Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.23 no.2 Buenos Aires set. 2010
ARTÍCULOS ORIGINALES
Development and in vitro evaluation of biopolymers as a delivery system against periodontopathogen microorganisms
Aida Rodriguez-Garcia, Luis J. Galan-Wong, Katiushka Arevalo-Niño
Institute of Biotechnology, Faculty of Biological Science, Autonomous University of Nuevo Leon.
CORRESPONDENCE Dr. Katiushka Arevalo Nino Instituto de Biotecnologia - Facultad de Ciencias Biologicas Universidad Autonoma de Nuevo Leon Pedro de Alba y Av. Manuel L. Barragan S/N. Cd. Universitaria San Nicolas de los Garza, NL. Mexico. CP. 66450. e-mail: karevalo01@hotmail.com
ABSTRACT
Periodontal disease is the major cause of tooth loss in adults. Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans are considered key pathogens in periodontitis. The treatment consists of oral hygiene education, instrumentation for removal of calculus (scaling), chemotherapy and periodontal surgery. Several agents are commercially available; these chemicals can alter oral microbiota and have undesirable sideeffects such as vomiting, diarrhea and tooth staining. Hence, the search for alternative products continues and natural phytochemicals isolated from plants used as traditional medicine and the use of biomaterials are considered good alternatives. Chitosan and pullulan are polymers that have been proposed due to their favorable properties such as biocompatibility, biodegradability, and adhesion ability. They can be used as local delivery systems of active principles of plant extracts. Thymus vulgaris, Matricaria chamomilla, Croton lechleri, Calendula officinalis L. and Juliana adstringens Schl. are known to have medicinal activity, and they are used in Mexican traditional medicine. Their extracts were tested in vitro for antimicrobial activity against P. gingivalis and A. actinomycetemcomitans, using agar diffusion and microdilution methods. The antimicrobial activity of films from biopolymers with plant extracts was evaluated by measuring the zones of inhibition against the tested organisms. The aim of this study was to develop bioadhesive films from chitosan and pullulan with added plant extracts and determine the antimicrobial activity of films against periodontal pathogens.
Key words: Biopolymers; Plant extracts; Delivery system; Antimicrobial activity; Periodontopathogen microorganisms.
RESUMEN
Desarrollo y evaluación in vitro de biopolímeros como un sistema de liberación contra microorganismos periodontopatógenos
La enfermedad periodontal es la principal causa de perdida de dientes en los adultos. Los agentes causales comunmente identificados con la enfermedad son Porphyromonas gingivalis y Aggregatibacter actinomycetemcomitans. El tratamiento de la enfermedad consiste en educacion sobre higiene oral, remocion de calculos por medio de instrumentacion (raspado y alisado de la raiz), la administracion de medicamentos y cirugia. Hay multiples agentes quimicos disponibles comercialmente; estos pueden alterar la microflora oral y tener efectos secundarios indeseables como vomito, diarrea y pigmentacion dental. Por lo tanto, los productos naturales como los fitoquimicos aislados de plantas que son usadas como medicinas tradicionales y los biomateriales, son considerados buenas alternativas. El quitosan y el pululan son polimeros que han sido propuestos debido a sus propiedades de biocompatibilidad, biodegradabilidad, habilidad de adhesion y que pueden ser usados como sistemas de liberacion de los principios activos de extractos de plantas. Los extractos de Thymus vulgaris, Matricaria chamomilla, Croton lechleri, Calendula officinalis L. y Juliana adstringens Schl. son conocidos por tener actividad medicinal y se usan en la medicina tradicional Mexicana. La actividad antimicrobiana de sus extractos fue probada in vitro contra P. gingivalis y A. actinomycetemcomitans usando los metodos de difusion en agar y de microdilucion. La actividad antimicrobiana de peliculas a base de biopolimeros con extractos de plantas fue evaluada midiendo las zonas de inhibicion de crecimiento de los organismos probados. El proposito de este estudio fue desarrollar peliculas bioadhesivas de quitosan y pululan adicionadas con extractos de plantas y evaluar su actividad antimicrobiana contra periodontopatogenos.
