Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.26 no.1 Buenos Aires abr. 2013
ARTÍCULOS ORIGINALES
Growth inhibition in rats fed inadequate and incomplete proteins: repercussion on mandibular biomechanics
Clarisa Bozzini, Graciela M. Champin, Carlos E. Bozzini, Rosa M. Alippi
Department of Physiology, University of Buenos Aires School of Dentistry, Buenos Aires, Argentina
CORRESPONDENCE Dr.Rosa Maria Alippi, Department of Physiology, University of Buenos Aires Faculty of Odontology Marcelo T. de Alvear 2142, Buenos Aires 1122, Argentina. E-mail: roal@fisio.odon.uba.ar
ABSTRACT
This study describes the effects of feeding growing rats with a diet containing inadequate and incomplete proteins on both the morphological and the biomechanical properties of the mandible. Female rats aged 30 d were fed freely with one of two diets, control (CD, 301 Cal/100g) and experimental (ED, 359 Cal/100g). CD was a standard laboratory diet, while ED was a synthetic diet containing cornflower supplemented with vitamins and minerals. Both diets had the same physical characteristics. Control (C) and experimental (E) animals were divided into 4 groups of 10 animals each. C40 and E40 rats were fed CD and ED, respectively, for 40 d; C105 were fed the CD for 105 d; and E105 were fed the ED for 40 d and then the CD for the remainder of the experimental period (65 d). Mandibular growth was estimated directly on excised and cleaned bones by taking measurements between anatomical points. Mechanical properties of the right hemimandible were estimated by using a 3-point bending test to estimate the structural properties of the bone. Geometric properties of both the entire bone and the cross-section were determined. Bone material properties were calculated from structural and geometric properties. The left hemimandibles were ashed and the ash weight obtained. Rats fed the ED failed to achieve normal body weight gain. Complete catch-up was observed at the end of nutritional rehabilitation. Mandibular weight and length were negatively affected by the ED, as were the cross-sectional area, the mineralized cortical area, and the cross-sectional moment of inertia. All of these parameters showed incomplete catch-up. The structural bone mechanical properties indicative of strength and stiffness were negatively affected. Intrinsic material properties, as assessed by the modulus of elasticity and maximal elastic stress, were within normal values. In summary, the experimental bone was weaker than the control and structurally incompetent. The bone considered was smaller than the control bone, showing a significant reduction in the cross-sectional area and the moment of inertia. However, material properties as well as the ash fraction and degree of mineralization were similar in E and C bones. Therefore, the E bone was weaker than the C bone because of its smaller bone mass, which appears to have been negatively influenced by the ED in relation to its effects on overall body mass.
Key words: Mandible; Bone; Biomechanics; Nutrition.
Inhibicion del crecimiento en ratas alimentadas con proteinas inadecuadas e incompletas: repercusion sobre las propiedades biomecanicas de la mandibula
RESUMEN
El trabajo presente describe los efectos de la alimentacion de ratas en crecimiento con una dieta, que contiene proteinas inadecuadas e incompletas, sobre las caracteristicas morfologicas y las propiedades biomecanicas de la mandibula. Ratas hembras jovenes (30 d) fueron alimentadas ad lib con una de las siguientes dietas: Control (CD, 301 Cal/g) o Experimental (ED, 359 Cal/g). CD fue una dieta estandar de laboratorio, mientras que ED fue una dieta sintetica conteniendo harina de maiz suplementada con vitaminas y minerales. Ambas dietas presentaron caracteristicas fisicas (dureza, humedad) similares. Los animales controles (C) y experimentales (E) fueron asignados a uno de cuatro grupos (n = 10/grupo). Las ratas de los grupos C40 y E40 recibieron, respectivamente, las dietas CD y ED durante 40 d. Los animales pertenecientes al grupo C105 fueron alimentados con CD durante 105 d, mientras que los del grupo E105 recibieron la dieta ED durante 40 d y la CD por el resto del periodo experimental (65 d). El crecimiento mandibular fue estimado directamente sobre el hueso (hemimandibula) previamente despojado de las sustancias blandas, mediante mediciones entre puntos anatomicos. Las propiedades biomecanicas estructurales de la hemimandibula derecha fueron estimadas mediante el test mecanico de flexion a 3 puntos. Las propiedades geometricas del hueso entero y de la seccion transversal fueron tambien determinadas. Las propiedades del material oseo fueron calculadas a partir de las geometricas y las estructurales. La hemimandibula izquierda de cada animal fue calcinada para la obtencion de cenizas, que fueron pesadas. Las ratas alimentadas con dieta ED mostraron una tasa de crecimiento subnormal, observandose crecimiento compensador (catch-up) completo hacia el final del periodo de rehabilitacion nutricional. El peso y la longitud mandibulares fueron negativamente afectados por