Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Odontológica Latinoamericana
versão On-line ISSN 1852-4834
Acta odontol. latinoam. vol.23 no.2 Buenos Aires set. 2010
ARTÍCULOS ORIGINALES
The use of ozone to lighten teeth. An experimental study
Jerónimo Tessier1, Patricia N. Rodriguez2, Fima Lifshitz3, Silvia M. Friedman2, Eduardo J. Lanata1
1 Department of Operative Dentistry. Faculty of Dentistry. University of Buenos Aires. Argentina.
2 Department of Biochemistry and Oral Biology. Faculty of Dentistry. University of Buenos Aires. Argentina.
3 Pediatric Sunshine Academics & Sansum Medical Research Institute. Santa Barbara, CA. USA.
CORRESPONDENCE Friedman Silvia Maria 3 de Febrero 1856. Apt. 5 A 1428. Buenos Aires. Argentina. 54 11 4784 -1111 Fax: 54 11 4 508- 3958 friedman@bioquimica.odon.uba.arfriedman@odon.uba.ar
ABSTRACT
Tooth-whitening agents are available for therapeutic use in the dental office or at home. However, whitening more severe stains, such as those caused by systemic ingestion of tetracycline, constitutes a challenge. The aim of this study was to evaluate, in an experimental model of growing rats, the efficacy of using ozone to lighten tetracycline-stained incisors. At weaning, male Wistar rats (n=40) were randomly assigned to one of three groups. Two control groups, C21 and C60 (n=8, each) were used to document the usual age-related color. The third group (n=24) received 0.25 g% of oxytetracycline (O) until 60 days of age. These rats were subsequently divided into three further groups: O0, O3 and O5 (n=8, each). These rats were anesthetized; O3 and O5 groups received ozone application to the lower incisors for 3 (group O3) or 5 minutes (group O5), respectively; while O0 did not receive the ozone treatment. Teeth were then photographed and the incisors from the control (C60) and treatment groups (O0, O3 and O5) were cut, and compared to a standard color guide (there were eight shades numbered 0 to 7, lightest to darkest) to assess the hue visually. The teeth were then placed in phosphoric acid to quantify the color by spectrophotometry. The data (mean ± SD) were analyzed by One-Way Analysis of Variance (ANOVA) followed by Tukey’s test or Dunnett test. The visual observation, analyzed blindly by one investigator, showed that O3 and O5 groups had diminished yellowing of the teeth as compared to the untreated O0 group (P<0.001). The color quantified by spectrophotometry also detected significant differences among groups (O3 < O0, P<0.01; O5 < O0,P < 0.001 and O5 < O3, P<0.01). C21 and C60 were significantly different among groups (P<0.001). This is the first experimental study to show that ozone can be successfully used for lightening the yellowish tinge of tetracycline- stained rat incisors. Further studies are required for its potential use in the dental clinic.
Key words: Ozone; Tetracycline; Bleaching-agent.
RESUMEN
Uso del ozono para blanquear dientes. Estudio experimental
Los agentes blanqueadores dentales estan disponibles para tratamientos que se realizan en el consultorio odontologico o en el domicilio. Sin embargo, aclarar manchas severas, como las causadas por la ingestion sistemica de tetraciclina, constituyen un desafio. El objetivo de este estudio fue evaluar en un modelo experimental de ratas en crecimiento, la eficiencia del uso de ozono para aclarar los incisivos oscurecidos por el uso de tetraciclina. Ratas macho Wistar al destete (N=40) fueron asignadas aleatoriamente a uno de tres grupos. Dos de ellos grupos controles, C21 y C60 (N=8, cada uno), para documentar el color habitual de los incisivos, correspondiente a la edad del animal. El tercer grupo (N = 24) recibio 0,25 % de oxitetraciclina (O) hasta los 60 dias de edad. Entonces, el grupo O se dividio aleatoriamente en tres grupos O0, O3 y O5 (N = 8, cada uno) y las ratas se anestesiaron. Los grupos O3 y O5 recibieron en los incisivos inferiores la aplicacion de ozono durante 3 y 5 minutos, respectivamente; mientras que O0 no recibio tratamiento. Los incisivos de C60, O0, O3 y O5 fueron fotografiados. Luego se cortaron y se contrastaron con una guia estandar de ocho colores (ordenados de 0 a 7, desde el mas claro a mas oscuro) para cuantificar visualmente el color de los incisivos. Luego, se colocaron en acido fosforico para cuantificar el color por espectrofotometria. Los resultados (media ± SD) se analizaron por medio de ANOVA y prueba de Tukey o Dunnett (α =0.05) para determinar el efecto del tratamiento. El analisis visual de las imagenes mostro que los grupos O3 y O5 disminuyeron el color amarillo intenso respecto a O0. Dicha diferencia de color fue evaluada a traves de la guia (G) y cuantificada mediante espectrofotometria (E). Segun G, la mayor diferencia de color respecto a C60 fue para O0 (P<0.001), disminuyo en O3 (P<0.001) y aun mas en O5 (P<0.01). De acuerdo a E, O3<O0, P<0.01; O5<O0, P< 0,001 y O5<O3, P<0.01. C21 y C60 resultaron significativamente menores por ambos metodos (P<0.01). Este primer estudio experimental evidencia que el tratamiento con ozono puede aclarar los incisivos de rata tratadas con tetraciclina. Se requieren estudios adicionales para su uso en la clinica odontologica.
