Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.26 no.1 Buenos Aires abr. 2013
ARTÍCULOS ORIGINALES
Presence and antimicrobial profile of gram-negative facultative anaerobe rods in patients with chronic periodontitis and gingivitis
Fredy Gamboa1, Dabeiba-Adriana García2, Adriana Acosta2, Deborah Mizrahi3, Andreína Paz3, Diana Martínez2, Azucena Arévalo2, Fabio Aristizabal4, Martín Abba5
1 Department of Microbiology (School of Sciences) and Dental Research Centre Group (School of Dentistry),
2 Dental Research Centre Group,
3 Periodontologist. Pontificia Universidad Javeriana. Bogotá, Colombia.
4 Universidad Nacional de Colombia. Bogotá, Colombia.
5 Centro de Investigaciones Inmunológicas Basicas y Aplicadas (Ciniba), Universidad Nacional de La Plata, La Plata, Argentina.
CORRESPONDENCE Dr. Fredy Gamboa Departamento de Microbiologia (Facultad de Ciencias) y Centro de Investigaciones Odontologicas (Facultad de Odontologia) Pontificia Universidad Javeriana Carrera 7 No. 40-62 Bogota- Colombia E-mail: gamboa@javeriana.edu.co
ABSTRACT
Chronic periodontitis is a multifactorial infectious disease associated with Gram-negative anaerobes which are part of the subgingival microflora. In recent years, studies have been conducted to assess the presence of Gram-negative facultative anaerobes (Enterobacteriaceae) and their participation in the development and progression of chronic periodontitis. The aim of this study was to determine the presence of Enterobacteriaceae in patients with chronic periodontitis and gingivitis and to assess antimicrobial susceptibility of clinical isolates. A descriptive, observational study was performed including 64 patients with chronic periodontitis and 22 patients with gingivitis. Microbiological samples were taken from the gingival sulcus using paper points, which then were placed in thioglycollate broth. Samples were incubated for 4 hours at 37oC and finally replated on MacConkey agar. Bacteria were identified using the API-20E system (Biomerieux, France) and antimicrobial susceptibility was determined using the disk diffusion method. The evaluation of samples showed presence of 29 enterobacterial species distributed as follows: 7 in the group with gingivitis and 22 in the group with chronic periodontitis. In the chronic periodontitis group the most common species were: K. oxytoca n=5, S. liquefaciens n=4 and K. pneumoniae and E. coli with n=3. The gingivitis group had the highest frequency of Erwinia sp. (n= 2). Clinical isolates showed very low sensitivity levels to β-lactam ampicillin and amoxicillin/ clavulanic acid, 17.2% and 27.6%, respectively, and higher sensitivity levels to ciprofloxacin (96.6%), amikacin (79.3%), gentamicin (68.9%) and ceftazidime, ceftriaxone, kanamycin and trimethoprimsulfa (65.5%). In conclusion, the existence of a high frequency of enterobacteria in patients with chronic periodontitis and gingivitis shows that periodontologists should pay greater attention to prevention protocols, and develop mechanical and antimicrobial therapies in which antimicrobial susceptibility profile reports should be considered as part of periodontal treatment.
Key words: Gram-Negative Aerobic Rods and Cocci; Enterobacteriaceae; Chronic periodontitis.