ED, asi como el area de seccion transversal, el area de tejido mineralizado cortical y el momento de inercia de la seccion. Todos estos parametros mostraron crecimiento compensador incompleto durante la rehabilitacion proteica.. Las propiedades oseas estructurales indicadoras de la resistencia y la rigidez mandibulares fueron afectadas negativamente. Las propiedades materiales intrinsecas, evaluadas a partir del modulo elastico de Young y el estres elastico maximo mostraron valores normales. En resumen, el hueso considerado fue mas pequeno que el normal, mostrando una reduccion significativa a nivel del area de seccion transversal y el momento de inercia. Sin embargo, las propiedades materiales, el contenido fraccional de cenizas y el grado de mineralizacion fueron similares en los huesos C y E. Por lo tanto, el hueso E fue menos resistente que el C debido a su menor masa osea, la que pareciera estar negativamente influenciada por la ED en relacion con sus efectos sobre la masa corporal general.
Palabras clave: Mandibula; Biomecanica osea; Nutricion.
INTRODUCTION
Most of the bones that form the skeleton have a support function, thus playing a critical functional role in bearing functional loads (body weight, mastication, somatic muscle tension). The criterion for adequate support function is the formation and maintenance of sufficient quantity and quality of bone tissue adequately distributed in individual bones to support the body throughout life and to withstand ordinary stresses to which skeletal components are subject1. Bone is considered as a composite material, thus formed of several components that give it the capacity to deform under applied loads. Biomechanics is the branch of physics that analyzes the effects of forces to which a living structure is subject. In this study, the static aspect of biomechanics will be analyzed, in which bones are considered as support organs and not as participants in either locomotion or postural reflexes (in the mandible, as participant of mastication).
Both body weight and regional somatic muscle contraction can be considered as the most important "mechanical factors" in the determination of "bone strength" in the so called "weight-bearing bones", such as the axial or appendicular skeletal bones. The mandible is both morphologically and functionally different from the other bones of the axial skeleton. Moreover, it arises from a different embryonic germ layer (neuroectoderm) than the bones of the axial or appendicular skeleton, which arise from the mesoderm. It has been shown that the mechanical loading of the mandible during mastication has an impact on the mass, density and microarchitecture of the mandibular alveolar bone2,3.
The mandible is not a weight-bearing bone. However, since it is influenced by mechanical masticatory loading, it can be considered as a "load-bearing bone" that presents similarities to the weight-bearing bones from the mechanical point of view.
It is assumed that the "load-carrying behavior of bone" or "mechanical properties" of bones integrated as organs (structural properties) are directly related to both the amount (bone mass) and the architectural distribution of the mineralized tissue (geometric properties) and to the mechanical quality of bone material (material properties). The structural properties are the strength (assessable as the bone's ability to support loads) and the stiffness (measurable as the load / deformation relationship). While structural properties are dependent on bone size and shape, material properties are not. The latter are usually evaluated by assessing two important properties, namely the stiffness of the mineralized tissue (Young's modulus of elasticity) and the maximum elastic stress. These properties are determined by matrix mineralization as well as by other mineralization-unrelated, microstructural factors, such as crystal size and packing and disposition of collagen fibers4. The structural stiffness, and indirectly the strength of bones, is thought to be controlled by a "bone mechanostat" 5. This is a feedback mechanism that optimizes the bone design through permanent re-distribution of the mineralized tissue. Mechanical factors are thus the primary factors determining bone strength However, there are also other "non-mechanical factors" that can modulate bone physiology, by either establishing or maintaining the mechanical competence of bones. Dietary protein is one of them. In this sense, we have recently reported6-10 the results of experiments conducted to establish the effects of dietary protein concentration and quality on bone biomechanical properties (femoral shaft and mandible) in growing rats. The general conclusion was that dietary protein affects bone quality through its effects on bone mass without affecting either bone architecture or the quality of the bone mineralized material. The effects on bone mass were associated with the general effects on body growth rate. Only when protein was absent from the diet was the reduction of bone mass associated with deterioration of the stiffness of the bone tissue11.
