Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Odontológica Latinoamericana
On-line version ISSN 1852-4834
Acta odontol. latinoam. vol.23 no.1 Buenos Aires Apr. 2010
ARTÍCULOS ORIGINALES
Effects of five carbamide peroxide bleaching gels on composite resin microhardness
André L.F. Briso1, Inger T.C. Tuñas2, Letícia C.A.G. de Almeida1, Vanessa Rahal1, Glaucia M.B. Ambrosano3
1 Department of Restorative Dentistry, Araçatuba Dental School–UNESP, Araçatuba, São Paulo, Brazil.
2 Department of Restorative Dentistry, Estácio de Sá University - UNESA, Rio de Janeiro, Rio de Janeiro, Brazil.
3 Department of Community Health, Piracicaba Dental School–UNICAMP, Piracicaba, São Paulo, Brazil.
CORRESPONDENCE Andre Luiz Fraga Briso Rua Jose Bonifacio 1193, Aracatuba, Sao Paulo, Brazil. 16015-050. Phone/fax: +55 18 36363346 e-mail: alfbriso@foa.unesp.br
ABSTRACT
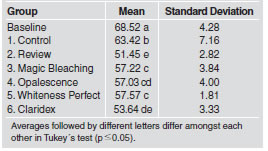
The purpose of this study was to evaluate the effects of five home bleaching products containing 15-16% carbamide peroxide on the microhardness of microhybrid composite resin Z-250 (3M/Espe). A total of 72 specimens were fabricated in cylindrical acrylic matrices (4×2 mm), filled with composite resin and photo-activated for 40 seconds. They were divided in 6 study groups (n=12), according to the bleaching product: Review (SS White), Magic Bleaching (Vigodent), Opalescence (Ultra dent), Whiteness Perfect (FGM), Claridex (Biodinamica), and a control group (not bleached). Specimens were exposed to 1 cc of bleaching gel for 6 hours daily for 2 weeks. The control group specimens were kept in artificial saliva throughout this time. All the specimens were then analyzed in a microhardness tester. Knoop hardness measurements were performed, and the results were submitted to parametric statistical analysis (analysis of variance and Tukey´s test). Mean Knoop values and standard deviation were: baseline, 68.52a (4.28); control, 63.42b (7.16); Whiteness Perfect, 57.57c (1.81); Magic Bleaching, 57.22c (3.84); Opalescen ce, 57.03cd (4.00); Claridex, 53.64de (3.33); Review, 51.45e (2.82). Identical letters mean statistical equality according to Tukey’s test at the 5% significance level. The products significantly decreased Z-250 (3M/Espe) microhardness.
Key words: Hardness; Tooth bleaching; Composite resins.
RESUMO
Efeitos de cinco géis clareadores a base de peróxido de carbamida na microdureza de resina composta
O objetivo deste estudo foi avaliar os efeitos de cinco produtos clareadores a base de peroxido de carbamida 15-16% na microdureza da resina composta microhibrida Z-250 (3M/Espe). Setenta e dois especimes foram confeccionados com o uso de matrizes cilindricas (4×2 mm), preenchidas com resina composta e fotoativadas por 40 segundos. Eles foram divididos em 6 grupos de estudo (n=12), de acordo com o produto clareador utilizado: Review (SS White), Magic Bleaching (Vigodent), Opalescence (Ultra dent), Whiteness Perfect (FGM), Claridex (Biodinamica), e um grupo controle (nao clareado). Os especimes receberam 1 cc de produto clareador por 6 horas diarias durante 2 semanas, e durante todo o tempo eles foram mantidos em saliva artificial. Depois dos procedimentos clareadores todos os especimes foram analisados em um microdurometro. As medidas de dureza Knoop foram submetidas a analise estatistica parametrica (analise de variancia e Teste de Tukey). Os valores de dureza Knoop e seu desvio padrao foram: baseline, 68,52a (4,28); controle, 63,42b (7,16); Whiteness Perfect, 57,57c (1,81); Magic Bleaching, 57,22c (3,84); Opalescen ce, 57,03cd (4,00); Claridex, 53,64de (3,33); Review, 51,45e (2,82). Letras semelhantes significam resultados estatisticos semelhantes segundo o teste de Tukey, nivel de significancia 5%. Os produtos clareadores diminuiram significativamente a microdureza da resina composta microhibrida Z-250 (3M/Espe).