Palabras clave: Biopolimeros; Extractos de plantas; Sistema de liberacion; Actividad antimicrobiana; Microorganismos periodontopatogenos.
INTRODUCTION
Periodontal disease is an undesirable condition which has widespread occurrence. It is an inflammatory disease that affects the periodontum, which consists of the investing and supporting tissues surrounding a tooth (i.e., the periodontal ligament, the gingiva, and the alveolar bone1. Aggregatibacter actinomycetemcomitans, Tannerella forsythensis and Porphyromonas gingivalis, are among the principal microorganisms associated with the process of periodontal diseases2,3. Microorganisms contribute to both the initiation and progression of gingivitis, plaque, and periodontal disease. Therapeutic approaches include oral hygiene instruction, surgical techniques and mechanical debridement of the root surfaces, scaling and/or treatment of the infection with systemic or local antibiotics4. As a public health concern, the notion of periodontal disease being a risk factor for cardiovascular disease, stroke and premature birth brings increased urgency to the need for controlling and preventing the disease in a cost-efficient manner5.
Chitosan a (1→4)-linked 2-amino-2-deoxy-s-D-glucan, is prepared by N-deacetylation of chitin, which is the second most abundant polysaccharide found in nature6 and distributed widely in nature, especially in marine invertebrates, insects, yeast, and fungi. There are numerous works on the use of chitosan in a number of biomedical applications, including drug-delivery systems, tissue engineering, and orthopedics7. Pullulan is a neutral linear polyssacharide composed of (1→6)-linked α-D-maltotriose residues synthesized from starch or sugar by Aureobasidium pullulan, which has adhesive properties and can be used in wound-healing compositions8. Medicinal plants have been used as traditional treatments for numerous human diseases for thousands of years in many parts of the world. Mexico has a great wealth of medicinal plants and it has been popular tradition to use plants for scientific investigation, many of which deal with the antimicrobial properties of the plant extracts and their potential as a clinically relevant antimicrobial therapy. Thymus vulgaris (thyme), Matricaria chamomilla (chamomile), Croton lechleri (sangre de drago), Calendula officinalis L. (marigold) and Juliana adstringens Schl. (cuachalalate) are known to have antimicrobial, antiviral, anti-inflammatory and antifungal properties9-11.
Many compounds derived from plants used in traditional medicinal systems have been recorded in pharmacopeias as agents used to treat infections, and a number of these have been recently investigated for their efficacy against oral microbial pathogens. Essential oil and extracts from fresh leaves and flowers of T. vulgaris can be used as aromatic additives in foods, pharmaceuticals, and cosmetics. Its primary constituents are thymol and carvacrol. The constituents of M. chamomilla thought to have antimicrobial properties include alpha-bisabolol, luteolin, quercetin, and apigenin11. The chemical composition of C. lechleri includes a considerable number of compounds, including several simple phenols and diterpenes, proanthocyanidins, and phytosterols12. Marigold is a phytotherapeutic plant rich in biologically active metabolites, such as sesquiterpens, triterpens, flavonoids, carotenoids and tannins. These components confer antiseptic action, antiinflammatory, anti-edematous, immunomodulatory activity and antimicrobial effects13. J. adstringens Schl. is an endemic plant of Mexico. Its principal compounds are masticadienonic acid; 3alpha-hydroxymasticadienonic acid; 3-epi-oleanolic; as well as the sterol beta-sitosterol14,15. Due to the safety risk of systemic uptake of drugs administered by local delivery16,17, the use of natural, safe plant medication11 provides an attractive alternative for treating oral diseases with potentially high compliance. The plant extracts mentioned in this work are effective in treating various oral disorders such as plaque reduction, candidiasis, canker sores and alveolitis15,18,19.