Cornflour or cornstarch is ground from the white endosperm at the heart of corn kernels. It is a yellow flour made of dried and ground corn. In maize, the ethanol-soluble or prolamin fraction, the zeins, constitutes as much as half of the total protein in the endosperm12. Zein is thus the main protein of maize, being deficient in lysine and tryptophan, two of the twenty essential amino acids that allow normal growth in monogastric animals. Proteins lacking an essential amino acid are inadequate proteins. According to its adequacy, zein is considered as an incomplete protein because it is incapable of either maintaining life or supporting growth. In fact, experimental animals fed zein as their sole protein lose weight. If tryptophan is added to the zein, weight is maintained but growth does not occur. Only after lysine and tryptophan are added can normal growth take place. It has been suggested that all of the essential amino acids in zein are utilized poorly and that depression of appetite may account for the effect of corn in depressing growth13.
The present study was designed to explore the mechanical behavior of the rat mandible in postweaning female rats stunted by being fed cornstarch supplemented by vitamins and minerals. The possibility of catch-up growth after the end of the nutritional insult was also investigated.
MATERIALS AND METHODS
Forty female Sprague-Dawley rats aged 30 d and weighing 47.75 } 4.23 g at the start of the experiment were housed in stainless-steel cages under natural light-dark photoperiod in a temperature-controlled (23oC) room. Rats were fed freely with one of two diets, control (CD, 301 Cal/100g) and experimental (ED, 359 Cal/100g). The CD was prepared as follows: 30 g of gelatin dissolved in 800 ml of distilled water was added to 1.0 kg of crushed pellets of the standard rat laboratory diet and mixed thoroughly. The homogeneous paste obtained was treated as explained below. The ED was prepared as follows: 1.0 kg of cornstarch, 10 g of vitamins (AIN Vitamin Mixture 76, MP Biomedicals, Ohio, USA), 35 g of minerals (AIN Mineral Mixture 76), 80 ml of soy oil and 1.78 mg of choline were mixed thoroughly. Thirty g of gelatin were dissolved in 800 ml of distilled water and added to the first preparation. The homogeneous paste obtained was maintained in a refrigerator until its consistency was similar to the standard pellet. It was then cut in small portions to be offered to the experimental animals in the usual food containers. Gelatin was added to both diets because of its agglutinating effect. Its constituent proteins have low biological value because they lack many essential amino acids, and thus do not meet protein demands or represent complete nutritional value. Analytical determination of a sample of the ED provided the following results (g/100g): Humidity: 11.6; Ashes: 0.5; Carbohydrates: 80.2; Proteins: 6.2; Lipids: 1.5; and Ca: 0.8 mg.
Control (C) and Experimental (E) animals were divided into 4 groups of 10 animals each. C40 and E40 rats were fed CD and ED, respectively, for 40 d; C105 were fed CD for 105 d; and E105 were fed ED for 40 d and then CD for the remainder of the experimental period (65 d). During this period, body weight and food intake were recorded periodically. At the end of the period, final body weight and length were determined. The rats were then killed by anesthesia overdose. The hemimandibles were dissected and cleaned of adhering soft tissue, weighed in a Mettler scale and stored at -20oC wrapped in gauze soaked with Ringer's solution in sealed plastic bags, following Turner and Burr14. Each bone was thawed at room temperature before analysis. Mandibular growth was estimated directly on the right hemimandible by taking measurements (to the nearest 0.05 mm) with digital calipers, following Eratalay et al.15 with some modifications6. Dimensions were as follows (Fig. 1): a) mandibular area was calculated from a triangle formed between three points: the most anterior inferior bone point of the interdental space (I), the most posterior point of the angular process (II), and the most superior point of the coronoid process (III); b) the length of the base of the jaw was estimated by the distance I-II ; c) the length of the mandible was estimated by the distance between the most anterior superior point of the interdental space (IV) and the most posterior point of the angular process (II); d) the mandibular height corresponded to the distance between the most posterior point of the angular process (II) and the most superior point of the coronoid process (III); e) the alveolar length was the distance between two points on the alveolar process immediately anterior to the anterior surface of the first molar (V) and immediately posterior to the posterior surface of the third molar (VI); f) the interdental length (incisor alveolar process) was the distance from the most anterior superior bone point of the interdental spine (IV) to the anterior surface of the first molar (V). The mandibular length was divided into anterior and posterior parts by a vertical line drawn perpendicular to the oclusal plane of the molars immediately posterior to the posterior surface of the third molar. These specific measurements were chosen because they provide information on the growth of the bone as a whole without considering its morphological units16.

