Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.25 no.1 Buenos Aires abr. 2012
ARTÍCULOS ORIGINALES
Periodontal response to subgingival restorations in dogs with periodontitis
Diego V. Saldanha, Sabrina C. Gomes, Daniela M. Souza, J. Cavagni, Rui V. Oppermann
School of Dentistry, Federal University of Rio Grande do Sul (UFRGS)- Porto Alegre-RS-Brazil
CORRESPONDENCE Dr. Sabrina Carvalho Gomes Av. Ramiro Barcelos, 2492 Porto Alegre/RS, Brazil Zip Code: 90035-003 email: sabrinagomes.perio@gmail.com
ABSTRACT
The aim of this study was to evaluate the periodontal response to subgingival restorations in dogs with naturally occurring periodontitis. At the baseline, the experimental teeth from three dogs (2nd and 3rd upper premolars and 2nd, 3rd and 4th lower premolars) were randomly assigned to Resin-modified Glass Ionomer Cement (RMGIC) and Amalgam (AM) restorations or controls (CT) at the buccal sites with (SUPRA+) or without mechanical supragingival plaque control (SUPRA-) and maintained for 90 days. Clinical [Periodontal Probing Depth (PPD), Clinical Attachment Loss (CAL), and Gingival Margin Recession (GMR)], histological (connective tissue inflammatory and epithelium condition) and histometric evaluation (distance between the apical border of the cavity and the bone level and between the apical extension of the epithelium and the bone level) were performed by a calibrated blinded examiner. Better clinical (especially regarding CAL) and histological results (unaltered epithelium and less severe inflammatory connective tissue) were observed associated with RMGIC sites. Histometric evaluation showed less bone loss associated to RMGIC. Overall, SUPRA+ sites presented less inflammatory response. It could be concluded that in dogs with periodontitis, subgingival RMGIC restorations, especially in the presence of supragingival plaque control, elicited better periodontal response than AM restorations.
Key words: Dental Amalgam; Glass Ionomer; Dogs; Periodontitis.
RESUMO
Resposta periodontal a restaurações subgengivais em cães com periodontite
O objetivo do presente estudo foi avaliar a resposta do periodonto a restaurações subgengivais em cães com diagnóstico de periodontite. No início do estudo, os dentes experimentais de três cães (2o e 3o molares superiores e 2o, 3o e 4o premolares inferiores) foram randomicamente designados para restauração com cimento de ionômero de vidro modificado por resina (CIVMR), amálgama (AM) ou Controle (CT) nos sítios vestibulares submetidos (SUPRA+) ou não (SUPRA-) a controle mecânico de placa supragengival e mantidos por 90 dias. Avaliações clínicas [Profundidade de Sondagem (PS), Nível de Inserção Clínica (NIC), Recessão Gengival (RG)], histológicas (condições inflamatórias do tecido epitelial e conjuntivo) e histométricas (distância entre a margem apical da cavidade e o nível ósseo e entre a extensão apical do epitélio e o nível ósseo) foram realizadas por examinador calibrado e cego. Melhores resultados clínicos (especialmente quanto à PI) e histológicos (epitélio sem alterações e tecido conjuntivo com menor severidade de infiltrado inflamatório) foram observados em associação a sítios restaurados com CIVMR. A avaliação histométrica mostrou menor perda óssea associada a restauração com CIVMR. Todos os sítios SUPRA+ exibiram menor resposta inflamatória. Pode ser concluído que, em cães com periodontite, restaurações subgengivais realizadas com CIVMR, especialmente na presença de controle mecânico de placa supragengival, apresentaram melhor resposta do periodonto quando comparadas com restaurações de amálgama.
Palavras chave: Amálgama; Ionômero de Vidro; Cães; Periodontite.
