Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.24 no.1 Buenos Aires abr. 2011
ARTÍCULOS ORIGINALES
The effect of spiramycin on Porphyromonas gingivalis and other “classic” periopathogens
Verónica Chiappe1, Mariel Gómez1, Liliana Fernández-Canigia2, Hugo Romanelli1
1Department of Biomedical sciences and Diagnosis. CEBBAD. Maimonides University. Buenos Aires. Argentina.
2Clinical Investigation and Medical Education Centre “Dr. Norberto Quirno”, Buenos Aires, Argentina.
CORRESPONDENCE Dr. Veronica Chiappe Facultad de Odontologia, Universidad Maimonides Hidalgo 765. C 1405 BCK. Ciudad Autonoma de Buenos Aires. E-mail: vbchiappe@gmail.com
ABSTRACT
In clinical trials, Spiramycin has shown additional benefit over scaling and root planing on pocket depth reduction, but its effect on periodontal microbiota was evaluated only by darkfield microscopy. Therefore, this study was conducted to determine the effect of Spiramycin administration on Porphyromonas gingivalis and other periodontopathic bacteria using 16S rARN PCR technique. Thirty two non-smoker adults with untreated periodontitis and pocket depth ≥ 7 mm. were evaluated to participate in this randomized placebo-controlled clinical trial. Clinical measurements were performed on day -15, 15, 30 and 90 from baseline. Subgingival samples were analyzed for detection of Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), Treponema denticola (Td) and Aggregatibacter actinomycetemcomitans (Aa) on days -15, 30 and 90. On day 0, 25 Pg positive subjects were randomly assigned to receive either systemically administered Spiramycin for 7 days (Test group SP) or identical placebo tablets (Placebo group PL). After Spiramycin administration Pg, Tf and Td were suppressed showing statistically significant difference (p<0.05) with the Placebo group. None of the groups showed changes in Aa detection. These data indicate that bacteria currently associated with advanced periodontitis (Pg, Tf and Td) are suppressed after 7 days of systemic administration of Spiramycin.
Key words: Spiramycin; Porphyromonas gingivalis; Periodontitis; Antibiotics.
RESUMEN
Efecto de la espiramicina sistémica sobre Porphyromonas gingivalis y otros periopatógenos “clásicos”
Los estudios clinicos indican un efecto adicional beneficioso de la Espiramicina cuando se la utiliza junto con el raspaje y alisado radicular. Hasta el presente su efecto sobre la microbiota periodontal ha sido estudiado solo con microscopio de campo oscuro. El objetivo del presente estudio fue evaluar el efecto de la Espiramicina sobre Porphyromonas gingivalis y otros patogenos periodontales “clasicos” con la tecnica PCR utilizando el gen 16S del ARNr. Participaron de este estudio clinico-controlado randomizado 32 sujetos adultos, no fumadores, con enfermedad periodontal no tratada, con bolsas periodontales ≥ a 7 mm. Se registraron parametros clinicos en el dia -15, 15, 30 y 90 de iniciado el estudio. Se analizo el biofilm periodontal subgingival con PCR, identificandose Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), Treponema denticola (Td) y Aggregatibacter actinomycetemcomitans (Aa), en el dia 30 y 90. En el dia 0, 25 sujetos positivo para Porphyromonas gingivalis se dividieron aleatoriamente en dos grupos: Grupo Test (Espiramicina 7 dias) y Grupo Control (Placebo 7 dias). La Espiramicina fue efectiva sobre Pg, Tf, Td. Hubo diferencia (p<0,05) con el grupo control. Ninguno de los grupos mostro diferencias para Aa. Estos datos indicarian que la Espiramicina fue efectiva sobre las bacterias que actualmente se asocian con periodontitis severas (Pg, Tf, Td) luego de la administracion sistemica durante 7 dias.
Palabras clave: Espiramicina; Porphyromonas gingivalis; Periodontitis; Antibioticos.