Fig. 1: Medial aspects of the right hemimandible showing the bony points between which measurements were taken (see text).
Mechanical properties of the rat right hemimandible were determined using a three-point bending mechanical test14. Each bone was placed on two lower supports (11 mm span) with the lateral aspect facing down and centered along its length. Loads were applied transversally to the bone axis at a point immediately posterior to the posterior surface of the third molar. The test machine (Instron model 4442, Instron Corp., Canton, MA, USA) was operated in stroke control at a rate of 5.00 mm/min., which is useful to describe the static properties of the bone structure. For this biomechanical test, load / deformation (W / d) curves (Fig. 2) showing both the elastic (Hookean behavior) and the plastic (non-Hookean behavior) phases, separated by the yielding point, enabled graphic determination of the main structural mechanical properties of the bones, which essentially measure the resistance to both deformation (stiffness) and fracture (strength). They are the following: (A) structural properties (whole-bone properties, as derived from the slope of the W/d curve in the linear region of the elastic behavior): (1) maximal stress deflection (yield deflection dy, elastic limit , or load at the yielding point Wy) represents the end point of the elastic deformation (yielding point) and defines a threshold about which unrecoverable permanent deformation occurs, marking the initiation of damage accumulation with the first appearance of the first microcracks that occur on the periosteal surface of the bone; it is a measure of the bone strength; (2) structural elastic stiffness (load/deflection relationship, diaphyseal stiffness, bone rigidity, or slope of the linear phase of the W/d curve) represents the rigidity of the bone or the resistance to deformation; and (3) structural strength (whole-bone strength, maximal supported load, ultimate load, load at fracture Wf) represents the value of the load at fracture and expresses directly the resistance of the whole bone to fracture, incorporating both the elastic and the plastic behaviors. (B) geometric properties (bone design characteristics): (1) bone length and diameters; (2) crosssectional area (CSA): using an Isomet low-speed diamond saw (Buheler, Lake Bluff, Il, USA) the fracture section was regularized to perform micromorphometrical determinations of the vertical (load direction) and horizontal (right angle to load direction) outer (VOD, HOD) and inner (VID, HID) diameters of the fracture sections. Measurements were taken directly using a stereomicroscope (Stenu DV4, Carl Zeiss Microimagen, Gottingen, Germany) with an accuracy of } 0.001 mm. CSA was calculated by applying the equation: CSA = 3.14 (VOD . VID - HOD . HID) /4. (3) second moment of inertia of cortical bone (with reference to the anterior-posterior bending axis, xCSMI) as estimated by the equation: xCSMI = (3.14 [VOD3 . HOD - VID3 . HID / 64]). CSMI captures both bone mass and distribution on the cross section. The larger the CSMI, the further the disposition of bone cortical mass from a given reference axis. (C) Bone material properties (intrinsic properties of the mineralized tissue) as calculated from structural and geometric properties. Thus, bone material properties were not directly determined by mechanical means: (1) Young's modulus of elasticity (Bone material stiffness, intrinsic stiffness, strain-stress relationship) calculated by the formula: E = WyL3 / 48 dy.Ix (Wy = load at the yielding point, L = distance between supports, dy = maximal elastic deflection, Ix = second moment of inertia of the cross-section in relation to the horizontal axis; and (2) maximal elastic stress, which expresses the reacting force opposed by the deformed bone to the deforming load. It was calculated by the formula: δ = LBWy / 8Ix (B = vertical outer diameter of the regularized fracture section.