INTRODUCTION
The early studies by Waerhaug brought some understanding of the periodontal response to restorations1-3. According to these studies, the "weak point" in this relationship was the interface between the restorations and the cavities, filled with bacterial plaque. On the other hand, other authors related the observed inflammatory response to the composition of the restorative material4 or to its surface roughness and degree of polishing1,5, 6. Traditionally, restored sites were always associated with more attachment loss than non-restored sites7, 8 while subgingival restorations have been shown to be associated with more severe periodontal destruction than supragingival restorations or non-restored surfaces7, 9, 10. Overhangs also contribute to a worse periodontal condition11 even thought Parsell et al.12 questioned this. Other authors have shown that the elimination of overhangs, in association with improved supragingival plaque control, leads to better clinical periodontal response 13, 14.
In the seventies, new adhesive materials were introduced to periodontal studies with interesting results compared to "traditional" materials such as silicate and amalgam15,16. Van Dijken and Sjöstrom17 recently observed, during an experimental gingivitis period, more gingival crevicular fluid associated with restored sites with adhesive materials (resin, compomer or resin-modified glass ionomer cement) than with enamel surfaces. On the other hand, Paolantonio et al.18 observed no differences between Class V restorations in healthy subjects, during a one-year investigation, although they reported an increase in Gram-negative bacteria associated to subgingival resin restored sites. Glass ionomer cement (GIC) is recognized as an important biocompatible material used in retro-filling restorations19, 20 and subgingival restorations. Dragoo21 and White22 found a favorable periodontal response to these subgingival restorations. Breault et al.23 showed reduction in probing depth and in the percentage of bleeding upon probing after the replacement of subgingival resin-modified glass ionomer cement (RMGIC) restorations. Dragoo21 also observed a good histological response to these subgingival restorations. A clinical and histological evaluation performed on healthy dogs by Gomes et al.24 demonstrated a better periodontal response to subgingival RMGIC restorations than to Amalgam. These results were particularly noticeable when supragingival plaque control was performed. Nevertheless, it should be kept in mind that the susceptibility to periodontal disease may influence such a response to these restorations. Earlier studies performed by Lindhe et al. 25 pointed to the fact that development of periodontitis in this animal model is not predictable and may influence the impact on the experimental design.
Dogs with naturally occurring periodontitis are rarely used in the studies and the influence of the disease on the periodontal response in seldom discussed. Bogle et al.26 showed different response to periodontal therapy in dogs with artificial or naturally occurring periodontitis. The authors postulated that the worse results found in the naturally occurring periodontitis group was due to the "chronic nature of the disease". In this sense, it would be interesting to evaluate the impact of the placement of subgingival restorations in periodontitis in dogs. The aim of the present study was to investigate the clinical and histological periodontal response to subgingival restorations in dogs with naturally occurring periodontitis.
MATERIALS AND METHODS
Ethical considerations
The research was approved by the Ethical and Research Committee of the Federal University of Rio Grande do Sul, RS, Brazil.
Sample description
Three healthy female mongrel dogs (3 to 5 years old) weighing approximately 8 to 10 kg, with periodontitis [Clinical Attachment Loss (CAL) associated with Bleeding on Probing (BOP)] were included. The animals were kept on a soft diet and received water ad libitum throughout the study.
Experimental Procedures
All the experimental procedures are summarized in Fig. 1.

Fig. 1: Flowchart of the experimental procedures.
Animal preparation and Clinical examination
Initially, the animals were sedated with 1% azeprimazin (Acepran, Univet S/A, Brazil) and anesthetized with Halotan gas (Cristália Ltda., Brazil). Experimental teeth (2nd and 3rd upper premolars and 2nd, 3rd and 4th lower premolars) were randomly assigned to RMGIC and Amalgam (AM) restorations or controls (CT). Clinical examinations were performed on Baseline (day 0) and at the end of the experimental period (day 90). Visible Plaque Index (VPI), Gingival Bleeding Index (GBI), Periodontal Probing Depth (PPD), BOP and CAL were recorded. Local anesthesia was provided (2% mepivacaine with noradrenaline, Scandicaine, DFL, Brazil) and a notch was made on the buccal aspect of the selected experimental teeth to identify the gingival margin position in order to measure the Gingival Margin Recession (GMR) accurately after the healing period.