The aim of this work was to provide a self-bioadhesive film for a topical application that adheres to the oral mucosal tissues and investigate the in vitro antimicrobial effects against two pathogenic microorganisms that have been associated with periodontal diseases, in order to assess the potential for developing them into agents that can be used as preventive or treatment therapies, either alone or in combination with conventional treatments.
MATERIAL AND METHODS
Bacteria Growth conditions
Porphyromonas gingivalis ATCC 33277 and Aggregatibacter actinomycetemcomitans ATCC 43718 were activated in trypticase soy broth and brain heart infusion in the anaerobic chamber and incubated at 35°C for 48 hours in anaerobic and 5% CO2 atmosphere conditions respectively. For long-term storage, strains were made on glycerol stocks.
Plant material
Different parts of Thymus vulgaris, Matricaria chamomilla, Croton lechleri, Calendula officinalis L. and Julliana adstringens Schl. were collected and extracted with hydroalcoholic solvents to obtain aqueous and organic extracts. The extracts were filtered with filters role of Whatman Ltd., sterilized by filtration by 0.22 μm Millipore filters Durapore R GV and stored in sterile amber bottles.
To evaluate the antimicrobial activity of each extract, 1:2 and 1:3 dilutions were prepared and the Minimum Inhibitory Concentration (MIC) was determined by microdilution technique NCCLS (National Committee for Clinical Laboratory Standards, 1997).
Antimicrobial activity
In vitro antimicrobial activities of aqueous extracts of T. vulgaris, Matricaria chamomilla, C. lechleri, Calendula officinalis L., J. adstringens Schl and Chlorhexidine 0.12% (as positive control) were evaluated against Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. The minimum inhibitory concentrations (MIC) were determined by the microdilution method using 96-well plates. The inoculum was prepared and adjusted to 0.5 McFarland standard turbidity. The correct turbidity density was measured at 540nm using a spectrophotometer. All the extracts were dissolved in trypticase and brain heart infusion broth (for P. gingivalis and A. actinomycetemcomitans respectively) and then serial two-three fold dilutions were made. The 96-well plates were prepared by dispensing 100 μL of each dilution into double well and adding 100 μL of the inoculum. The final volume in each well was 200 μL. Two wells were left with 200 μL of media, two more with 200 μL of inoculum and two with 200 μL of chlorhexidine (as positive control). The plates were incubated at 35°C for 24 hours according to the requirements of each strain. The MIC was determined from the lowest concentration of extracts to inhibit the growth of microorganisms. Inhibition of proliferation was assessed by optical density measurements (625 nm).
For minimum bactericidal concentrations (MBC), inoculum from clear wells was resuspended in PBS to give a standard concentration of cells (1x10-1) and 10 μL were inoculated into Petri dish pouring molten trypticase or brain heart infusion agar to 45- 50°C and swirled to distribute the medium homogeneously. The inoculated plates were incubated at 37°C for 24 hours in anaerobiosis and 5% CO2 atmosphere conditions. Absence of bacterial growth noted on the solid medium was considered to be indicative of bactericidal activity of the extracts. Petri dishes containing media and 10 μL of chlorhexidine from the control wells were used as control. All assays and controls were made three times in triplicate.
Preparation of the polymers
Chitosan with deacetylation degree 85% was purchased from Sigma Chemical CO. St. Louis MO. USA. Pullulan (molecular weight=200,000) was donated by Hayashibara Company (Okayama, Japan). Three different formulations of each biopolymer were tested to obtain the best properties (thickness and solubility) for the production of films. 1.0, 1.2 and 0.8 g of chitosan powder were dissolved in 100mL 1% (vol/vol) acetic acid aqueous solution for final concentrations of 1%, 1.2% and 0.8%. Pullulan was used at 10%, 12% and 10%, plus sorbitol (as plasticizer) dissolved in bidistilled water. The average pH was 3.9 for chitosan and 5.7 for pullulan. From the three formulations tested, the final selected concentration for chitosan was 1% and 10% plus 0.3% of sorbitol for pullulan. These formulations of biopolymer were added to aqueous extracts in concentrations of about 10% of the MBC of the plants that showed the best antimicrobial activity (T. vulgaris, J. adstringens Schl and C. lechleri) and stirred until the mixture became homogeneous. The method used was “casting”, pouring the formulation into acrylic moulds, spreading the mixture with a spoon and drying at room temperature. Films were recovered manually after 24 hours. Once recovered, the films were cut with a 6 mm diameter hole puncher and stored at 25°C and 25% relative humidity until they were used.