Presencia y perfil antimicrobiano de bacilos gram-negativos anaerobios facultativos en pacientes con periodontitis crónica y gingivitis
RESUMEN
La periodontitis cronica es una enfermedad infecciosa multifactorial asociada a bacilos Gram-negativos anaerobios estrictos que hacen parte de la microflora subgingival. En los ultimos anos se han realizado estudios para valorar la presencia de bacilos Gramnegativos anaerobios facultativos (enterobacterias) y su importancia en el desarrollo y progresion de la periodontitis cronica. El objetivo de este estudio fue determinar la presencia de enterobacterias en pacientes con periodontitis cronica y gingivitis y conocer la susceptibilidad antimicrobiana de los aislamientos clinicos. Se realizo un estudio observacional y descriptivo en el que se incluyeron 64 pacientes con periodontitis cronica y 22 pacientes con gingivitis. Las muestras tomadas en el surco gingival con conos de papel se depositaron en caldo tioglicolato, se incubaron durante 4 horas a 37 oC y se resembraron finalmente en Agar MacConkey. En la identificacion de las bacterias se utilizo el sistema API-20E (Biomerieux, France) y la susceptibilidad antimicrobiana se realizo por el metodo de difusion en disco. En los dos grupos se identificaron 29 especies enterobacterianas, 7 en el grupo con gingivitis y 22 en el grupo con periodontitis cronica. En el grupo de periodontitis cronica las especies mas frecuentes fueron: K. oxytoca n=5, S. liquefaciens n=4 y K.pneumoniae y E. coli con n=3. En el grupo con gingivitis, Erwiniasp tuvo la mayor frecuencia (n=2). Los aislamientos clinicos presentaron niveles muy bajos de sensibilidad a los B-lactamicos ampicilina y amoxicilina/ ac.clavulanico, 17.2% y 27.6%, respectivamente, y mayor sensibilidad a ciprofloxacina (96.6%), amicacina (79.3%), gentamicina (68.9%) y a ceftacidima, ceftriaxona, kanamicina y trimetoprim-sulfa (65.5%). En conclusion, la alta frecuencia de enterobacterias en pacientes con periodontitis cronica y gingivitis debe conducir a los profesionales en periodoncia a trabajar con mayor determinacion en protocolos de prevencion y a desarrollar terapias mecanicas y antimicrobianas en las cuales se tengan en cuenta, como parte del tratamiento periodontal, los perfiles antimicrobianos reportados.
Palabras clave: Bacilos gram-negativos; Enterobacterias; Periodontitis cronica.
INTRODUCTION
Chronic periodontitis is a multifactorial infectious disease with high prevalence worldwide, in which the leading role of Gram-negative subgingival microflora has been defined, with a group of fully identified bacteria including P. gingivalis, T. denticola, T. forsythia, A.actinomycetemcomitans, P. intermedia, E. corrodens, C. rectus, F. nucleatum, Capnocytophaga sp. and P. micra 1, 2.
Factors inherent to the host such as heredity, smoking and environmental factors are important and determinant in the progression and severity of the disease1.
Although strict anaerobe periodontal pathogenic microorganisms are directly involved in the onset and progression of chronic periodontitis, it is also important to mention that for several years, facultative anaerobe Gram-negative enteric rods (enterobacteria) have also been found in the gingival sulcus of patients with chronic periodontitis3-10. In the human oral environment, in addition to enterobacteria being found in the gingival sulcus of patients with chronic periodontitis, they have been isolated from mucosa and teeth3,11,12. The presence of enterobacteria in the oral cavity is basically due to orofecal transmission, deficient oral hygiene, or contamination from grooming accessories, food or drink13,14.
Another outstanding feature of enterobacteria is their implication as key pathogens in some cases of refractory periodontitis4,5. Similarly, these opportunistic microorganisms may be major players as a result of the inadequate use of antibiotics, which may suppress normal oral microbiota, leading to persistent colonization by opportunists15.
It is important to mention that if antimicrobial therapy is needed in chronic periodontitis to eradicate enterobacteria as well as regular periodontal pathogenic microorganisms, their resistance or sensitivity to antibiotics needs to be known7. In this regard, a variety of patterns of antimicrobial resistance have been found for these Gram-negative microorganisms isolated from the oral cavity, which may also be implicated in hospital-acquired, systemic and multi-resistant infections7.
To sum up, many studies suggest that enteric bacteria can play a major part in periodontal disease3,6,10. New studies therefore aim to assess the importance of enterobacteria in the progression of periodontal disease, their frequency of appearance in patients with chronic periodontitis and gingivitis, relationship with age and socio-demographics of study populations, and antimicrobial susceptibility or resistance profile3,16-18.
The working hypothesis for this study suggests that there is high frequency of enterobacteria in patients with gingivitis and chronic periodontitis and high antimicrobial resistance. Thus, the aim of this study was to determine presence of enterobacteria in patients with gingivitis and chronic periodontitis, and to assess the antimicrobial susceptibility of clinical isolates. Finding these microorganisms and knowing their antimicrobial susceptibility profile could enable dentists to take them into account in prevention and control processes and in designing and applying new therapies, which should include antimicrobial therapy.