Palabras claves: Ozono; Tetraciclina; Agente blanqueador.
INTRODUCTION
Tooth-whitening agents are available for use in the office or at home. The demand for tooth bleaching dental treatments is increasing as patients wish to improve their appearance1. The efficacy of tooth whitening is a major concern in dental practice, since its cosmetic result is immediately noticeable2, though it may not be the only aspect involved in good facial appearance. However, whitening more severe stains, such as those caused by systemic ingestion of tetracycline, constitutes a challenge. A number of tooth whitening agents and methods have been described in the literature for the treatment of tetracycline-stained teeth. Different bleaching agents have been utilized, e.g. carbamide peroxide3 or hydrogen peroxide plus carbamide peroxide4. There are also variations in the way these agents are applied, such as exposure time, e.g., months of carbamide peroxide treatment5 ; or concentration, e.g. 10%, 15% or 20% carbamide peroxide6. Additionally the mode of activation or methods of application of the whitening compound may differ7-9.
In dentistry, ozone has proved to be successful in the treatment of root caries10, non-cavitated fissure carious lesions11-13, early carious lesions in teeth14, dental surgery or following tooth extraction processes15,16 and reduction of pathogenic micro-organisms of carious dentine17-19. Ozone has also been used to whiten teeth in individuals with dental sensitivity and mucousal ulcerations20; in those who consume tobacco, coffee or chocolate; and in those who have extrinsically stained teeth due to brown-colored melanoidins or chlorhexidine use21. However, ozone has not been used to bleach intrinsically stained teeth. The aim of this study was to evaluate the efficiency of ozone to lighten tetracycline-stained incisors in an experimental model of growing rats.
MATERIALS AND METHODS
This study was performed in accordance with the Code of Ethics in Research of the University of Buenos Aires and the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the University of Buenos Aires. At weaning (21 days of age), 40 male Wistar rats from the animal laboratory of the Department of Biochemistry, Faculty of Dentistry, University of Buenos Aires, Argentina, were studied. The rats were housed in galvanized cages with meshed floors in order to maintain hygienic conditions and to avoid coprophagia. They were exposed to a 12-h light, 12-h dark cycle throughout the study. Room temperature was maintained at 21±1°C with a humidity of 50-60%. Rats were fed a rodent stock diet ad libitum. Animals were randomly assigned to one of three groups. Two control groups (n=8, each) (C21 and C60) remained untreated in order to document the spontaneous evolution of the color of the jaw incisors at age 21 and 60 days, respectively. Tap water was provided ad libitum. At 21 days of age, when the rats were weaned (group C21), their lower incisors were cut and saved to determine the baseline color. At age 60 days, the lower incisors from the C60 group were photographed and cut in order to assess their color as described below. The third group of rats (O, n=24) received 0.25 g% of oxytetracycline (Holliday, Scott) in drinking water and were fed ad libitum from the time of weaning to age 60 days. At 60 days of age, group O was randomly divided into three further groups (n=8, each) O0, O3 or O5. The rats were anesthetized with ketamine (70 mg/kg) and xylazine (13 mg/kg), administered intraperitoneally. The anesthesia persisted for at least 60 minutes and allowed sufficient time to administer the ozone application. O0 was given no ozone treatment while in groups O3 and O5, ozone was applied to the jaw incisors for 3 (group O3) or 5 minutes (group O5), respectively. The ozone system used for the application was the HealOzone 2130C (KaVo Dental, Biberach, Germany). This is a self-contained device that produces ozone at a fixed concentration of 2100 p.p.m. ozone ± 5% at a flow rate of 615 cc min-1.