INTRODUCTION
The presence of periodontal pathogens such as Porphyromonas gingivalis, Tannerella forsythia and Aggregatibacter actinomycetemcomitans has been linked to ongoing periodontal destruction by several authors 1-5. Given the microbial etiology of periodontal diseases, several studies have indicated the effectiveness of adjunctive antibiotics to scaling and root planing, particularly in terms of pocket depth reduction and attachment level gain, not only in Aggressive Periodontitis but also in advanced Chronic Periodontitis 6,7.
Spiramycin is a macrolide antibiotic. It has been considered active mainly against Gram positive bacteria and, to a lesser extent, against Gram negative bacteria. In vitro studies that evaluated Spiramycin activity against periodontopathic bacteria showed that spirochetes were sensitive, Aggregatibacter actinomycetemcomitans, Eikenella corrodens were resistant and Porphyromonas gingivalis, Fusobacterium nucleatum showed variable results 8-12. Although Spiramycin has been used in the treatment of periodontal disease since 1956 13, few controlled clinical trials have been published so far 14-18. After a systematic evaluation of the use of systemic antimicrobials, Herrera et al.6 concluded that patients with progressive or active disease could further benefit from systemic antimicrobial therapy as an adjunct to scaling and root planing, and observed that Spiramycin clearly produced an added benefit in pocket depth reduction. Chin Quee et al 16 and Gomez et al 18 demonstrated that systemically administered Spiramycin in combination with mechanical therapy was more effective in reducing spirochetes than scaling and root planing alone.
Until now, the microbiological outcome has been evaluated only by darkfield microscopy. The present study was designed to evaluate the in vivo effect of the systemic administration of Spiramycin on Porphyromonas gingivalis and other “classic” periodontopathic bacteria: Tannerella forsythia, Treponema denticola and Aggregatibacter actinomycetemcomitans, using PCR technique as the microbiological method.
MATERIAL AND METHODS
The protocol used in this study was approved by the Ethics and Research Committee from Maimonides University. A total 32 volunteers who were referred to the Periodontology Service at Military Central Hospital Dr. Cosme Argerich for diagnosis and treatment of periodontitis were evaluated. Inclusion criteria were: age ≥ 30 years, non-smoker, clinical diagnosis of advanced chronic periodontitis characterized by the presence of at least 3 sites in different teeth with pocket depth ≥ 7mm and ≤ 10 mm, and presence at baseline microbiological examination of Porphyromonas gingivalis. Exclusion criteria included: known allergy to Spiramycin, professional scaling and root planing or surgical periodontal therapy in the past 6 months, systemic or topical antibiotic therapy 6 months prior to the initiation of the study, pregnancy, nursing, systemic disease such as diabetes or HIV and acute necrotizing disease. This study was designed as a randomized, placebo controlled clinical trial (RCCT) according to the CONSORT criteria 19. Following a full mouth screening visit, the most accessible site of all sites with pocket depth ≥ 7mm and ≤ 10 mm was selected for examination. On day -15, clinical measurements and subgingival samples were taken from the selected site in 32 subjects. 25 subjects, each contributing with one positive site for P. gingivalis entered into the study after having given their written informed consent. On day 0 (baseline) subjects were randomly assigned 20 to receive either systemically administered Spiramycin (Test group SP, n: 13) or identical placebo tablets (Placebo group PL, n: 12).