MATERIALS AND METHODS
Study Features
This was an observational, descriptive study which finally included 22 patients diagnosed with gingivitis and 64 patients diagnosed with untreated chronic periodontitis, who visited the pre- and post-graduate Periodontology clinics at the School of Dentistry of the Pontificia Universidad Javeriana from January 2010 to October 2011. One of the researchers performed a complete periodontal evaluation (full mouth) on all patients, using a Williams probe (Williams colorcoded probe PQW, Hu-Friedy, Chicago-Illinois, USA), including gingival margin, bleeding on probing, probing depth and clinical attachment level, and classified them according to the recommendations of the 1999 International Consensus of the American Academy of Periodontology 19.
Inclusion criteria were: patients diagnosed with chronic periodontitis or gingivitis, with at least 10 teeth, without systemic compromise, over 20 years old, who had not received prior periodontal treatment (within the last 6 months). Exclusion criteria were: patients undergoing antibiotic or corticosteroid therapy within the last three months, pregnant or lactating women and smokers. The study was approved by the Ethics and Research Committee of the School of Dentistry of the Pontificia Universida Javeriana. All patients signed an informed consent form which explained the nature of the project and its associated benefits. A survey was conducted to determine each patient's systemic condition and whether the patient met the inclusion and exclusion criteria.
Sampling
The clinical director of the study performed calibration, after which periodontal probing was done, 6 surfaces of all teeth were measured in order to select which surface to include in the sample (mesial vestibular, mesial lingual / palatal and vestibular / palatal interproximal surfaces). To take the sample from the gingival sulcus, 5 sites with pocket probing depth ≥ 4mm and clinical attachment level ≥ 2mm were selected for the group with chronic periodontitis, and pocket probing depth ≤ 3mm and absence of gingival inflammation for the group with gingivitis. Supragingival film was removed with sterile gauze, the zone was isolated with sterile cotton, and paper points (New SteticR) were placed depending on the depth of the gingival sulcus. Paper points were removed after 1 minute in the gingival sulcus, and placed in Eppendorf tubes containing 900 μl thioglycollate broth (BBL™ Fluid, Becton, Dickinson and Company) supplemented with hemin and menadione.
Isolation, identification and assessment of antimicrobial susceptibility
The Eppendorf tubes containing the samples were taken to the laboratory and incubated under anaerobic conditions at 37°C for 4 hours, after which they were centrifuged (EppendorfR centrifuge) at 4000 rpm for 10 minutes. After centrifuging, 300μl were discarded and the remaining 600μl were vortexed (Maxi mix II ThermolyneR) to mix the sample homogenously. Immediately 50μl were plated on a Petri dish with MacConkey agar (OxoidR) and incubated under aerobic conditions at 37oC for 48 hours. After microbial growth, the macroscopic characteristics of the colonies were observed and a Gram stain was performed. Oxidase test, nitrate test and OF test in glucose and lactose were performed on Gram-negative colonies. Enterobacteria are oxidase-negative, glucose-fermenting, nitrate-reducing Gram-negative rods. Species were identified using an API 20E (Biomerieux R S.A., Marcy-l`Etoile/France) identification system. Clinical isolate antimicrobial susceptibility was assessed using the Kirby-Bauer disk diffusion method following the recommendations of the Clinical and Laboratory Standards Institute (CLSI). The following antimicrobial agents were assessed: amikacin, ampicillin, amoxicillin/clavulanic acid, cefotaxime, ceftazidime, ceftriaxone, gentamicin, kanamycin, tobramycin, trimethoprim-sulfamethoxazole and ciprofloxacin.
Statistical analysis
Univariate and bivariate descriptive statistical analyses were performed (frequency distribution of categorical variables, mean, median and standard deviation of continuous variables). The chi-square test was used to establish a bivariate analysis between presence or absence of enterobacteria with the demographic variables age and sex (male, female). Nonparametric Mann-Whitney-Wilcoxon test was used to establish differences in pocket probing depth, attachment level and severity of periodontitis and presence or absence of enterobacteria. Values of P < 0.05 were interpreted as statistically significant.
RESULTS
The study sample consisted of 86 patients, of whom 44 (51.2%) were female and 42 (48.8%) male. Out of the 86 patients, 22 (25.6%) had gingivitis and 64 (74.4%) had chronic periodontitis. In the group of patients with gingivitis there were 16 females and 6 males, while in the group with chronic periodontitis there were 28 females and 36 males.