Visual Analysis
Once the experimental period was completed, the lower incisors (C60, O0, O3 and O5) were photographed (Digital Camera Nikon D80, lent AF-S UR Micro-Nikkor 105 mm f/2.86. Nikon Corporation, Japan and electronic flash Macro Sigma EM-140D6, Carl-Zeiss, Rodermark, Germany) to assess the color of the teeth. The visual evaluation was carried out using a modification of the Vita Classic shade guide (Vita Classic, Lumin Vacuum Shade Guide, Vita Zahnfabrik H. Rauter GmbH & Co.KG, Germany), which includes shades arranged by value, and was developed for human teeth. In the present study, the shades were modified to conform to the range of colors of rat teeth, ranging from the slightly yellow color of the rats at weaning (C21) to the darkest yellow tinge available. There were eight shades numbered 0 to 7, lightest to darkest, using a standard color guide. The color difference was calculated by subtracting the tab number corresponding to the experimental group (O0, O3 and O5) from that of C60 (paired by age). The data were analyzed statistically. The color of the teeth of each rat was determined by a single investigator, in order to avoid individual variations in the estimate. The colors of the incisors were analyzed blindly as the investigator did not know the source of the teeth of the rats in each of the groups studied.
Spectrophotometric Analysis
The incisors of C21, C60, O0, O3 and O5 groups were cut and prepared for quantitative assessment of tooth color. They were placed in one milliliter of 37% phosphoric acid. The solutions, tested in duplicate, as well as the blank solution (37% phosphoric acid) were vortexed and then incubated at room temperature for 1 hour. In order to obtain the best readability and accuracy, the scale was set to read zero absorbance with a blank. Each sample was transferred to a cuvette for reading at wavelength 425nm (spectrophotometer Metrolab-1600 plus, Metrolab Argentina). This peak was chosen because it was the most prominent when the solutions were scanned for absorption from 400nm to 800nm. The data were presented as mean absorbance units (AU) ± standard deviation, where absorbance is a measure of the quantity of light absorbed by a sample, which is proportional to the amount of chromophores present.
Statistical analysis
Color difference by age (experimental groups minus C60) obtained from visual assay was analyzed by One-Way Analysis of Variance (ANOVA) followed by Tukey’s test. Comparison of readings between control (C60) and experimental O0, O3 or O5 groups were performed using a One-Way Analysis of Variance (ANOVA) followed by Dunnett test. In order to detect differences between the five groups, One-Way Analysis of Variance (ANOVA) followed by Tukey’s test was used. The significance level was set at 5%. The Statistical Product and Service Solutions for Windows 9.0 (SPSS, Inc., Chicago, IL) were used for statistical analyses.
RESULTS
At weaning, the lower incisors acquired a slight yellow color and by 60 days of age, this color was more intense, as expected (P< 0.001)22. After tetracycline treatment (age 60 days), the lower incisors in the O0 group were dark yellow-orange. The results of the visual observation of tooth color showed that the ozone procedure lightened the pigmentation of the incisors of groups O3 and O5. The teeth of groups O3 and O5 were lighter than those of group O0 but darker than those of C60 (Fig. 1). Additionally, using the color scale developed from the standard shades, the color difference between C60 and O0, O3 and O5 was significantly higher for O0 (5.25+0.46) and decreased from O3 (3.25+0.50) to O5 (1.75+0.39) (p<0.01); with significant differences between O5 and O3 (p<0.01). C21 numbered as zero was significantly different among groups (p<0.001).

Fig. 1: Color comparison of the lower incisors shown in photographs of one rat of each group tested.
C60: Rat 60 days of age to document the natural age-related color (yellow).
O0: Rat 60 days of age that received oxytetracycline from weaning. The antibiotic used in this study stained teeth yellow- orange.
O3: Rat 60 days of age that received oxytetracycline from weaning and ozone treatment for 3 minutes.
O5: Rat 60 days of age that received oxytetracycline from weaning and ozone treatment for 5 minutes.