Clinical measurements were repeated on days 15, 30 and 90. Bacteriological samples were repeated on days 30 and 90. On day 30, Test group SP received instructions for oral hygiene, which were reinforced every 15 days. Subjects of Placebo group PL were no longer included in the study and they received conventional mechanical therapy. At the end of the trial (day 90) subjects of Test group SP received conventional mechanical therapy (Fig. 1). Test group SP received systemic Spiramycin beginning with two tablets (loading dose), continuing with 1 tablet every 12 hours to complete 7 days. Placebo group PL received placebo tablets following the same pattern as the Test group SP. Spiramycin was provided by Aventis Pharma Laboratory in its commercial formula RovamycineR. Each tablet contained 3.000.000 I.U. (650.4 mg of Spiramycin). The placebo tablets were identical to the Spiramycin tablets in shape, size and color. A labeled vial was provided to each patient containing the exact quantity of tablets for 7 days and the instructions for their use. Randomization was generated using random tables. The randomization list was kept by one of the authors (MG). The study was designed as double blinded concerning the patients, the clinical examiner (VCH) and the microbiologist (LFC), who were unaware of the treatment the patients had received. The following clinical parameters were recorded at the selected sites using a manual probe (Hu-Friedy, Marquis CPC 12): Plaque Index (PI) 21, Gingival Index (GI) 22, Bleeding within 30s on slight pocket probing (BOP), assigning positive (+) to present and negative (-) to absent, Probing pocket depth (PPD) and clinical attachment level (CAL) from the cement-enamel junction. Measurements were rounded to the nearest mm. The same probe was assigned to each subject throughout the study, to reduce variability 23.

Fig. 1: Flow chart of subjects through the experimental period.
All clinical measurements and microbiological sampling were performed by one clinical examiner (VCh), who was unaware of the results of the microbiological analysis at every point of the study. Sampling procedures: subgingival sampling was carried out after the PI assessments and before BOP, PPD and CAL. The selected site was isolated with cotton rolls and supragingival plaque was carefully removed with a curette. The subgingival plaque sample was taken by inserting 2 sterile paper points (Sharpys) consecutively into the periodontal pocket for 15 seconds each, which were then placed in a sterile tube containing 250 μl of sterile phosphate buffered saline solution (PBS). Samples were sent to the laboratory within 3 hours. Microbiological procedures: a Multiplex PCR, using conserved and species-specific 16S ribosomal RNA gene primers (Biodynamics) for simultaneous detection of A.actinomycetemcomitans, T.forsythia and P.gingivalis, were used 24. The nucleotide sequences of the primers were as follows: A.actinomyctemcomitans specific forward primer (Aa F), 5’-ATT GGG GTT TAG CCC TGG TG-3’, T.forsythia specific forward primer (Tf F), 5’-TAC AGG GGA ATA AAA TGA GAT ACG-3’, P.gingivalis specific forward primer (Pg F), 5’-TGT AGA TGA CTG ATG GTG AAA AACC- 3’; and conserved reverse primer (C11R) 5’-ACG TCA TCC CCA CCT TCC TC-3’. The expected product lengths were 360 bp for A.actinomycetemcomitans, 745 bp for T.forsythia and 197 bp for P.gingivalis.
The Multiplex PCR reactions were performed in a total volume of 50 μl, consisting of 0.7 μl of HotStar Taq DNA polymerase (Invitrogen), 5 μl of 10mM Tris-HCl, 50mM KCl (10X PCR Buffer), 2.0 mM MgCl2, 0.2 mM dNTP (Invitrogen), 0.4 μM primer C11R, 0.2 μM primer Aa F, 0.8 μM primer Pg F, 1 μM Tf F and 5 μl of template DNA. For detection of T.denticola, species-specific primers (Biodynamics SRL) based on 16S rRNA (Td F) 5’- TAA TAC CGA ATG TGC TAC TTT ACA T-3’ and (Td R) 5’-TCA AAG AAG CAT TCC CTC TTC TTC TTA-3’ 25, were used. The amplicon length expected was 316 bp. Amplifications were performed in 50 μl total volume, containing 0.4 μl of HotStar Taq DNA polymerase (Invitrogen), 5 μl of 10mM Tris-HCl, 50mM KCl (10X PCR Buffer), 2.5 mM MgCl2, 0.2 mM dNTP (Invitrogen), 0.1 μM of each primer and 5 μl of template DNA. The cycling parameters for PCR reactions consisted of 35 cycles (1min at 94oC, 1 minute at 61oC and 1 min at 74oC) and an initial denaturation step of 15 min at 94oC preceded the amplification cycles. PCR amplification was carried out in an automated thermal cycler (Perkin Elmer). A positive control using a DNA extract from the target species as template and a negative control (water) were both included in each PCR set. PCR products were analyzed by 2% agarose gel electrophoresis, stained with ethidium bromide (1μg/ml) and inspected under UV light translumination. A positive or negative identification was based on the presence of clear bands of the expected molecular size, using a 100 bp DNA ladder (Biodynamics). Data Analysis: the statistical analysis of the data was carried out with the software package SPSS (10.0). The significant level was set at 0.05 for all tests. Clinical and microbiological data were analyzed with the site/subject as the observational unit P.gingivalis negative by PCR was set as the primary outcome variable.