Table 1 describes the clinical and demographic characteristics of patients with chronic periodontitis with or without enterobacteria. In this group, enterobacteria were present at a frequency of 34.4% (22/64), strongly associated to male patients (P<0.05). There was no statistically significant difference in age regarding the chronic periodontitis group without enterobacteria (P>0.05). There was no statistically significant difference (P>0.05) in clinical characteristics (pocket probing depth, attachment level and severity of chronic periodontitis) between chronic periodontitis patients with or without enterobacteria. The 29 enterobacteria isolated from the two study groups were distributed as follows: 7 (24.1%) in the group with gingivitis (6 females and 1 male) and 22 (75.9%) in the group with chronic periodontitis (15 males and 7 females). No patient with enterobacteria (n=29) had more than one species. There was no statistically significant difference (P>0.05) in enterobacteria frequency between the two groups of patients (34.4% vs. 31.8%).
Table 1: Main demographic and clinical findings for patients with chronic periodontitis. aValues show mean ± standard deviation

Figure 1 shows that the greatest presence of enterobacteria in patients diagnosed with gingivitis was at ages 20 to 30 years (n=5), while in patients diagnosed with chronic periodontitis the greatest frequency of enterobacteria was at ages 41 to 50 years (n=9). In chronic periodontitis patients with and without enterobacteria, mean pocket probing depth was 5.60 } 1.75 and 5.57 } 1.78 respectively (P>0.05) (Table 1).

Fig. 1. Presence of enterobacteria according to age of patients with gingivitis and chronic periodontitis.
Mean attachment level in chronic periodontitis patients with and without enterobacteria was 5.71 } 1.89 and 5.75 } 1.76, respectively (Table 1). Table 2 shows distribution and frequency of enterobacteria species in each group. In the chronic periodontitis group the most frequent species were K. oxytoca n=5, S. liquefaciens n=4, and K. pneumoniae and E. coli n=3, while in the gingivitis group, Erwinia sp. had the highest frequency (n=2).
Table 2: Frequency of enterobacteria in the groups studied.

Table 3 summarizes the in vitro susceptibility profiles of the 29 enterobacteria to the 10 antimicrobial agents. Sensitivity to the β-lactams ampicillin and amoxicillin/clavulanic acid was very low, at 17.2% y 27.6 %, respectively. The highest sensitivity was to ciprofloxacin (96.6%), followed in decreasing order by amikacin (79.3%), gentamicin (68.9%), ceftazidime, ceftriaxone, kanamycin and trimethoprim- sulfa (65.5%) and cefotaxime (62.1%).
Table 3: Susceptibility to antimicrobial agents in vitrofor the 29 enterobacteria strains isolated from patients with gingivitis and chronic periodontitis.
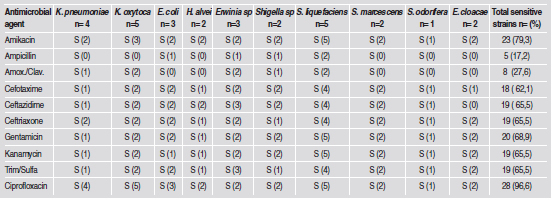
Serratia species (S. liquefaciens, S. marcensens, S. odorifera) were highly sensitive (87.5% - 100%) to the antimicrobial agents, except ampicillin and clavulanic amoxicillin (25%). Similarly, the two E. cloacae strains were highly sensitive to 6 of the 10 antimicrobial agents (100%), with the exception of cefotaxime (50%) and ampicillin, amoxicillin/ clavulanic acid and ceftazidime (0%). Klebsiella species (n=9) were less susceptible to antimicrobial agents; nevertheless, their susceptibility to ciprofloxacin and amikacin (100 and 55.6 %, respectively) is outstanding. E. coli strains (n=3) were sensitive to antimicrobial agents with values ranging from 33% to 100%.
DISCUSSION
Enterobacteria are important not only because they may complicate the clinical picture of patients with chronic periodontitis, since they are key in cases of refractory periodontitis, but also because due to their pathogenic capacity they could produce systemic infections if they persisted after periodontal therapy and surgery4,5,7,20.
This study shows the frequency and antimicrobial susceptibility patterns of enterobacteria species isolated from patients with gingivitis and chronic periodontitis. It is noteworthy because it found enterobacteria in 35.3% and 31.8% of patients with chronic periodontitis and gingivitis, respectively. These frequencies were very similar to values reported for Sweden (34.9 %) and Brazil (31.2%), but differed from frequencies reported for Chile (17.6%), USA (28%), China (57%) and Rumania (61.1%)12,21-25.