The color quantified by spectrophotometry showed significant differences between groups C60 and O3, O5 and O0; C60 was lower than experimental groups (P<0.01). Additionally, differences were observed for O3, O5 and O0 groups (O3 < O0, P<0.01; O5 < O0,P < 0.001). Five minutes of ozone exposure resulted in better lightening (O5 < O3, P<0.01). C21 color was significantly lower than all groups (p<0.01) (Figure 2).
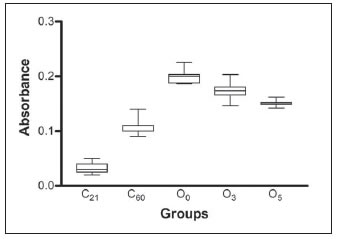
Fig. 2: Color quantification of the lower incisors by spectrophotometric analysis. The box and whiskers graph show the absorbance values of the different groups. C21 and C60: control rats color of lower incisors to document the natural agerelated color at 21 and 60 days.
O0= experimental group at 60 days of age that received oxytetracycline from weaning and remained ozone untreated.
O3 and O5 = experimental groups at 60 days of age that received oxytetracycline from weaning and ozone treatment for 3 or 5 minutes, respectively.
Data were statistically significant according to one-way analysis of variance and a) Tukey´s test a posteriori:
***P<0.001, C21 and C60 differed from O0, O3 and O5.
** P<0.01, O0 vs. O3
*** P<0.001, O0 vs. O5
** P<0.01, O3 vs. O5. b) Dunnett test a posteriori. C60 was considered as a control group:
** P<0.01, C60 vs. O0 ** P<0.01, C60 vs. O3 ** P<0.01, C60 vs. O5
DISCUSSION
This is the first experimental study designed to evaluate the use of ozone to lighten tetraclycline stained incisors. The whitening response to ozone of the lower incisors of the rats was impressive, clearly evident by visual evaluation. After three minutes of ozone exposure, the incisors were whitened and the tetracycline staining was reduced to a more suitable color. Furthermore, after five minutes’ treatment the incisors continued to lighten. The quantitative spectrophotometric analysis of tooth color confirmed the visual impression of tooth whitening. Three minutes of ozone exposure reduced the yellow color by 28%, while five minutes achieved a 56% reduction. The mechanism of this process may involve the ozonation of double bond systems, which contribute to the chromophoric properties of the product23.
Most of the clinical studies that have used bleaching agents to whiten teeth have been based on visual assessments; this methodology is subjective and potentially influenced by a number of factors. In the present study, the blind use of a standard visual color guide allowed quantification of differences in color of the incisors as natural evolution with advancing age as well as with the ozone treatment employed. The quantitative spectrophotometric assessment of tooth color confirmed the differences detected visually and provided further details of the degree of coloring among groups. The specific model selected for assessing tooth color and the response to ozone application was the rat incisor which, unlike most teeth, is continually erupting. This characteristic makes these teeth suitable for evaluating the whitening properties of ozone. Despite some small differences between rat and human incisor dentine24, a close resemblance in enamel morphology has been established25. Additionally, the relatively thick enamel layer in the mandible incisor makes it suitable for chemical and biochemical analyses26.
It has been shown that rat incisors start out white in the young rat, but by age 21 days (initial time of the present study) the upper incisors have a slight yellow tinge. By 25 days these teeth are distinctly yellow while the lower incisors have acquired a slight yellow shade. By 38 days of life, these colors are more intense, though the upper incisors remain with more color than the mandible teeth. The relationship between more pigmented upper incisors and less pigmented lower incisors remains true throughout the rat’s life. In adult rats, the upper teeth are dark yellow-orange and the lower incisors are yellow27. Since the color of the rat incisors changes throughout life, we performed baseline biochemical measurements, at weaning (C21) and at the end of the experimental period (C60). In the experimental groups given the antibiotic in the drinking water, we demonstrated that their teeth were stained yellow-orange in group O0. Tetracycline interferes with odontogenesis by attaching irreversibly to calcified tooth structures28. It has been suggested that the color is derived from photo-oxidation of tetracycline molecules bound within the tooth structures29. In infants and children, administration of tetracycline may cause not only permanent staining of teeth, but enamel hypoplasia and decreased linear skeletal growth30. Although it is known that certain types of stains can be eliminated by a number of methods31; tetracycline staining is more difficult to bleach32. Conventional whitening of tetracycline-stained teeth may require longer sessions at the dental office4,33 due to the resistance of tetracycline stains to bleach34.