The Kruskal Wallis test was used for ordinal data at baseline (PI, GI). Appropriate analysis of variance (ANOVA) was used for numerical data (PPD, CAL). PI was analyzed as numerical data. When interaction between main factors was found, 95% confidence intervals were calculated to evaluate differences under specific conditions. Fisher exact probability and Chi-square tests were employed to compare frequencies whenever nominal data were registered (BOP, Presence / Absence of pathogens).
RESULTS
25 out of the 32 subjects initially evaluated with PPD ≥ 7 mm, presented P. gingivalis (78.1%) and were included in the study. Of these subjects, 90% were also positive for T. forsythia, 85% for T. denticola, and 30% for A.actinomycetemcomitans. 5 subjects were lost to follow-up. Exiting the study was due to non-compliance with medication intake or non-attendance to visit. 20 subjects (12 female), average age 55 years, completed the study. These patients did not manifest any adverse reaction to Spiramycin (Fig. 1).
Baseline data showed no statistically significant difference between test and Placebo groups (p>0.05) for the subgingival species investigated (Table 1) or for any of the clinical parameters (Table 2). After medication, no change was observed in PI on days 15 and 30 in any group (Table 3).
Table 1: Number of subjects positive for investigated species al baseline.

Table 2: Mean (SD) baseline clinical measurements.
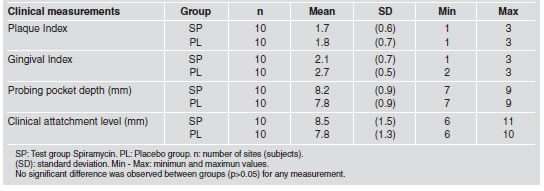
Table 3: Change in Plaque Index (PI), Probing pocket depth (PPD) and Clinical attachment level (CAL).

On day 90, PI was reduced to score ≤ 1 in the Test group after oral hygiene instruction. In the Test group, PPD values were significantly reduced from baseline, on days 15, 30 and 90. Reduction from baseline to day 90 was -3.4 mm (SD 1.0). In the Placebo group, PPD values were significantly reduced on day 15 (-0.8 mm SD 0.9) and not significantly on day 30 (-0.4 mm SD 1.0). Comparison between placebo and Test groups showed statistically significant difference in both evaluations (15 and 30 days) (p<0.05) (Table 3). CAL values were significantly reduced on day 15, 30 and 90 from baseline in the Test group. In the Placebo group, CAL values did not change significantly over time. Comparison between the Placebo group and the Test group showed no difference on day 15 and statistically significant differences on day 30 (p<0.05) (Table 3).
The number of sites with BOP was reduced in the Test group on days 15, 30 and 90 (p<0.05). No change in the number of bleeding sites was observed in the Placebo group over time. The difference between the Test and Placebo groups was statistically significant (p<0.05) (Table 4).
Table 4: Bleeding on probing (BOP). Change in frequency of bleeding sites.