Prior studies conducted in Colombia report frequencies of enterobacteria in patients with chronic periodontitis of 34.5, 27.9, 21.05, 16.7 and 13.1% 6,9,16,17,26. It would appear that variations in frequency are due to specific socio-cultural and demographic features, as well as to differences in methods of sampling, transportation, processing and isolation by bacteriological culture9,16. The differences may also be due to situations created in the oral microbial ecosystem leading to overgrowth of these opportunistic microorganisms, including deficient hygiene habits and attitudes, chronic baseline diseases, tobacco use, alcohol use, and prior antimicrobial therapies20.
In contrast to studies by Slots et al.20 and Barbosa et al.3, who report greater presence of enterobacteria in females, this study found greater presence of enterobacteria in males (66.7%). The difference might be related to behavioral factors, primarily to the fact that male patients visit the periodontologist less frequently, with the consequent effect on eradication or control of these microorganisms6; however, this information was not available for this study. In recent years many efforts have been made to prove that enterobacteria worsen the prognosis and clinical picture in periodontitis4-6, 10. However, as there is no study on their presence in patients with gingivitis, their role in this pathology and subsequent implication in oral pathological processes is unknown. In this study on gingivitis patients, it is interesting to note the presence of enterobacteria in 31.8%. Further studies should be conducted to determine the composition of the subgingival microbiota and establish its significance to gingivitis patients.
In this study, the most frequent enterobacteria in patients with chronic periodontitis were K. oxytoca (n= 5, 22.8%), S. liquefaciens (n=4, 18.2%), K.pneumoniae (n=3, 13.7%) and E. coli (n=3, 13.7%), in contrast to the frequencies reported by Ardila et al.,6 which were K. pneumoniae (n=12, 75%) and S. marcescens (n= 2, 12.5%) and the frequencies reported by Martinez-Pabon et al.9, which were E. cloacae (n=6, 37.5%), S. marcescens (n=4, 25%) and K. pneumoniae (n=3, 18.75%). All three studies establish that K. pneumoniae, K. oxytoca, S. marcescens, S. liquefaciens, E. cloacae and E. coli are the most frequently found enterobacteria, representing 75% of all strains found. The variability in species and frequencies may lead to identification of the source of contamination and transmission.
Regarding susceptibility to antimicrobial agents, this study found high sensitivity to ciprofloxacin (96.6%), amikacin (79.3%) and gentamicin (68.9%) and low sensitivity to ampicillin (17.2%) and amoxicillin/ clavulanic acid (27.6%). These results in enterobacteria are very similar to those found by Ardila et al.6, who report high sensitivity to ciprofloxacin (100%) and low sensitivity to amoxicillin/ clavulanic acid (25%); Botero et al.17, who report high sensitivity to ciprofloxacin (100%) and low sensitivity to amoxicillin (12.5%) and Gaetti- Jardim et al.7, who report sensitivity to ciprofloxacin (93.4%) and amoxicillin/clavulanic acid (71.7%).
A study on 201 strains of enterobacteria and pseudomonas isolated from the oral cavity in different clinical situations (healthy, gingivitis and periodontitis) reported 42.6%, 65.4%, 97.6 %, 98.1 % and 63.5% sensitivity to ampicillin, amoxicillin/ clavulanic acid, imipenem, meropenem and tetracycline, respectively, and β-lactamase production in 41.2% of the organisms assessed, with the highest frequency of the genetic determinant blaTEM as resistance indicator27. Although β-lactamases were not detected in our study, it is clear that the therapy options for infections caused by enteric Gram-negative rods that express these hydrolytic enzymes are limited, because they are usually resistant to all β- lactam antibiotics except carbapenems. It is also important to consider that bacteria in the oral cavity that are resistant to antimicrobial agents may be a source of transmission of antimicrobial resistance genes to other pathogenic bacteria18. However, the strains assessed are highly sensitive to ciprofloxacin and the aminoglycosides evaluated (amikacin and gentamicin). Aminoglycosides, also known as amino sugars, are heterocyclic antibiotics that act on bacterial protein synthesis. They are not recommended individually in the treatment of infections of the oral cavity against Gram-negative microorganisms; nevertheless, they are used jointly with other antimicrobial agents, particularly β-lactams, in oral surgery7. Gaetti-Jardim et al. report high sensitivity to amikacin (80.6%) and gentamicin (91.4%)7. These studies leave an open window for considering the use of these antimicrobial agents against enterobacteria. Ciprofloxacin is a synthetic antimicrobial agent of the fluorinated quinolone group, whose spectrum of action includes Gram-positive and Gram-negative bacteria. The results of this study are consistent with the high sensitivity found in other studies, suggesting that ciprofloxacin has high potential for eradicating enterobacteria in patients with chronic periodontitis7, 18. Because of their great pathogenic power, contaminating, colonizing and proliferating capacity, considerable frequency, facultative anaerobe characteristics and ability to use the nutrient richness of the gingival sulcus, enterobacteria could interact with periodontal pathogens when both are present in the micro-environment of the periodontal pocket, causing damage and complicating the clinical picture and prognosis of chronic periodontitis4,5,10,18. In this situation, it would be timely to consider the use of combined antimicrobial agents against the enterobacteria and classical periodontal pathogens implicated in periodontal disease.