An important advantage of the ozone procedure utilized in our experimental model was the short time needed to achieve successful bleaching. Future studies will be needed to elucidate the ozone effects on morphology and/or substance of the enamel that could result from a limited application to the teeth. Other bleaching agents that have been used to treat tetracycline- stained teeth have been shown to produce morphological alterations in the enamel or on the subsurface of enamel35. Carbamide peroxide bleaching induces surface erosion, depressions, porosity and increased depth of enamel grooves and partial removal of enamel prisms. However, most bleaching agents differ from ozone since they are acidic, which is not favorable to enamel, dentin and cement2.
CONCLUSION
This is the first study to demonstrate that tetracycline- stained incisors can be successfully lightened by the use of ozone. A simple, safe and non-invasive ozone treatment would provide an alternative therapeutic agent to current methods; however, further morphological assessments will be required for its use in the dental clinic.
ACKNOWLEDGEMENTS
The authors would like to thank Ricardo Orzuza of the Department of Biochemistry and Oral Biology, School of Dentistry, University of Buenos Aires, for his technical support and taking care of the animals. This study was supported by the University of Buenos Aires, grant O410 and Pediatric Sunshine Academics Inc, Santa Barbara, California, Partially supported by Kavo Dental Joinville, Brasil; Plus Dental Buenos Aires, Argentina.
1. Braun A, Jepsen S, Krause F. Spectrophotometric and visual evaluation of vital tooth bleaching employing different carbamide peroxide concentrations. Dent Mater 2007;23:165-169 [ Links ]
2. Miranda CB, Pagani C, Benetti AR, Matuda F da S. Evaluation of the bleached human enamel by scanning electron microscopy. J Appl Oral Sci 2005;13:204-211. [ Links ]
3. Haywood VB, Leonard RH, Dickenson GL. Efficacy of sixmonths nightguard vital bleaching of tetracycline-stained teeth. J Esthet Dent 1997; 9:13-19. [ Links ]
4. Kugel G, Aboushala A, Zhou X, Gerlach RW. Daily use of whitening strips on tetracycline-stained teeth: Comparative results after 2 months Compend Contin Educ Dent 2002; 23:29-34. [ Links ]
5. Leonard RH Jr, Van Haywood B, Caplan DJ, Tart ND. Nightguard vital bleaching of tetracycline-stained teeth: 90 months post treatment. J Esthet Restor Dent 2003;15:142-152. [ Links ]
6. Matis BA, Wang Y, Eckert GJ, Cochran MA, Jiang T. Extended Bleaching of Tetracycline-Stained Teeth: A 5- Year Study. Oper Dent 2006;31:643-651. [ Links ]
7. Wang XD, Chen SL, Yu JB. Clinical survey of a combined in-office cold light bleaching and nightguard vital bleaching system for tetracycline stained teeth. Hua Xi Kou Qiang Yi Xue Za Zhi 2008;26:409-412. [ Links ]
8. Matis BA, Wang Y, Jiang T, Eckert GJ. Extended at-home bleaching of tetracycline-stained teeth with different concentrations of carbamide peroxide. Quintessence Int 2002; 33:645-655. [ Links ]
9. Shin DH, Summitt JB. The whitening effect of bleaching agents on tetracycline-satined teeth. Oper Dent 2002;27: 66-72. [ Links ]
10. Baysan A, Lynch E. The use of ozone in dentistry and medicine. Part 2. Ozone and root caries. Prim Dent Care 2006; 13:37-41. [ Links ]
11. Huth KC, Paschos E, Brand K, Hickel R. Effect of ozone on non-cavitated fissure carious lesions in permanent molars. A controlled prospective clinical study. Am J Dent 2005;18(4):223-228. [ Links ]
12. Baysan A, Beighton D. Assessment of the ozone-mediated killing of bacteria in infected dentine associated with non-cavitated occlusal carious lesions. Caries Res 2007;41:337-341. [ Links ]