Microbiological findings are presented in Table 5. On days 30 and 90 after antibiotic intake, the number of subjects positive for P.gingivalis, T.forsythia and T.denticola in the Test group showed a statistically significant decrease (p<0.05). The decrease was not statistically significant between the two time points (p>0.05). In the Placebo group, the number of positive sites for the investigated species showed no change over time. Comparison between the Test and Placebo groups on day 30 yields statistically significant difference (p<0.05). No change was observed in the number of sites with A.actinomycetemcomitans, either in the Test or in the Placebo group. Comparison between groups showed no statistically significant difference (p>0.05).
Table 5: Number of subjects positive for Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), Treponema denticola (Td) and Aggregatibacter Actinomicetemcomitans (Aa) during the experimental period.

DISCUSSION
The primary outcome variable selected in the present study was suppression of Porphyromonas gingivalis detected in deep periodontal pockets after the administration of Spiramycin and the secondary outcomes included the effect of this antibiotic on T.forsythia, T.denticola and A.actinomycetemcomitans. This study was not designed to evaluate this antimicrobial for treating periodontitis. Our results showed that Spiramycin was effective in suppressing P.gingivalis, T.forsythia and T.denticola, but not A.actinomycetemcomitan. On day 90, a slight tendency to regrowth was observed only for T.forsythia. It is worth noting that oral hygiene instructions were given on day 30, and reinforced as necessary. The role of meticulous plaque control to limit pathogen regrowth has been described previously 26. It should also be considered that pocket depth reduction modifies the subgingival environment and limits colonization by anaerobic bacteria 27. The reduction of P.gingivalis, T.forsythia and T.denticola was reflected in the clinical condition: decrease of pocket depth, gain of clinical attachment level and reduction in the number of sites with bleeding on probing. Sites with persistence of A.actinomycetemcomitans also showed clinical improvement. Favorable clinical response to mechanical periodontal therapy has been described in Chinese chronic periodontitis patients at sites infected with A.actinomycetemcomitans 28. It should be taken into account that different serotypes of A.actinomycetemcomitans vary in virulence and pathogenic potential 29.
Controlled clinical trials found significant reduction in probing pocket depth14,17 and bleeding on probing 15. Microbiological findings showed significant reduction in motile rods and spirochetes, favoring Spiramycin when compared with placebo, as a monotherapy 15,18 and when combined with mechanical therapy 16,18. Comparison of our results with the studies mentioned above is limited, due to variability in dosages, the microbiological technique used and patient selection. These studies administered 500 to 1000 mg twice a day for 14 days; microbiological evaluation was by darkfield microscopy and patients had Adult Periodontitis (Criteria described by the America Academy of Periodontology 1989) 30. We administered 650.4 mg (as RovamicyneR is manufactured nowadays) twice a day for 7 days; microbiological evaluation was done by PCR and the selection of patients followed the current classification of the AAP 1999 31. Nevertheless, the major reduction in the percentage of spirochetes previously reported 15,16,18 is in agreement with the reduction of T.denticola that we observed with PCR technique. We administered Spiramycin for 7 days compared to its traditional dosage of 14 days. Interestingly, only 7 days of medication showed suppression of the bacteria studied. Fewer days of medication would benefit compliance. The recognition that bacteria residing in biofilms are far more resistant to antibiotics than the same species in planktonic state 32 gave rise to the concern that systemic antimicrobials as monotherapy may have little or no effect on the subgingival microbiota. However, the antimicrobial effect observed in this study occurred on bacteria belonging to a biofilm. The mechanisms of increased resistance in biofilms differ according to the species, the antibiotic and the different habitats where biofilms grow. One mechanism of resistance to antibiotics is the capacity of the exopolymer matrix to retard diffusion. Positively charged hydrophilic antibiotics may be inactivated by enzymes. Macrolides are unaffected by this process even if positively charged, because they are hydrophobic 33.