One of the strengths of this study was to have determined the presence of enterobacteria not only in chronic periodontitis patients but also in gingivitis patients. The latter points to the need for implementing better strategies to reduce enterobacteria in gingivitis patients, so that they will not subsequently associate with periodontal pathogens in periodontal disease. Similarly, when antimicrobial therapy is used, the knowledge of susceptibility profiles to antimicrobial agents reported for the enterobacteria isolated from gingivitis and periodontitis patients should be considered. The main limitation of the study, due to its observational descriptive character, was that it did not determine the presence of enterobacteria longitudinally, with the aim of ascertaining whether colonization was persistent or transient.
CONCLUSIONS
This study found high frequency of enterobacteria in chronic periodontitis and gingivitis patients. The clinical isolates identified had high sensitivity to ciprofloxacin and aminoglycosides and low sensitivity to the β-lactams ampicillin and amoxicillin/ clavulanic acid.
ACKNOWLEDGMENTS
This study was financed by Colciencias (Departamento Administrativo de Ciencia, Tecnologia e Innovacion) within the project "Variabilidad Genetica por AFLP y perfiles de expression genica en aislamientos de Porphyromonas gingivalis sensibles y resistentes al metronidazole y/o tetraciclina provenientes de pacientes con periodontitis cronica", financing code 1203-493-26230.
1. Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000 1994;5:7-25. [ Links ]
2. Holt SC, Ebersole JL. Porphyromonasgingivalis, Treponemadenticola, and Tannerella forsythia: the "red complex", a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2005;38:72-122. [ Links ]
3. Barbosa FC, Mayer MP, Saba-Chujfi E, Cai S.. Subgingival occurrence and antimicrobial susceptibility of enteric rods and pseudomonads from Brazilian periodontitis patients. Oral Microbiol Immunol 2001;16:306-310. [ Links ]
4. Slots J, Feik D, RamsTE. Prevalence and antimicrobial susceptibility of Enterobacteriaceae, Pseudomonadaceae and Acinetobacter in human periodontitis. Oral Microbiol Immunol 1990;5:149-154. [ Links ]
5. Slots J, Rams TE, Listgarten MA. Yeasts, enteric rods and pseudomonads in the subgingival flora of severe adult periodontitis. Oral Microbiol Immunol 1988;3:47-52. [ Links ]
6. Ardila CM, Fernandez N, Guzman IC. Antimicrobial susceptibility of moxifloxacin against Gram-negative enteric rods from Colombian patients with chronic periodontitis. J Periodontol 2010;81:292-299. [ Links ]
7. Gaetti-Jardim EC, Marqueti AC, Faverani LP, Gaetti-Jardim E Jr. Antimicrobial resistance of aerobes and facultative anaerobes isolated from the oral cavity. J Appl Oral Sci 2010;18:551-559. [ Links ]
8. Slots J, Feik D, Rams TE. In vitro antimicrobial sensitivity of enteric rods and pseudomonads from advanced adult periodontitis. Oral Microbiol Immunol 1990;5:298-301. [ Links ]
9. Martinez-Pabon MC, Isaza-Guzman DM, Mira-Lopez NR, Garcia-Velez C, Tobon-Arroyave SI. Screening for subgingival ocurrence of gram-negative enteric rods in peridontally diseased and healthy subjects. Arch Oral Biol 2010; 55:728-736. [ Links ]
10. Ardila CM, Lopez MA, Guzman IC. Positive correlations between presence of Gram-negative enteric rods and Porphyromonas gingivalis in subgingival plaque. Acta Odontol Latinoam 2011;24:15-19. [ Links ]
11. Winn Junior WC, Allen SD, Janda WM, Koneman EW, Procop GW, Schreckenberger PC, Woods GL: Color Atlas and Textbook of Diagnostic Microbiology. Baltimore, MD, USA: Lippincott Williams and Wilkins 1997;211-302.