Palavras-chave: Microdureza; Clareamento de dente; Resinas compostas.
INTRODUCTION
One of the main reasons that patients seek esthetic dental treatment is the real or perceived discoloration of anterior teeth1. Therefore tooth whitening is one of the most rapidly growing areas in dentistry2. Tooth bleaching, when properly indicated and performed, is safe, effective, and preserves tooth structure3. Since a publication by Haywood and Heymann (1989)4 introduced nightguard vital bleaching, bleaching has become widespread and increasingly popular. In daily clinical practice, tooth color restorations are frequently observed in teeth that have been bleached5. The restorative filling materials used in dentistry require long-term durability to survive in the oral cavity6. Therefore, it is important for dentists to understand the effects of bleaching agents on the physical properties of restorative materials. Surface hardness is one of the most important physical characteristics of dental materials6. Since hardness is related to a material´s strength, proportional limit, and ability to abrade or to be abraded by contralateral dental structures/materials, any chemical softening resulting from bleaching may have implications for the clinical durability of restorations7. Recent studies5,8 report that the increase or decrease in surface microhardness of resin composite materials after contact with bleaching agents depends on the material composition; thus, some restorative materials may be more susceptible to these alterations. These studies also report that certain bleaching agents are more likely to cause such alterations. For these reasons it is appropriate to investigate the effects of different compositions of bleaching agents on composite resin microhardness, an issue that has not been addressed in detail in the literature to date.
No consensus has been reached regarding the effect of bleaching agents on composite resin microhardness1-3,9,10, suggesting the necessity of further investigation. The purpose of this study was to investigate the effects of five home bleaching products containing 15-16% carbamide peroxide on composite resin microhardness. The null hypothesis considered was that composite resin microhardness would not be affected by bleaching agents.
MATERIALS AND METHODS
Experimental design
A total of 72 specimens were fabricated and divided into 6 study groups (n=12). Twelve of these specimens were assigned to the control group that remained stored in artificial saliva without being exposed to the action of bleaching gels. The other specimens were randomly divided in 5 groups that received 15-16% carbamide peroxide treatment. The bleaching agents used in the study groups are listed in Table 1: Group II- Review (SS White, Rio de Janeiro, RJ, Brazil), Group III- Magic Bleaching (Vigodent, Rio de Janeiro, RJ, Brazil), Group IV- Opalescence (Ultradent, South Lake, UT, USA), Group V-Whiteness Perfect (FGM Product Odontol Ltda., Joinvile, SC, Brazil) and Group VI- Claridex (Biodinamica Quimica e Farmaceutica Ltda., Ibipora, PR, Brazil). Filtek Z-250 (3M ESPE, St. Paul, MN, USA) was the composite resin selected for testing.
Table 1: Materials used in the study.