Surgical and restorative procedures
Full thickness flaps were raised at the buccal side of all the experimental teeth. Then, the exposed roots were thoroughly scaled and planed with periodontal files and curettes (Neumar, SP, Brazil). In sequence, roots with more extensive bone loss were assigned to receive the restorations (RMGIC and AM) or serve as CT. In the RMGIC and AM teeth, after the surgical procedures, cavities were prepared (#1011 diamond burs, KG Sorensen, Brazil) and the apical wall the preparations were extended to the bone crest. The bur diameter determined the cavity size. AM (Dispersalloy, Dentsply Caulk, USA) and RMGIC (Vitremer, 3M, Brazil) were performed according to the manufacturer's instructions. In sequence, flaps were repositioned and sutured.
Post-surgical period
Sutures were removed in the 10th day. During the first 15 days post-surgery, chemical plaque control was performed, once daily, with 2% chlorhexidine gel (Bellafarma, Caxias do Sul, Brazil). After this period, daily mechanical plaque control was performed (SUPRA +), in quadrants 2 and 3 by a trained professional, with a soft baby brush (Dental Baby, Sonae Distribuidora, Brazil), without toothpaste. Quadrants 1 and 4 were kept throughout the study without supragingival plaque control (SUPRA -). At the end of the 90th day the animals were anesthetized and the clinical parameters (VPI, GBI, GMR, PPD and BOP) were recorded.
Histological procedures
The animals were euthanized with an overdose of anesthetic and immediately perfused with 10% formalin. Block sections were decalcified in Morse solution and routinely processed for paraffin embedding. For each experimental site, four equidistant zones were composed in the proximalproximal orientation: mesial; middle-mesial; middle- distal and distal. Sequential 4 µm ƒnspeciemens were obtained in each one of these zones. The middle specimen in each zone was selected for further analysis. If the selected specimen was not adequate enough for analysis and measurements (precise limits of the cavity, integrity of the pocket and junctional epithelium, well-defined bone crest and the absence of "artifacts"), an immediately anterior or posterior section was selected.
Data analysis
Clinical data
Medians of the percentage of VPI, GBI and BOP were calculated for each quadrant and dog at the beginning and end of this period. Means and standard deviations in mm of the differences between day 90 and baseline for PPD, GMR and CAL scores were calculated for the experimental groups.
Histological data
A single, calibrated, trained examiner (at the Pathology Laboratory of the Dental Faculty of the Federal University) conducted all the histological analyses. This examiner was blinded for the experimental groups and had high agreement in performing to the measurements as demonstrated by the Kappa value (0.788) and the Pearson coefficient (0.997). The sections were analyzed in a computer-assisted microscope. In order to describe the connective tissue, 6 areas were initially composed (Fig. 2). The inflammatory condition of the connective tissue was assessed according to Gomes et al. (25): the sections were analyzed with the aid of a four-score system: Absent (Ab): collagen fibers and fibroblasts are predominant; Mild (Mi): reduced number of collagen fibers and fibroblasts, inflammatory cells are cells are predominant, the presence of collagen and fibroblasts is greatly reduced; Severe (Se): inflammatory cells are predominant, collagen fibers and fibroblasts are absent.

Fig. 2: Areas defined for connective histological description: 1: close to the root/restoration surface; 2: middle vertical; 3: facing the oral epithelium; 4: close to the gingival margin; 5: middle horizontal and 6: facing the bone crest.
The pocket epithelium was divided into three areas: Coronal third (CO), middle third (MIDD) and apical (API) third and received the following scores: A (altered epithelium: presence of epithelium proliferation towards the connective tissue, hydropic degeneration or ulceration) or UN (unaltered epithelium: epithelium integrity). The percentage of observations of each score was calculated for the three zones and the experimental groups. The histometric analysis consisted of the measurement, in millimeters, from the apical border of the cavity (CAV-BC) and from the apical limit of the pocket epithelium to the bone crest. Mean values for each experimental group were calculated.
