;23:244-250. [ Links ]
17. Oliver TL, Haywood VB. Efficacy of nightguard vital bleaching technique beyond the borders of the shortened tray. J Esthet Dent 1999;11:95-102. [ Links ]
18. Carvalho and MOF, Robazza CRC, Lage-Marques JL. Analise espectrofotometrica e visual do clareamento dental interno utilizando laser e calor como fonte catalisadora. Pesqui Odontol Bras 2002;16:337-342. [ Links ]
19. Bhaskar SN. Histologia and Embriologia Oral de Orban. 8th ed. Rio Grande do Sul: Artes Medicas, 1978. [ Links ]
20. Hasegawa A, Ikeda I, Kawaguchi S. Color in translucence of in vivo natural central incisors. J Prosthet Dent 2000; 83:418-423. [ Links ]
21. Gerlach RW, Gibb RD, Sagel PA. A randomized clinical trial comparing a novel 5.3% hydrogen peroxide bleaching strip to 10%, 15% and 20% carbamide peroxide tray-based bleaching systems. Compendium of Continuing Education in Dentistry 2002;15:7-12. [ Links ]
22. O’Brien WJ, Groh CL, Boenke KM. A new, small-colordifference equation for dental shades. J Dent Res 1990; 69:1762-1764.
23. Ruyter IE, Nilner K, Moller B. Color stability of dental composite resin materials for crown and bridge veneers. Dent Mater 1987;3:246-251. [ Links ]