Fig. 2: The mechanical test generates a "load / deformation" curve (W/d) from which several parameters can be measured. These parameters can be normalized after adjusting for the sample size (cross sectional area or moment of inertia), allowing load conversion to "stress" and deformation to "strain", and obtaining the "stress / strain" curve (S/S). The first linear portion of the curve is known as the "elastic region", where there is a proportional deformation (or strain) with increasing load (or stress) exerted; when the load is removed, bone returns to its original shape. After the "yielding point", increasing load causes permanent damage to the bone structure: relatively small increments of load cause relatively large increments of deformation (plastic region). The "point of fracture" corresponds to the maximum load the bone can sustain without breaking. The slope of the curve within the elastic region is a measure of the "stiffness" of the whole bone (extrinsic bone property) when obtained from the W/d curve. When obtained from the S/S curve, it is called "Young's modulus of elasticity" and is an index of the stiffness of the bone material (intrinsic bone property). "Strength", the other important bone property, can be defined either by the point of fracture or by the load at yield. Wf = load at fracture; Wy = load at the yielding point; df = deformation at the fracture point; dy = deformation at the yielding point.
The left hemimandible of each animal was ashed at 600oC in a muffle furnace for 18 h and the ash weight obtained. The degree of mineralization (α) was estimated as the ratio between ash mass and dry bone mass.
Results were summarized as means } SD and were considered statistically significant at the level of p < 0.05. Comparisons between parameters were performed by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test by using Graph- Pad Prism Software (GraphPad Software Inc., San Diego, CA, USA). Correlation was analyzed by using the same software. The experiment was conducted in accordance with the principles outlined in the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes, and approved by the University of Buenos Aires Ethics Committee.
RESULTS
Rats fed the ED did not attain the same weight as the age-matched C rats (Fig. 3-A, C40 vs. E40). In fact, body weight gain in E animals fed the ED for 40 d was undistinguishable from that of the same rats at the beginning of the experimental period (Fig.3, INI vs. E40). During this period, C rats grew at a rate of 3.91 } 0.2 g/d, while E rats grew at a rate of 0.06 } 0.06 g/d. During the recovery period, E rats grew at a rate of 4.09 } 0.31 g/d while C rats did so at a rate of 1.39 } 0.18 g/d (days 40 to 75 of the experimental period (data not shown). Growth rate was 0.70 } 0.19 g/d and 1.44 } 0.18 g/d in C and E rats, respectively, from day 75 to 105. Final body weight reached a value that was about 90% of the C value by the end of the recovery period (Fig. 3, C105 vs. E 105, p > 0.05). The lower body weight found in E than C animals was accompanied by a significant 31.3% reduction in body length (Fig. 3-B). The parameter recovered totally by the end of the recovery period. Like body size, mandibular dry weight as well as mandibular length, height and area (Fig. 4, A, B, C and D, respectively), which collectively represent the size of the bone, were significantly lower in E40 than in C40 rats. With the exception of mandibular height, which reached normal values by the end of the recovery period (day 105), the other three parameters in the E105 group were about 10% (p < 0.01) lower than in the C105 group. It is worth noting that a high correlation (r = 0.9762) was found between mandible and femur weights (Fig. 3-C) when data from animals were put in the same graph. When the length of the bone was divided into an anterior and posterior part by a line drawn immediately posterior to the posterior surface of the third molar, it was remarkable that in E40 rats the anterior part was significantly reduced by 9% while the posterior part was significantly reduced by 33% (Fig. 4-E and 4-F). The analysis of the regularized fracture section indicated that the cross-sectional area (CSA) and the cross-sectional moment of inertia (xCSMI) (Figs. 6-A and 6-B, respectively) were markedly reduced in E40 rats and the recovery was incomplete for both parameters in the E105 animals. Structural properties (Fig. 5), as derived from the slope of the load/deformation curve in the linear region of the elastic behavior, were drastically affected by treatment. The values for both the yielding and the fracture loads and the structural stiffness were less than 50% in the E40 rats than in C40 rats. These structural properties recovered totally by the end of the recovery period (E105 vs. C105). Bone material quality indicators, or pre-yielding bending stiffness (elastic modulus, E) (Fig. 6-C) and maximal elastic stress (Fig. 6-D) did not differ significantly between C and E rats, as was the degree of mineralization (Fig. 6-E), during the first and the second phases of the experiment. The hemimandible ash content, which represents the mineral mass of the bone, was highly diminished in E40 rats, recovering totally in E105 rats.