Antimicrobial activity by disc diffusion assay
The discs (6 mm in diameter) of biopolymers with the added extracts where applied by pressing slightly on the solid trypticase and brain heart infusion agar medium inoculated with 1 μL of bacterial suspension (108 CFU/mL) of P. gingivalis and A. actinomycetemcomitans. One disc impregnated with chlorhexidine was used as positive reference in each plate. The inoculated plates were incubated at 37°C for 24 hours in anaerobiosis and 5% CO2 atmosphere conditions respectively. Antimicrobial activity was evaluated by measuring the inhibition zones formed on the media in millimeters with a ruler. All inhibition assays and controls were repeated three times with each test conducted in duplicate. The collected data were analyzed by descriptive statistics ANOVA test using SPSS 17.0 software. The results were presented as mean±S.D. Statistical significance was accepted at the p<0.05 level.
RESULTS AND DISCUSSION
Periodontal diseases are the most frequent infections in humans. Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans are among the microorganisms most commonly identified with the process1. Increasing resistance to drugs and the side effects of some antibiotics and mouthwashes such a chlorhexidine, used in the treatment of periodontitis, justify the development of novel options as alternative of dental treatments. Plant extracts used in different countries as traditional medicines have been studied extensively -due to their properties as antimicrobials20. In this work, we tested the antimicrobial activity of aqueous extracts of T. vulgaris, M. chamomilla, C. lechleri, C. officinalis and J. adstringens Schl. by microdilution and agar diffusion method. The antimicrobial effect of the two biopolymers (chitosan and pullulan) with added aqueous extract of the plants on cultures of P. gingivalis and A. actinomycetemcomitans was tested using the disc diffusion method. The plants used in this work showed antimicrobial activity against the two oral pathogens tested.
Figure 1 shows the results obtained on the inhibitory activity of the different concentrations of T. vulgaris, M. chamomilla, C. lechleri, C. officinalis L. and J. adstringens Schl. against the tested strains. Our results show that minimal inhibitory concentration (MIC) of J. adstringens Schl. was 37 mg/mL for P. gingivalis and the same concentration of C. lechleri for A. actinomycetemcomitans. MIC of T. vulgaris and M. chamomilla were 62.5 mg/mL for both strains, since C. officinalis L. was 62.5 mg/mL for P. gingivalis and 250 mg/mL for A. actinomycetemcomitans and C. lechleri 111 mg/mL for P. gingivalis.
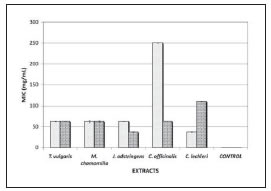
Fig. 1: Minimum inhibitory concentration (MIC) of the plant extracts on cultures of periodontopathogen microorganisms tested. A. actinomycetemcomitans; P. gingivalis. CONTROL (chlorhexidine 0.12%).
1.2 mg/mL of chlorhexidine was effective for both strains. The MBC value of J. adstringens and C. lechleri was 37 mg/mL for P. gingivalis and A. actinomycetemcomitans respectively. Concentrations of 62.5 mg/mL of T. vulgaris for P. gingivalis and J. adstringens for A. actinomycetemcomitans were needed. MBC of T. vulgaris were 111 mg/mL for A. actinomycetemcomitans and C. lechleri for P. gingivalis. M. chamomilla required higher concentrations (250 mg/mL) for both microorganisms. MBC of C. officinalis was 250 mg/mL for A. actinomycetemcomitans and 111 mg/mL for P. gingivalis. For chlorhexidine, 1.2 mg/mL was effective for both strains tested (Fig. 2).