In addition to knowing the microbicidal spectrum of an agent, as determined by use of the kill kinetics assay with planktonic organisms, it is also important to know how the agent acts when confronted with the challenge of penetrating a biofilm and killing the component bacteria. Antimicrobial susceptibility determined in vitro does not correlate with in vivo effect if bacteria grow in a biofilm 34-36. In this study we analyzed the outcome of Spiramycin on bacteria belonging to periodontal pocket biofilm. The findings of the present study have indicated that the use of systemic Spiramycin for 7 days suppresses Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola but not Aggregatibacter actinomycetemcomitans. Further studies evaluating this dosage of Spiramycin adjunct to scaling and root planing are needed to establish its benefit in the treatment of periodontitis patients.
ACKNOWLEDGMENTS
Special thanks to Dr. Ricardo Macchi for the statistical support, Dr. Paulina Mosquera, Dr. Analia Paolinelli for clinical assistance and Dr. Alfredo Vitullo for reviewing the manuscript. The investigation support and drug supply was provided by Aventis Pharma Laboratory SA. Argentina.
1. Ali RW, Bakken V, Nilsen R, Skaug N. Comparative detection frequency of 6 putative periodontol pathogens in sudanese and norwegian adult periodontitis patients. J Periodontol 1994; 65: 1046-1052. [ Links ]
2. Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000 1994; 5:78-111. [ Links ]
3. Machtei EE, Dunford R, Hausmann E, Grossi SG, Powell J, Cummins D, Zambon JJ, Genco RJ. Longitudinal study of prognostic factors of established periodontitis patients. J Clin Periodontol 1997; 24:112-109. [ Links ]
4. Faveri M, Figueiredo LC, Duarte PM, Mestnik MJ, Mayer MPA, Feres M. Microbiological profile of untreated subjects with localized aggressive periodontitis. J Clin Periodontol 2009; 36:739-749. [ Links ]
5. Sanz M, Quirynen M. Advances in the aetiology of periodontitis. Group A consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol 2005; 32:54-56. [ Links ]
6. Herrera D, Sanz M, Jepsen S, Needleman I, Roldan S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planning in periodontitis patients. J Clin Periodontol 2002; 29 (Suppl 3): 136-159. [ Links ]
7. Haffajee AD, Socransky SS, Gunsolley JC. Sistemic antiinfective periodontal therapy. A systematic review. Ann Periodontol 2003; 8:115-181. [ Links ]
8. Slots J, Rams TE. Antibiotics in periodontal therapy: advantages and disadvantages. J Clin Periodontol 1990; 17:479-493. [ Links ]
9. Baker PJ, Evans R, Slots J, Genco R. Antibiotic susceptibility of anaerobic bacteria from human oral cavity. J Den Res 1985; 64:1233-1244. [ Links ]
10. Mouton C, Dextraze B, Mayran D. Sensibilite au metronidazole a la Spiramycine et a leur association de bacteries a potentiel parodontopathique.J Biol Buccale 1984; 12:17-20. [ Links ]
11. Chin Quee T, Roussou T, Chan E. In vitro activity of Rodogyl against putative periodontopatic bacteria. Antimicrob Agents Chemother 1983; 24:445-447. [ Links ]
12. Lakhssassi M, Elhajoui N, Lodter JP, Pineill JL, Sixou M. Antimicrobial susceptibility variation of 50 anaerobic periopathogens in aggressive periodontitis: an interindividual variability study. Oral Microbiol Immunol 2005; 20:244-252. [ Links ]
13. Daligand PD. Le paradentosis: resultats de 3 annees de traitements par la Spiramycine. Revue Stomatol 1958; 59: 784-787. [ Links ]
14. Mills WH, Thompson GW, Beagrie GS. Clinical evaluation of Spiramycin and Erithromycin in control of periodontal disease. J Clin Periodontol 1979; 6:308-316. [ Links ]