Fig. 3: Body weight (A), body length (B), and correlation between mandible and femur weights in rats treated as described in Materials and Methods. Bars represent Mean } SD of 7 rats. Equal letters on top of bars indicate p > 0.05.

Fig. 4: Morphometric properties of the rat mandible. For explanation of groups see Materials and Methods. Bars represent Mean } SD of 7 rats. Equal letters on top of bars indicate p > 0.05.

Fig. 5: Mandibular structural properties in female rats, as derived from the slope of the load / deformation curve in the linear region of the elastic behavior. For explanation of groups, see Materials and Methods. Bars represent Mean } SD of 7 rats. Equal letters on top of bars indicate p > 0.05.
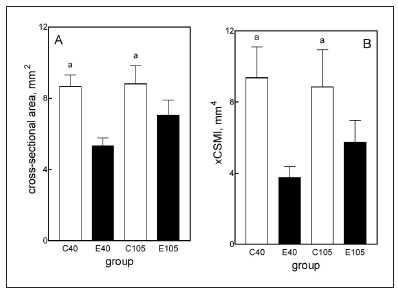
Fig. 6: Characterization of the cross-section (A, B).

Fig. 6 cont.: Material intrinsic properties (C, D) and degree of mineralization (E) and hemimandible ash content (D). For explanation of groups, see Materials and Methods. Bars represent Mean } SD of 7 rats. Equal letters on top of bars indicate Mean } SD of 7 rats. Equal letters on top of bars indicate p > 0.05.
DISCUSSION
This study estimated mechanical properties of the entire hemimandible as a structure, which approaches the behavior of the mandible in vivo. The study examined the effect of a diet (ED) containing maize proteins as a sole source of proteins on body and mandibular growth and mandible biomechanics in female rats from days 30 to 70 of postnatal life. A high body growth rate occurs during this period. Mechanical testing at the chosen level of bone organization measures properties of the entire bone as a structure, which incorporates the properties of the materials that compose the bone, as well as its internal and external geometry17. These properties were measured at the level of a line drawn perpendicularly to the bone behind the posterior part of the third molar. The rat mandible has the capacity to achieve complete catch up during protein recovery at the end of a short period of dietary protein restriction18. This study thus also analyzed whether body and mandibular growth accelerated during nutritional rehabilitation after the imposition of the experimental diet and evaluated the possible recovery of mandible biomechanical behavior.
The ED freely offered to the experimental rats during the first 40-day period of the study contained 6.2% of proteins from maize which, taken together, are considered both inadequate (deficient in essential aminoacids) and incomplete (unable to support body growth and/or maintain life). The diet, therefore, did not contain adequate and complete proteins, which were also present in a concentration that does not allow normal growth in the rat. In fact, it has been demonstrated that 5% concentration of high quality protein is necessary to maintain body weight in an immature rat, although it does not permit growth19, 20. These characteristics of the ED explain the absolute lack of overall body growth (as assessed by body weight) and body length (an index of longitudinal skeletal growth) in the treated animals.
Catch-up growth has been defined as growth at a velocity above the statistical limits of normality for age during a defined period that follows a period of impaired growth21. Such an increase in growth rate may or may not allow an animal to attain its normal adult size. If it does, catch-up is said to be complete and if it does not, incomplete. In this study, the severe growth restriction seen in the rats fed on ED was immediately reversed when the diet was changed to CD. During the first 35 days of nutritional rehabilitation, E rats grew at a rate that was 2.94 times greater than that of the C rats at the same age, which fulfills the requisites for catch-up growth. Body growth rate in E rats decreased over the next 30 days, although was still 2.06 times higher than the rate for the C rats. These changes in body growth rate enabled E rats to approach a final body weight (mature weight) that was not significantly different from that of C rats and give reasons for the conclusion that catch-up growth was complete after the end of the recovery period.