RESULTS
Overall, the supragingival plaque control determined a reduction of the median values of IPV from 100% to 16.66% in SUPRA+ sites. The final median values observed to GBI were 58.33% in SUPRA+ sites. The BOP was not affected in SUPRA+ sites, maintaining the 100% values observed at baseline. The highest PPD values were associated to AM and CT sites in SUPRA- sites. CAL occurred in all experimental sites, less meaningfully at CT SUPRA+ followed by RMGIC SUPRA+. GMR was more expressive in RMGIC, especially at SUPRA- sites (Table 1).
Table 1: Clinical variables.

The supragingival plaque control did not interfere with the CAV-BC distance in AM sites, but the SUPRA+ values to RMGIC were lower than the others (Table 2). The results in Table 2 also show the EPI-BC. It can be observed that the lower values are related to RMGIC, regardless the SUPRA control. Table 3 shows the predominant scores for each of the areas evaluated histologically, as described in Fig. 2. In general, A1 and A4 showed a similar tendency regarding connective tissue description. CT sites in A1 did not show Se scores, regardless of the SUPRA control. In group A4, Mo (41.7%) was the most expressive score in SUPRA+ sites, followed by Se (16.67%) in SUPRA- sites. On the other hand, AM showed dominating Se scores in both areas (A1: 53.33% and 87.50%; A4: 80% and 100%, respectively in SUPRA+ and SUPRAsites). In A1, better observations were associated RMGIC, especially, with SUPRA+ (46.7% Mi and 33.3%Ab) compared to 61.5% of Se in SUPRAsites. In A4, Se scores were present in 57.7% of SUPRA-RMGIC and in 33% of the SUPRA+ sites. A2 and A3, in general, showed the best connective condition associated to SUPRA+ sites. RMGIC sites were associated to dominating Mi and Ab scores (60% and 40%, respectively) in A2 and to Mi and Mo scores in A3 (60% and 40%, respectively). AM, on the other hand, showed Mo (50%) and Mi (50%) scores in A2 mostly associated to SUPRA+. A5 and A6 demonstrated dominating Ab and Mi scores, Se being observed in 6.7% of the AM sites in A5 (6.7%) and to RMGIC in A6 (3.8%), both in SUPRA-. CT sites showed the greatest tendency to Ab scores, varying from 55% to 91.7% of the observations.
Table 2: Histometric Analysis.
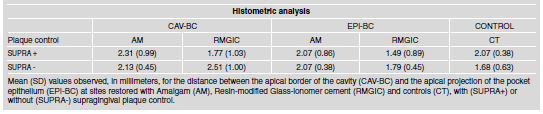
Table 3: Median percentage of predominant scores (Severe, Moderate, Mild and Absent).

The condition of the epithelium varied between experimental groups. In the CO third, the AM, RMGIC and CT sites showed predominating altered epithelium (almost 100% of the observations). In the MIDD third, RMGIC SUPRA- showed the highest altered epithelium percentage (76.9%), slightly greater than AM SUPRA+ (66.7%). The API third showed UN dominating percentage, except AM, which had dominating Al, especially at SUPRA- sites (100%).
Table 4: Median percentage of predominant scores to unaltered and altered epithelium.

DISCUSSION
The present study evaluated the periodontal response to subgingival restorations in periodontitis susceptible dogs. Taken together, the results showed that RMGIC, rather than AM, especially when supragingival plaque control is adequately performed, is related to better periodontal response. The naturally occurring periodontitis model was chosen based in earlier observations by Lindhe et al.25, who pointed to the fact that experimental periodontitis is not fully predictable and may consequently interfere with the observations. Bogle et al.26 also verified that the response to periodontal therapy may vary between dogs with artificial or naturally occurring periodontitis. It is important to observe that the animals received the root planing before the cavities were made and restored, possibly preventing the expected influence of established and unaltered subgingival biofilm during the healing phase27. These observations were made on a limited number of animals. Nevertheless, more recent studies focusing on histological analysis in dogs, have used similar number of animals24,28.