Fig. 2: Minimum bactericidal concentration (MBC) of the plant extracts on cultures of periodontopathogen microorganisms. A. actinomycetemcomitans; P. gingivalis. CONTROL (chlorhexidine 0.12%).
Previous works indicate that chitosan and pullulan have certain in vitro antibacterial activity21. Other studies show that the exposure of A. actinomycetemcomitans to chitosan resulted in the disruption of cell membranes and that it could be considered for the treatment of periodontal diseases22. In this study, we tested the antimicrobial activity of two biopolymers (chitosan and pullulan) with added aqueous extract of T. vulgaris, J. adstringens Schl. and C. lechleri on cultures of P. gingivalis and A. actinomycetemcomitans using the disc diffusion method. Thickness of chitosan films was an average of 0.03 mm and for pullulan 0.07 mm.
Figure 3 shows the inhibition zones formed by the biopolymers chitosan and pullulan with added aqueous extracts of T. vulgaris, J. adstringens Schl. and C. lechleri, against A. actinomycetemcomitans cultures.

Fig. 3: A histogram plotting the width of the inhibition zones of the biopolymers with aqueous extracts on A. actinomycetemcomitans.
For chitosan, the inhibition zones were 4.5 mm without extract (CONTROL), 4 mm with T. vulgaris, 4.3 mm with J. adstringens Schl., and 6 mm with C. lechleri. For pullulan, the inhibition zones were 9.3 mm without extract (CONTROL), 9.6 mm with T. vulgaris, and 7.3 mm with J. adstringens Schl.
Figure 4 shows the inhibition zones formed by the biopolymers with plant extracts against P. gingivalis. For chitosan, the inhibition zones were 4 mm without extract (CONTROL), 3 mm with T. vulgaris, 3.6 mm with J. adstringens Schl. and 4.5 mm with C. lechleri. For pullulan, the inhibition zones were 4 mm without extract (CONTROL), 6 mm with T. vulgaris and C. lechleri, and 5 mm for J. adstringens Schl.

Fig. 4: A histogram plotting the width of the inhibition zones of the biopolymers with aqueous extracts on P. gingivalis.
CONCLUSIONS
The findings suggest that the use of pullulan with plant extracts had synergistic effects, enhancing the antimicrobial activity of the films. Chitosan films without extracts showed inhibitory effect against the growth of both strains. Our results reveal that both biopolymers with added plant extracts have antibacterial activity and can be used as bio-adhesive film against the periodontopathogens tested. These in vitro data still need to be validated as natural treatment for periodontal diseases by assessing clinical performance.
Data on the antimicrobial activity on pathogens are important for the future application of these polymers and plant extracts in medicine and dentistry, especially for J. adstringens, which showed the best result. This is an endemic plant of Mexico which is in danger of extinction.
ACKNOWLEDGEMENTS
This work was supported in part by the National Research Council of Mexico (CONACYT) grant #204231. We would like to thank to Hayashibara Co. for the generous donation of Pullulan. We also thank Dr. Roberto Mercado and MS. Ismael Malagon for statistical assistance. We appreciate the technical support of Vilma Suarez and Mayra Trevino.
1. Oral Microbiology and Immunology. Nisengard and Newman. Second Edition. 1994. W. B. Saunders Company. [ Links ]
2. The oral microflora and human periodontal disease. Slots J, Chen C. In: Tannock GW, ed. Medical importance of the normal microflora. 1998. London: Kluwer Academic Publishers. 101-127. [ Links ]
3. Quirynen M, Vogels R, Pauwels M, Haffajee AD, Socransky S, Uzel N.G, and Steenberghe D. van. Initial Subgingival Colonization of ‘Pristine’ Pockets. J Dent Res 2005; 84:340-344.