Both final mandibular weight and mandible general morphology in this study were undoubtedly affected by growth retardation. This is clearly evidenced by the positive correlation (r = 09789, r2 = 0.9582, p < 0.0001) found between mandibular weight and body weight. A highly significant positive correlation (r = 0.9762) was also found between mandible (a non weight-bearing bone) weight and femur (a weight-bearing bone) weight, which suggests that the rate of body growth may be more important than the types of loads acting on the bones in the determination of their individual growth. Mandibular weight and area, both taken as indexes of bone size, caught up in E rats during nutritional rehabilitation. However, final values differed slightly thoug significantly from the C values, which indicates that catch-up was incomplete. It is suggested that bone would have caught up completely if the rehabilitation period had been longer. The differences in cross-sectional area (CSA) and crosssectional moment of inertia (xCSMI) indicate that the size of the bone, in terms of the cross-section, was significantly affected by subnormal body growth.
Here again, catch-up was incomplete during protein recovery. The rat mandible can be arbitrarily dividedinto anterior and posterior parts. The former comprises the alveolar and symphyseal regions, while the latter comprises the condyloid, the coronoid and the angular process. In the weaning rat, the length of the posterior part of the mandible is about one-half of the anterior part22. From this time on, the relative increase of the posterior part of the bone is more than twicw as high as that of the anterior part, because the condyle, the growth cartilage of the mandible, is situated in the posterior part. The difference in growth rates between the anterior and posterior parts of the bone is responsible for the observation that both portions have almost equal lengths at adulthood. The deleterious effect of ED on mandibular growth was more accentuated on the posterior part of the bone. Therefore, the "anterior/posterior ratio" differed in E (1.76 } 0.09)) and C rats (1.43 } 0.10) (p < 0.01), which indicates that ED induced some deformation of the mandible relative to age.
These alterations were paralleled by weakening of bone strength (Fig. 5A and B) and structural stiffness (Fig. 5C). The body weight or mass of animals is one of the most important factors influencing the ability of weight-bearing bones to develop or resist stress. A positive linear correlation (r = 0.8648, r2 = 0.7480, p < 0.0001) between the load at fracture of the mandible and the mandible area suggests that the dependence of bone strength to bone mass is also evident in a non weight-bearing bone such as the mandible. Therefore, it appears that mandible mass, and consequently the structural mandible strength, increased following the normal proportionality with body mass in all animals. In other words, growth retardation induced by maize proteins made animals have smaller bones. Therefore, the load at fracture, normalized by bone mass, was not different from that of similarly sized control rats.
The above discussion suggests that the impaired mechanical performance of the mandibular bone induced by the low quality of the dietary protein tested is the result of changes in the amount of cortical bone mass, although the spatial distribution of this cortical bone could be an additional factor. However, the high positive correlation between the strength of the bone and its size suggests that the main affected variable was the mandible mass. The lower value of xCSMI (which captures both bone mass and distribution) may only reflect the much lesser amount of bone mass in the cross-sections, and not necessarily the distribution of those small amounts in the E animals.
The large differences in mandibular strength between groups fed on the ED or C diets contrasted with the maintenance of normality of the elastic modulus and the maximum elastic stress, both indicative of the intrinsic properties of bone material, which depends on the constitution but not on its amount or spatial distribution, suggesting that the adverse effects caused by treatment may have been only quantitative in nature. The lack of effects of ED on both calcium concentration in ashes and the degree of mineralization could explain the normal mandibular bone rigidity. In conclusion, we have described a number of alterations in both morphological and biomechanical variables in the rat mandible resulting from feeding growing rats for 40 days on a diet containing maize proteins. The clear differences in strength and stiffness of the bone between treated and control rats seemed to be the result of an induced loss of gain in bone structural properties as a consequence of a correlative loss of gain in bone growth and mass, in the absence of changes in the quality of the bone mineralized material. Protein recovery in treated animals caused catch-up growth, which was complete or incomplete depending on the variable examined.
ACKNOWLEDGMENTS
This work was supported by Research Grants from the University of Buenos Aires (UBACYT 20020100100389 and 20020100100067). CEB and RMA are Career Investigators from National research Council (CONICET).