During the experimental period, two quadrants were submitted to a supragingival plaque control protocol. Our results showed that this protocol was effective in reducing VPI and GBI, but not BOP. These observations are in agreement to those reported for healthy dogs by Tal et al.29, who did not observe a reduction in subgingival bleeding despite using chemical plaque control. On the other hand, Gomes et al.24 found a profound benefit from supragingival plaque control on the incidence of BOP. It may be speculated that in dogs with periodontitis, the response of the subgingival area to the supragingival plaque control may be delayed. The mean PPD values varied among groups, but in general it was observed that supragingival plaque control was associated with better results. AM sites showed similar values to CT sites. In a similar experimental model, Gomes et al.24 suggested that when the number of observations is small, interpretations should be made carefully. Nevertheless, taking PPD together with CAL and GMR values, it can be speculated that as AM sites showed more extensive CAL (3.00 to 3.50mm) and less GMR (0.50 to 1.00), the PPD are probably related to inflammatory condition at 90 days rather than to the initial PPD. Our PPD values are greater than those reported by Gomes et al.24. The results are probably explained by the periodontitis status of the present sample. The PPD was smaller in RMGIC sites. Previous studies21, 22,24, also showed reductions in PPD values adjacent to RMGIC restorations. Dragoo21 and Gomes et al.24 reported the presence of non-functional periodontal ligament facing the RMGIC restorations.
In our histological sections these observations were not found, differing from Gomes et al.24, who not only observed the presence of the parallel nonfunctional ligament, but also bone filling at sites restored with RMGIC. The values observed for PPD, CAL and GMR in the control group were close to those observed for RMGIC. The CAL values in sites treated with AM were higher than those observed at RMGIC and CT sites. Older studies have reported severe inflammatory response adjacent to AM subgingival restorations 4, 30, 31. In the early 70's, Waerhaug2 reported that the CAL was related to the position of the subgingival biofilm and not to the apical extension of the restorations. In the present study, the restorations were performed to the bone crest level in both groups. It is therefore possible to assume, as stated previously2, that the interface between the restorations and the cavities, which harbors dental biofilm, more than the location of the apical border of restorations, may influence the level of clinical attachment. Other authors also stated that the restorative material per se may explain part of the clinical response observed in the periodontium, although there are some conflicting results in this field6,17,32. The condition of the connective tissue was investigated in the present study. Other studies focused on the description of the condition of the connective tissue. In general, the results point to a better histological or clinical response at non-restored than at restored sites33; in the presence of well-finished restoration margins5, 12 when supragingival restorations are performed7, 8, 10 and in the presence of supragingival plaque control14,24. Thus, in our study, SUPRA+ sites showed less inflammatory connective response than did SUPRA- sites. Such observations are in agreement with other studies1,3. The coronal third, bordering the supragingival environment, showed the more expressive inflammatory response. When the apical portions of the connective tissue near the osseous crest are evaluated, different results may be observed related to the supragingival plaque control or to the restorative material: overall, the results observed in SUPRA+ were better than those observed in the absence of plaque control, this tendency also being observed for RMGIC. Nevertheless, in the present study all the investigated histological or histometric parameters presented a worse response than the results of Gomes et al.24. The susceptibility to periodontitis in the present sample may explain the differences described. When the histological analysis was performed in the vertical directions, it is clearly observed that the closer we are to the pocket lumen, the more intensive is the connective exudate response. Once again, adhesive material and plaque control contributed to better results.