Specimen preparation
Cylindrical acrylic devices 4 mm in diameter and 2 mm in height were used. They were positioned on double-face tape, placed on a glass plate, and filled with composite resin. A Mylar strip was then placed on the composite resin surface and a glass slab was placed, in turn, on the Mylar strip. A constant pressure of 500 g weight was applied for 30 seconds. The test specimens were photo-activated in an apparatus containing a radiometer with a power of 650 mW/cm2 (Optilux 500, Demetron products, Sao Paulo, SP, Brazil) and were stored in a humid sterilizer at 37oC for 24 hours9. The test specimens that were exposed to the bleaching gels were covered in 1 cc of gel for 6 hours daily for a 2 week period. After exposure to the carbamide peroxide gel, the specimens were thoroughly washed under running water, dried with absorbent paper, and immersed in artificial saliva for 18 hours per day9. The specimens in the control group were kept in artificial saliva throughout the experiment, but the saliva was changed daily. During immersion in bleaching gels or artificial saliva, the test specimens were kept in plastic pots in a humid sterilizer at 37°C8. After 2 weeks of treatment, all the specimens were cleaned with an ultrasonic device (Cole Parmer 8891 Ultrasonic Cleaner, Vernon Hills, IL, USA) using distilled water at 37oC and detergent for 12 minutes. Next, the specimens were dried and analyzed in a microhardness tester (FM- Equilam Industria e Comercio Ltda., Ipiranga, SP, Brazil).
Hardness measurement
Knoop hardness measurements were performed on each specimen in three different places, as previously established. A load of 50 g was applied for 5 seconds. For each specimen, the values were read and transformed into a Knoop Hardness Number (KHN), calculated from the measured long diagonal length. The average values were then calculated.
Measurement of the pH of bleaching agents
The pH of bleaching agents was measured using a portable pH meter (Model 2A 14-KA Analyzer, Thermo Fisher Scientific Inc., Waltham, MA, USA) with a direct electrode, which was calibrated with standard buffer solutions at pH 4.0 and 7.0 and recalibrated for each product. pH measurement was performed after previous dilution of 0.5 g of each bleaching gel agent in 1.5 g of deionized water.
Statistical design
The results obtained were tabulated and submitted to one-way analysis of variance (ANOVA) and parametric statistical analysis, using Tukey’s test.
RESULTS
Three indentations were made on each sample, yielding 216 readings on the microhardness tester. Each specimen’s average value was tabulated and assigned to the study group. Table 2 shows the mean Knoop Hardness values (KHN) and the standard deviations (S.D.) for the baseline, control group (not bleached) and each bleached group. Tukey’s test showed that the baseline and control group had the higher Knoop hardness values (68.52 and 63.42, respectively). The bleached groups showed a significant reduction in microhardness after the bleaching treatment. The Whiteness Perfect (FGM) (57.57), Magic Bleaching (Vigodent) (57.22), and Opalescence (Ultradent) (57.03) bleaching products resulted in similar Knoop hardness values. The lowest values were found in specimens that were exposed to Claridex (Biodinamica Quimica e Farmaceutica Ltda.) (53.64) and Review (SS White) (51.45) bleaching gel.
Table 2: Mean Knoop hardness values (KNV) and standard deviation for the Filtek Z-250 (3M/Espe) composite resin for each group.
DISCUSSION
Microhardness has been related to the mechanical properties of composite resins, their degradation, and their predisposition to staining4. Thus, the surface microhardness of restorative materials, even after their insertion in the oral cavity, must remain unaltered and resistant to mechanical, chemical, and thermal stress – all of which are inherent to the oral environment. Nowadays, due to increasing patient demands for bleaching treatment, the ideal restorative material must remain unaltered throughout the chemical process of bleaching. This in vitro study evaluated the effect of homeapplied 15-16% carbamide peroxide bleaching agents on the surface microhardness of Filtek Z-250 (3M/Espe) composite resin, simulating a routine clinical condition: patients with resin restorations who are looking to improve their smile by bleaching their teeth. We immersed samples in the artificial saliva solution to simulate in situ conditions5,11. However, the artificial saliva solution may have contributed to hydrolytic degradation5, as revealed by the finding that the microhardness values of the control group were lower than baseline values. We verified that home bleaching agents should not be used indiscriminately when composite resin restorations are present: all bleaching gels used caused a significant decrease in the surface microhardness of the Z-250 (3M/Espe) composite. This information is in accordance with a recent study8, which also found a reduction in microhardness of this hybrid composite. On the other hand, some authors5,12 reported that no significant surface microhardness changes were found after application of bleaching agents for composite resin.