13. Schmidlin PR, Zimmermann J, Bindl A. Effect of ozone on enamel and dentin bond strength. J Adhes Dent 2005;7:29-32. [ Links ]
14. Lanata EJ. Intervencion sin invasion con ozono. In: Alfaomega Grupo Editor Argentino S.A., editors. Atlas de Operatoria Dental, Buenos Aires: Editorial Alfaomega S. A. 2008;46-66. [ Links ]
15. Stubinger S, Sader R, Filippi A. The use of ozone in dentistry and maxillofacial surgery: A review. Quintessence Int 2006;37:353-359. [ Links ]
16. Agrillo A, Sassano P, Rinna C, Priore P, Iannetti G. Ozone therapy in extractive surgery on patients treated with bisphosphonates. J Craniofac Surg 2007;18:1068-1070. [ Links ]
17. Baysan A, Whiley RA, Lynch E. Antimicrobial effect of a novel ozone- generating device on micro-organisms associated with primary root carious lesions in vitro. Caries Res 2000;34:498-501. [ Links ]
18. Lanata EJ. Tecnologias emergentes en Operatoria Dental. In: Grupo Guia, editors. Operatoria Dental. Estetica y Adhesion. Buenos Aires: Editorial Grupo Guia S.A. 2003;251-266. [ Links ]
19. Goncalves Cardoso M, Dias de Oliveira L, Yumi Koga-Ito C, Olavo A, Cardoso J. Effectiveness of ozonated water on Candida albicans, Enterococcus faecalis, and endotoxins in root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;105:e85-e91. [ Links ]
20. Ilzarbe IM. Nuevo metodo para blanqueamiento de dientes vitales mediante gases hiperoxidantes naturales. Revista Maxillaris 2000;25: ano III. [ Links ]
21. Holmes J, Grootveld M, Smith C, Claxson A, Lynch E. Bleaching of Components Responsible for Extrinsic Tooth Discoloration by Ozone J Dent Res 82 Spec Iss A abstract number 0615, 2003. (www.dentalresearch.org) [ Links ]
22. Halse A. Location and first appearance of rat incisor pigmentation. Scand J Dent Res 1972;80:428-33. [ Links ]
23. Friedman SM. Bases bioquimicas de la aplicacion del ozono. En: Alfaomega Grupo Editor Argentino. Atlas de Operatoria Dental, Estetica y Adhesion. Buenos Aires: Editorial Alfaomega S.A. 2008;4:123-130. [ Links ]
24. Manton DJ, Bhide R, Hopcraft MS, Reynolds EC. Tooth Mousse may be applied concurrently with the bleach, and not reduce bleaching effectiveness Aust DentJ 2008;53: 128-132. [ Links ]
25. Moinichen CB, Petter Lyngstadaas S, Risnes S. Morphological characteristics of mouse incisor enamel. J Anat 1996;189:325-333. [ Links ]
26. Robinson C, Kirkham J, Brookes SJ, Bonass WA, Shore RC. The chemistry of enamel development. Int J Dev Biol 1995;39:145-152. [ Links ]
27. Addison WH, Appleton JL.The structure and growth of the incisor teeth of the albino rat. J Morphol 1915;26:43-96. [ Links ]
28. Guggenheimer J. Tetracyclines and the human dentition. Compend Contin Educ Dent 1984;5:245-248. [ Links ]
29. Mello HS. The mechanism of tetracycline staining in primary and permanent teeth. J Dent Child 1967;34:478-487. [ Links ]
30. Baker KL. Tetracycline induced tooth changes. Part 5. Incidence in extracted first permanent molar teeth: a resurvey after four years. Med J Aust 1975;2:301-304. [ Links ]
31. Joiner A. The bleaching of teeth: A review of the literature. J Dent 2006;34:412-419. [ Links ]
32. Haywood VB. A comparison of at-home and in-office bleaching. Dent Today 2000;19:44-53. [ Links ]
33. Haywood VB. Historical development of whiteners: clinical safety and efficacy. Dent Update 1997;24:98-104. [ Links ]
34. Haywood VB, Leonard RH, Nelson CF & Brunson WD. Effectiveness, side effects and long-term status of nightguard vital bleaching. J Am Dent Assoc 1994;125:1219-1226. [ Links ]
35. Hegedus C, Bistey T, Flora-Nagy E, Keszthelyi G, Jenei A. An atomic force microscopy study on the effect of bleaching agents on enamel surface. J Dent 1999;27:509-515. [ Links ]