The condition of the pocket epithelium was also investigated. It was observed that the CO and MIDD thirds more frequently presented altered epithelium. Irrespective of this condition, the restorative material (RMGIC) and plaque control interfered positively. In this sense, MIDD and API thirds, showed fewer sites with altered epithelium. This pattern was also observed by Gomes et al.24 in SUPRA+ sites. Our histometric evaluation also showed better results associated to RMGIC and CT sites than to AM, even though caution must be taken due to the small number of observations. The distance between CAV-BC and EPI-BC in SUPRA+ sites was less pronounced in RMGIC. Comparisons similar to this one were not found in the literature. We can speculate that the conditions associated to these sites, such as smaller teeth/restoration interface observed for RMGIC restorations and its biocompatibility may explain the osseous crest position. Gomes et al.24 observed the bone crest coronal to the apical border of the RMGIC sites in the presence of plaque control. Tal et al.29 compared control (C: flap + subgingival AM restorations) and test sites (T: flap) and found 1.15mm of bone resorption at C sites as compared to 0.15mm at T sites. Gomes et al.24 investigated the distance between the apical borders of the cavity and the functional periodontal ligament. Their observations showed better results wit RMGIC restorations.
Taken together, our results suggest that a supragingival plaque control contributes to maintaining adequate clinical and histological periodontal response to subgingival restorations and that RMGIC may be an elective restorative material rather than AM in subgingival restorations.
1. Waerhaug J. Effect of rough surfaces upon gingival tissue. J Dent Res 1956;35:323-325. [ Links ]
2. Waerhaug J. Subgingival plaque and loss of attachment in periodontosis as evaluated on extracted teeth. J Periodontol 1977;48:125-130. [ Links ]
3. Waerhaug J. Tissue reactions around artificial crowns. J Periodontol 1953;24:172-185. [ Links ]
4. App GR. Effect of silicate, amalgam, and cast gold on the gingival. J Prosthet Dent 1961;11:522-532. [ Links ]
5. Matthews DC, Tabesh M. Detection of localized tooth-related factors that predispose to periodontal infections. Periodontol 2000. 2004;34:136-150. [ Links ]
6. Willershausen B, Köttgen C, Ernst CP. The influence of restorative materials on marginal gingiva. Eur J Med Res 2001;6:433-439. [ Links ]
7. Jansson L, Blomster S, Forsgardh A, Bergman E, Berglund E, Foss L, Reinhardt EL, Sjöberg B. Interactory effect between marginal plaque and subgingival proximal restorations on periodontal pocket depth. Swed Dent J 1997;21:77-83. [ Links ]
8. Nogueira-Filho GR, Stefani CM, Casati MZ, Nakai CM, Plaza CA, Nociti Junior FH, Sallum EA, de Toledo S.. Need of periodontal treatment evaluated with CPITN and its relation to the quality of the cervical margin of restorations. Pesqui Odontol Bras 2001;15:51-55. [ Links ]
9. Gullo CA, Powell RN. The effect of placement of cervical margins of class II amalgam restorations on plaque accumulation and gingival health. J Oral Rehabil 1979; 6:317-322. [ Links ]
10. Schätzle M, Land NP, Anerud A, Boysen H, Bürgin W, Löe H. The influence of margins of restorations of the periodontal tissues over 26 years. J Clin Periodontol 2001; 28:57-64. [ Links ]
11. Chen JT, Burch JG, Beck FM, Horton JE. Periodontal attachment loss associated with proximal tooth restorations. J Prosthet Dent 1987;57:416-420. [ Links ]
12. Parsell DE, Streckfus CF, Stewart BM, Buchanan WT. The effect of amalgam overhangs on alveolar bone height as a function of patient age and overhang width. Oper Dent 1998; 23:94-99. [ Links ]
13. Arneberg P, Silness J, Nordbo H. Marginal fit and cervical extent of class II amalgam restorations related to periodontal condition. A clinical and roentgenological study of overhang elimination. J Periodontal Res 1980;15:669-677. [ Links ]