It is also important to note that bleaching materials are extremely unstable. Products that contain 15-16% carbamide peroxide are degraded to approximately 5% hydrogen peroxide13. Following the initial degradation, hydrogen peroxide, which is considered to be the active agent14, breaks down into free radicals, which may induce oxidative cleavage of polymer chains5. Furthermore, free radicals may impact the resin-filler interface and cause filler-matrix debonding15. Therefore, bleaching agents are likely to affect the resin matrix1, whereas inorganic fillers are likely to be inert, even in an extremely acidic environment. Soderholm and colleagues16 reported that some aspects of this chemical process might have accelerated the hydrolytic degradation of composite17. This fact would explain why the baseline and control groups displayed higher microhardness values than the bleached groups. Chemical softening of restorative materials would also occur if the bleaching products had solubility parameters similar to those of resin matrix1. The bleaching gels used in this study have a similar composition: all of them contain carbamide peroxide, carbopol and humectants. The carbamide peroxide and components such as carbopol (carboxy polymethylene) that were added to thicken the gel, improve adherence to the tooth surface and prolong the release of oxygen, have not been listed as composite resin solvents18. The carbopol keeps the gel contained within the tray and slows the chemical reaction2. Furthermore, changes to the composite resin surface could have been caused by complex interactions within these multicomponent bleaching products, rather than by one specific component1. Such a pattern would explain the varying performance among the groups.
One factor that contributes to these interactions is the pH value (Table 2), which can also affect the microhardness of restorative materials19. Therefore some bleaching agents are more likely to cause such alterations7. As acidity increases, so does the potential for alteration20. Dental tissues are also affected by lower pH values, and it is known that increasing peroxide concentration is associated with increased acidity. Thus, some authors21 report that peroxide pH needs to be approximately 7.0, as verified in groups III, IV and V in our studies (Table 2). However, some recent studies22 showed that the refrigerated at-home gels showed higher pH values and concluded that the bleaching agents’ storage temperature affects their pH. We also verified that bleaching agents presenting similar pH values, such as Whiteness Perfect, Opalescence and Magic Bleaching, caused minimal alterations in the composite surface and exhibited higher Knoop hardness values. Moreover, our data support reports by Yap and Wattanapayungkul7 and Turker and Biskin23, who reported that the effects of bleaching depend on the types of resinous material and bleaching agents used. In addition, discrepancies verified in our study may be explained by differences between the bleaching agents used. Some restorative materials may be more susceptible to some alterations and certain bleaching agents are more likely to cause such alterations24.
On the other hand, some studies8 emphasize that the alteration that appeared after contact with carbamide peroxide was limited to the material surface, suggesting that the material should be repolished after bleaching. It may be suggested that home bleaching agents are safe, provided that they are used under professional supervision. Moreover, there is no sufficient indication for restoration replacement, except in cases where esthetic issues are involved. The null hypothesis was rejected, as composite resin microhardness was affected by bleaching agents. However, further studies are necessary to evaluate the effects of bleaching products on the other properties of restorative materials.
CONCLUSION
Under the conditions of this in vitro study, all the bleaching gels tested (Review, Magic Bleaching, Opalescence, Whiteness Perfect and Claridex), caused a decrease in Z-250 composite resin microhardness.