4. Slots J, Jorgensen M. Efficient antimicrobial treatment in periodontal maintenance care. J Am Dent Assoc 2000; 131:1293-1304. [ Links ]
5. Williams RC, Offenbacher S. Periodontal medicine. Periodontol 2000 2000; 23:9-156. [ Links ]
6. Kurita, K. Chemistry and Application of Chitin and Chitosan. Polym Degrad Stabil 1998;59: 117–120.
7. Hu Q, et al. Preparation and characterization of biodegradable chitosan/hydroxyapatite nanocomposite rods via in situ hybridization: a potential material as internal fixation of bone fracture. Biomaterials 2004; 25:779-785. [ Links ]
8. Leung S-HS, Leone RS, Kumar LD, Kulkarni N. Fast dissolving orally consumable films. PCT International Application 2000. WO 00/18365. [ Links ]
9. Nogueira JC, Diniz Mde F, Lima EO. In vitro antimicrobial activity of plants in Acute Otitis Externa. Braz J Otorhinolaryngol 2008; 74:118-124. [ Links ]
10. Ruiz-Bustos E, Velazquez C, Garibay-Escobar A, Garcia Z, Plascencia-Jatomea M, Cortez-Rocha MO, Hernandez-Martinez J, Robles-Zepeda RE. Antibacterial and antifungal activities of some Mexican medicinal plants. J Med Food 2009; 12:1398-1402. [ Links ]
11. Tratado de fitofarmacos y neutraceuticos. Alonso, J. 1a. Reimpresion corregida y renovada. 2007. Corpus Editorial y Distribuidora. [ Links ]
12. Chen Z, Cai Y, Philliphson J. Studies on the antitumor, antibacterial, and wound-healing properties of dragon’s blood. Planta Med 1994; 60:541-545.
13. Kishimoto S, Maoka T, Sumitomo K, Ohmiya A. Analysis of carotenoid composition in petals of calendula (Calendula officinalis L.) . Biosci. Biotechnol. Biochem 2005; 69: 2122-2128. [ Links ]
14. Navarrete A, Avula B, Joshi VC, Ji X, Hersh P, Khan IA. Quantitative determination of triterpenes from Amphitherygium adstringens by liquid chromatography and morphological analysis of cuachalalate preparations. Journal - Association of Official Analytical Chemists Int 2006; 89: 1-7. [ Links ]
15. Cowan, M. Plant products as antimicrobial agents. Clinical Microbiology Reviews, American Society for Microbiology 1999; 12:564–582.
16. Rams TE, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000 1996; 10:139-159. [ Links ]
17. Golomb G, Friedman M, Soskolne A, Stabholz A and Sela MN. Sustained release device containing metronidazole for periodontal use. Journal of Dental Research 1984; 63:1149-1153. [ Links ]
18. Pina-Vaz C, Goncalves Rodrigues A, Pinto E, Costa-de- Oliveira S, Tavares C, Salgueiro L, Cavaleiro C, Goncalves MJ, Martinez-de-Oliveira J. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol 2004; 18:73-78. [ Links ]
19. Olivera Ortega AG, Soto Hernandez M, Martinez Vazquez M, Terrazas Salgado T, Solares Arenas F. Phytochemical study of cuachalalate (Amphiptherygium adstringens, Schiede ex Schlecht). J. Ethnopharmacol 1999; 68:109-113. [ Links ]
20. More G, Tshikalange TE, Lall N, Botha F, Meyer JJ. Antimicrobial activity of medicinal plants against oral microor ganisms. Journal of Ethnopharmacology 2008; 119:473-477. [ Links ]
21. Tin S, Sakharkar K, Lim C, Sakharkar M. Activity of Chitosans in combination with antibiotics in Pseudomonas aeruginosa. International Journal of Biological Sciences 2009; 5:153-160. [ Links ]
22. Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH, Kim CY. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. International Journal of Antimicrobial Agents 2001; 18:553-557. [ Links ]