14. Gorzo I, Newman HN, Strahan JD. Amalgam restorations, plaque removal and periodontal health. J Clin Periodontol 1979;6:98-105. [ Links ]
15. Larato DC. Influence of a composite resin restoration on the gingiva. J Prosthet Dent 1972;28:402-404. [ Links ]
16. Sotres LS, Van Huysen G, Gilmore HW. A histologic study of gingival tissue response to amalgam, silicate and resin restorations. J Periodontol 1969;40:543-546. [ Links ]
17. van Dijken JW, Sjöström S. Development of gingivitis around aged restorations of resin-modified glass ionomer cement, polyacid-modified resin composite (compomer) and resin composite. Clin Oral Investig 1998;2:180-183. [ Links ]
18. Paolantonio M, D'ercole S, Perinetti G, Tripodi D, Catamo G, Serra E, Bruè C, Piccolomini R.. Clinical and microbiological effects of different restorative materials on the periodontal tissues adjacent to subgingival class V restorations. J Clin Periodontol 2004;31:200-207. [ Links ]
19. Chong BS, Owadally ID, Pitt Ford TR, Wilson RF. Cytotoxicity of potential retrograde root-filling materials. Endod Dent Traumatol 1994;10:129-133. [ Links ]
20. Peltola M, Salo T, Oikarinen K. Toxic effects of various retrograde root filling materials on gingival fibroblasts and rat sarcoma cells. Endod Dent Traumatol 1992;8: 120-124. [ Links ]
21. Dragoo MR. Resin-ionomer and hybrid-ionomer cements: part II, human clinical and histologic wound healing responses in specific periodontal lesions. Int J Periodontics Restorative Dent 1997;17:75-87. [ Links ]
22. White C Jr. Repair of a root resorption lesion. A case report. J Periodontol 1998;69:596-600. [ Links ]
23. Breault LG, Fowler EB, Lyons JC. Subgingival restorations with resin ionomer: a periodontal alternative. Compend Contin Educ Dent 2000;21:733-737. [ Links ]
24. Gomes SC, Miranda LA, Soares I, Oppermann RV. Clinical and histologic evaluation of the periodontal response to restorative procedures in the dog. Int J Periodontics Restorative Dent 2005;25:39-47. [ Links ]
25. Lindhe J, Hamp SE, Löe H. Plaque induced periodontal disease in beagle dogs. A 4-year clinical, roentgenographical and histometrical study. J Periodontal Res 1975;10:243-255. [ Links ]
26. Bogle G, Garrett S, Crigger M, Egelberg J. New connective tissue attachment in beagles with advanced natural periodontitis. J Periodontal Res 1983;18:220-228. [ Links ]
27. Karring T, Nyman S, Lindhe J. Healing following implantation of periodontitis affected roots into bone tissue. J Clin Periodontol. 1980;7:96-105. [ Links ]
28. Carnevale G, Sterrantino SF, Di Febo G. Soft and hard tissue wound healing following tooth preparation to the alveolar crest. Int J Periodontics Restorative Dent 1983; 3:36-53. [ Links ]
29. Tal H, Soldinger M, Dreiangel A, Pitaru S. Periodontal response to long-term abuse of the gingival attachment by supracrestal amalgam restorations. J Clin Periodontol 1989; 16:654-659. [ Links ]
30. Nasjleti CE, Castelli WA, Caffesse RG. Effects of amalgam restorations on the periodontal membrane in monkeys. J Dent Res 1977;56:1127-1131. [ Links ]
31. Parma-Benfenali S, Fugazzoto PA, Ruben MP. The effect of restorative margins on the postsurgical development and nature of the periodontium. Part I. Int J Periodontics Restorative Dent 1985;5:30-51. [ Links ]
32. Konradsson K, van Dijken JW. Interleukin-1 levels in gingival crevicular fluid adjacent to restorations of calcium aluminate cement and resin composite. J Clin Periodontol 2005;32:462-466. [ Links ]
33. Martins TM, Bosco AF, Nobrega FJ, Nagata MJ, Garcia VG, Fucini SE. Periodontal tissue response to coverage of root cavities restored with resin materials: a histomorphometric study in dogs. J Periodontol 2007;78:1075-1082. [ Links ]