15. Sznajder N, Piovano S, Bernat MI, Flores L, Macchi R, Carraro JJ. Effect of Spiramycin therapy on human periodontol disease. J Periodont Res 1987; 22:225-258. [ Links ]
16. Chin Quee T, Al-Jobouri W, Lautar-Lemay C, Chan ECS, Iugovaz I, Bourgoin J, Delorme F. Comparison of Spiramycin and Tetracycline used adjunctively in the treatment of advanced chronic periodontitis. J Antimicrob Chemother 1988; 22: (Suppl B) 171-177. [ Links ]
17. Bain CA, Beagrie GS, Bourgoin J, Delorme F, Holthuis A, Landry R, Roy S, Schuller P, Singer D, Turnbull R. The effects of Spiramycin and/or scaling on advanced periodontitis in humans. J Can Dent Assoc 1994; 60:209-217. [ Links ]
18. Gomez M, Romanelli H, Sznajder N, Chiappe V, Bernat MI, Lavandeira H, Macchi R. Accion de la Espiramicina en la periodontitis del adulto. R.A.O.A 1998; 86:574-582. [ Links ]
19. Altman DG, Schulz KF, Mohler D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T. The revised consort statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001; 134: 663-694. [ Links ]
20. Ramon-Torrel JM. Metodos de investigacion en Odontologia, España: Editorial Masson, 2000; 107-121 [ Links ]
21. Silness J, Loe H. Periodontal disease in pregnancy. Acta Odontol Scand 1964; 22:121-135. [ Links ]
22. Loe H, Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol Scand 1963; 21:533-551. [ Links ]
23. Van der Zee E, Davies EH, Newman HN. Marking width, calibration from tip and tine diameter of periodontal probes. J Clin Periodontol 1991; 18:516-520. [ Links ]
24. Tran SD, Rudney JD. Improved Multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actinomycetemcomitans, Bacteroides forsythus and Porphyromonas gingivalis. J Clin Periodontol 1999; 37:3504-3508. [ Links ]
25. Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol and Immunol 1996; 11:266-273. [ Links ]
26. Magnusson I, Lindhe J, Yoneyama T, Liljenberg B. Recolonization of a subgingival microbiota following scaling in deep pockets. J Clin Periodontol 1984; 11:193-207. [ Links ]
27. Socransky SS, Haffajee A. Ecologia microbiana periodontal. Periodontol 2000 (Ed Esp) 2006; 12:135-187. [ Links ]
28. Tong KSG, Zee KY, Lee DH, Corbet EF. Clinical responses to mechanical periodontal treatment in chinese chronic periodontitis patients with and without Actinobacillus actinomycetemcomitans. J Periodontol 2003; 74:1582-1588. [ Links ]
29. Yang HW, Asikainen S, Dogan B, Suda R, Lai CH. Relationship of Actinobacillus actinomycetemcomitans serotype b to aggresive periodontitis: frequency in pure cultured isolated. J Periodontol 2004; 75:592-599. [ Links ]
30. The American Academy of Periodontology. Proceedings of the World Workshop in Clinical Periodontics. Chicago: The American Academy of Periodontology 1989; 1/23-1/24. [ Links ]
31. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999; 4:1-6. [ Links ]
32. Steward P, Costerton J. Antibiotic resistance of bacteria in biofilms. Lancet 2001; 358:135-138. [ Links ]
33. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000 2002; 28:12-55. [ Links ]
34. Larsen T. Susceptibility of Porphyromonas gingivalis in biofilms to Amoxicillin, Doxicycline and Metronidazol. Oral Microbiol Immunol 2002; 17:267-271. [ Links ]
35. Noiri Y, Okami Y, Narimatsu M, Takahashi Y, Kawahara T, Ebisu S. Effects of Chlorhexidine, Minocycline and Metronidazol on Porphyromonas gingivalis strain 381 in biofilms. J Periodontol 2003; 74:1647-1651. [ Links ]
36. Eick S, Seltmann T, Pfister W. Efficacy of antibiotics to strains of periodontopathogenic bacteria within a single species biofilm. In vitro study. J Clin Periodontol 2004; 31:376-383. [ Links ]