1. Bailey SJ, Swift E Jr. Effects of home bleaching products on composite resins. Quintessence Int 1992;3:489-494. [ Links ]
2. Taher NM. The effect of bleaching agents on the surface hardness of tooth colored restorative materials. J Contemp Dent Pract 2005;6:1-8. [ Links ]
3. Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nigthguard vital bleaching technique. Quintessence Int 1992;23:471-488. [ Links ]
4. Haywood VB, Heymann HO.Nightguard vital bleaching. Quintessence Int 1989;20:173-176. [ Links ]
5. Yu H, Li Q, Hussain M, Wang Y. Effect of bleaching gels on the surface microhardness of tooth colored restorative materials in situ. J Dent 2008;36:261-267. [ Links ]
6. Okada K, Tosaki S, Hirota K, Hume WR. Surface Hardness change of restorative filing marerials stored in saliva. Dent Mater 2001;17:34-39. [ Links ]
7. Yap AUJ, Wattanapayungkul P. Effects of in-office tooth whiteners on hardness of tooth colored restoratives.Oper Dent 2002;27:137-141. [ Links ]
8. Lima DA, de Alexandre RS, Martins AC, Aguiar FH, Ambrosano GM, Lovadino JR. Effect of curing lights and bleaching agents on physical properties of a hybrid composite resin. J Esthet Restor Dent 2008;20:266-273. [ Links ]
9. Campos I, Briso AL, Pimenta LA, Ambrosano G. Effects of bleaching with carbamide peroxide gels on microhardness of restoration materials. J Esthet Restor Dent 2003;15:175-182. [ Links ]
10. Mujdeci A, Gokay O. Effect of bleaching agents on the microhardness of tooth colored restorative materials. J Prosthet Dent 2006;95:286-289. [ Links ]
11. Rodrigues JA, Oliveira GPF, Amaral CM. Effect of thickener agents on dental enamel microhardness submitted to at-home bleaching. Braz Oral Res 2007;21:170-175. [ Links ]
12. Garcia-Godoy F, Garcia-Godoy A, Garcia-Godoy F. Effect of bleaching gels on the surface roughness, hardness, and micromorphology of composites. Dent Mater 2002;50:247-250. [ Links ]
13. de Alexandre RS, Sundfeld RH, Briso ALF, Bedran-Russo AK, Valentino TA, Sundfeld MLMM. Effect of 10% carbamide peroxide dental bleaching on microhardness of filled and unfilled sealant materials. J Esthet Restor Dent 2006;18:273-278. [ Links ]
14. Haywood VB. Nightguard vital bleaching: current information and research. J Am Dent Assoc 1197;128:19S-25S. [ Links ]
15. Hanning C, Duong K, Becker K, Brunner E, Kahler E, Attin T. Effect of bleaching on subsurface micro-hardness of composite and a polyacid modified composite. Dent Mater 2007;23:198-203. [ Links ]
16. Soderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bergman M. Hydrolytic degradation of dental composites. J Dent Res 1984;63:1248-1254. [ Links ]
17. Kim JH, Lee YK, Lim BS, Rhee SH, Yang HC. Effect of tooth-whitening strips and films on changes in color and surface roughness of resin composites. Clin Oral Investig 2004;8:118-22. Epub 2004 Jul 9. [ Links ]
18. Polymer Handbook. Brandrup J, Immergt EH.. John Wiley and Sons, 1989;519-555. [ Links ]
19. Rodrigues JA, Basting RT, Serra MC, Rodrigues Junior AL. Effect of 10% carbamide peroxide dental bleaching on microhardness of filled and unfilled sealant materials. Am J Dent 2001;14:67-71. [ Links ]
20. Shannon H, Spencer P, Gross K, Tira D. Characterization of enamel exposed to 10% carbamide peroxide bleaching agents. Quintessence Int 1993;24:39-44. [ Links ]
21. Price RB, Sedarous M, Hiltz GS. The pH of tooth whitening products. J Can Dent Assoc 2000;66:421-426. [ Links ]
22. Freire A, Archegas LR, de Souza EM, Vieira S. Effect of storage temperature on pH of in-office and at-home dental bleaching agents. Acta Odontol Latinoam 2009;22:27-31. [ Links ]
23. Turker SB, Biskin T. The effect of bleaching agents on the microhardness of dental aesthetic restorative materials. J Oral Rehabil 2002;29:657-661. [ Links ]
24. Swift Jr EJ, Perdigao J. Effects of bleaching on teeth and restorations. Compend Contin Educ Dent 1998;19:815-820. [ Links ]