1. Ferretti JL, Capozza RF, Mondelo M, Zanchetta JR. Interrelationship between densitometric, geometric, and mechanical properties of rat femora: interferences concerning mechanical regulation of bone modeling. J Bone Min Res 1983;8:1389- 1396. [ Links ]
2. Mavropoulos A, Rizzoli R Ammamm P. Different responsiveness of alveolar and tibial bone to bone loss stimuli. Bone Min Metab, 2007; 22: 403-407. [ Links ]
3. van Eijden E. Biomechanics of the mandible. Crit Rev Oral Biol Med 2000;11:123-136. [ Links ]
4. Currey JD. The mechanical adaptation of bones. 1984 Princeton University Press, Princeton, NJ, USA. [ Links ]
5. Frost HM. Bone mass and the "mechanostat". A proposal. Anat Rec 1966;219:1-9. [ Links ]
6. Alippi, RM, Meta MD, Olivera MI, Bozzini C, Schneider P, Meta IF, Bozzini CE. Effect of protein-energy malnutrition in early life on the dimensions and bone quality of the adult rat mandible. Arch Oral Biol 2002;47:47-53. [ Links ]
7. Bozzini, CE, Champin G, Alippi RM, Bozzini C. Bone mineral density and bone strength from the mandible of chronically protein restricted rats. Acta Odon Latinoam 2011;24:223-228. [ Links ]
8. Bozzini, C, Bozzini CE, Alippi RM. Biomechanical properties of femoral shafts and bone tissue in protein-malnourished rats from weaning to adulthood. Comp Clin Pathol 2012;21: 1159-1165. [ Links ]
9. Alippi RM, Picasso E, Huygens P, Bozzini, CE, Bozzini C. Growth-dependent effects of dietary protein concentration and quality on the biomechanical properties of the diaphyseal rat femur. Endocrinol Nutr 2012;59:35-43. [ Links ]
10. Bozzini CE, Champin M, Alippi RM, Bozzini C. Biomechanical properties of the mandible, as assessed by bending test, in rats fed a low protein diet. Arch Oral Biol 2013;58: 427-434. [ Links ]
11. Ferretti JL, Tessaro RD, Delgado CJ, Bozzini CE, Alippi RM, Barcelo AC. Biomechanical performance of diaphyseal shafts and bone tissue of femurs from protein-restricted rats. Bone Miner 1988;4:329-339. [ Links ]
12. Geraghty D, Peifer MA, Rubenstein I, Messing J. The primary structure of a plant storage protein. Nucleic Acids Res 1981;9:5163-5174. [ Links ]
13. Benton DA, Harper AE, Elvehjen CA. Effect of isoleucine supplementation on the growth of rats fed zein or corn diets. Arch Biochem Biophys 1955;57:13-19. [ Links ]
14. Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone 1993;14:595-608. [ Links ]
15. Eratalay IK, Simons DJ, Mosty SK, Rosenberg GD, Nelson W. Bone growth of the rat mandible following everyday or alternate day methylprednisolone treatment schedules. Arch Oral Biol, 1981;26:769-777. [ Links ]
16. Moore MJ. An experimental study of the functional components of growth in the rat mandible. Acta Anat 1973;85: 378-385. [ Links ]
17. Liebschner MAK. Biomechanical considerations of animal models in tissue engineering of bone. Biomaterials 2004; 25:1697-1714. [ Links ]
18. Alippi RM, Meta MD, Boyer PM, Bozzini CE. Catch-up in mandibular growth after short-term dietary protein restriction in rats during the weaning period. Eur J Oral Sci 1999; 107:260-264. [ Links ]
19. Bozzini C, Barcelo AC, Alippi RM, Leal TL, Bozzini CE. The concentration of dietary casein required for normal mandibular growth in the rat. J Dent Res 1989;68: 840-842. [ Links ]
20. Thissen JP, Underwood LE, Maiter D, Maes M, Clemons DR, Ketelslegers JM. Failure of insulin-like growth factor- I (IGF-1) infusion to promote growth in protein-restricted rats despite normalization of serum IGF-1 concentrations. Endocrinology 1991;128:885-890. [ Links ]
21. Boersma B, Witt JM. Catch-up growth. Endocr Rev 1997; 18:646-661. [ Links ]
22. Olivera MI, Bozzini C, Meta IF, Bozzini CE, Alippi RM. The development of bone mass and bone strength in the mandible of the female rat. Growth Develop Aging 2003; 67:85-93. [ Links ]