12. Goldberg S, Cardash H, Browning H 3rd, Sahly H, Rosenberg M. Isolation of Enterobacteriaceae from the mouth and potential association with malodor. J Dent Res 1997; 76:1770-1775. [ Links ]
13. Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes infect 2000;2:897-906. [ Links ]
14. Betancourth M, Arce R, Botero J, Jaramillo A, Cruz C, Contreras A. Microorganismos inusuales en surcos y bolsas periodontales. Colombia Medica 2006;37:315-322. [ Links ]
15. Sixou M. Diagnostic testing as a supportive measure of treatment strategy. Oral Dis 2003;9:54-62. [ Links ]
16. Lafaurie GI, Contreras A, Baron A, Botero J, Mayorga- Fayad I, Jaramillo A. Demographic, clinical, and microbial aspects of chronic and aggressive periodontitis in Colombia: a multicenter study. J Periodontol 2007;78:629-639. [ Links ]
17. Botero JE, Contreras A, Lafaurie G, Jaramillo A, Betancourth M, Arce RM. Ocurrence of periodontopathic and superinfecting bacteria in chronic and aggressive periodontitis subjects in a Colombia population. J Periodontol 2007; 78:696-704. [ Links ]
18. Goncalves MO, Coutinho-Filho WP, Pimenta FP, Pereira GA, Pereira JA. Periodontal disease as reservoir for multiresistant and hydrolytic enterobacterial species. Lett Appl Microbiol 2007;44:488-494. [ Links ]
19. American Academy of Periodontology. 1999 International Workshop for a Classification of Periodontal Diseases and Conditions. Ann Periodontol 1999;4:8-38. [ Links ]
20. Slots J, Feik D, Rams TE. Age and sex relationships of superinfecting microorganisms in periodontitis patients. Oral Microbiol Immunol 1990;5:305-308. [ Links ]
21. Colombo AP, Teles RP, Torres MC, Rosalem W, Mendes MC, Souto RM, Uzeda Md. Effects of non-surgical mechanical therapy on the subgingivalmicrobiota of brazilians with untreated chronic periodontitis: 9-month results. J Periodontol 2005;76:778-784. [ Links ]
22. Herrera D, Contreras A, Gamonal J, Oteo A, Jaramillo A, Silva N, Sanz M, Botero JE, Leon R. Subgingival microbial profiles in chronic periodontitis patients from Chile, Colombia and Spain. J Clin Periodontol 2008;35:106-113. [ Links ]
23. Slots J, Lisgarten MA. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol 1988; 15:85-93. [ Links ]
24. Sedgley CM, Samaranayake LP, Chan JC, Wei SH. A 4- year longitudinal study of the oral prevalence of enteric gram-negative rods and yeast in Chinese children. Oral Microbiol Immunol 1997;12:183-188. [ Links ]
25. Ali RW, Velcescu C, Jivanescu MC. Prevalence of 6 putative periodontal pathogens in subgingival plaque samples from Romanian adult periodontitis patients. J Clin Periodontol 1996;23:133-139. [ Links ]
26. Mayorga-Fayad I, Lafaurie GI, Contreras A, Castillo D, Baron A, Aya M. Microflora subgingival en periodontitis cronica y agresiva en Bogota, Colombia: un acercamiento epidemiologico. Biomedica 2007;27:21-33. [ Links ]
27. Ramos MM, Gaetti-Jardim EC, Gaetti-Jardim Junior E. Resistance to tetracycline and B-lactams and distribution of resistance markers in enteric microorganisms and pseudomonads isolated from the oral cavity. J Appl Oral Sci 2009;17:13-18. [ Links ]














