Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.27 no.3 Buenos Aires dic. 2014
ARTÍCULOS ORIGINALES
Microbial diversity in dental unit waterlines
Guacyra M. Lisboa1, Yves R.M. Lisboa2, Telma M.L. Pinheiro2, Roberto C. Stegun3, Eurípedes A. da Silva-Filho1
1 Department of Biological Sciences, Federal University of Alagoas, Brazil
2 Department of Microbiology, Central Laboratory of Public Health of Alagoas, Brazil
3 Department of Dental Prosthesis, School of Dentistry, University of São Paulo, Brazil
CORRESPONDENCE Euripedes A. da Silva-Filho Av. Lourival Melo Mota, s/n, Cidade Universitaria 57072-900 - Maceio, AL, Brazil alvessf@gmail.com
ABSTRACT
Dental health care providers and patients are exposed during ongoing work to contamination by the water used in the dental units, due to accidental swallowing or aspiration of the sprays generated by the high-speed handpiece and the threeway syringe. This study evaluated the quality of water in dental units in the public dental care system of Maceio, Alagoas, Brazil, by conducting analyses of contamination by total coliforms, E.Coli, heterotrophic bacteria and filamentous fungi. We collected 200 mL of water at 5 sites in 6 dental offices of the Department of Health located in different parts of the city. A total 212 isolates and 16 genera of filamentous fungi were identified in the water collected from the dental units. Total coliforms indicated that the water used in dental units was not appropriate for human consumption. The high levels of contamination found in this study showed that water was a potential source of cross-infection.
Key words: Biofilms; Dental service Infection control; Water quality.
RESUMO
Diversidade microbiana nas linhas d'água das unidades dentais
Durante a rotina de trabalho, a equipe odontologica e os pacientes ficam expostos a possivel contaminacao pela agua utilizada nas unidades dentais, devido a ingestao acidental da agua ou pela aspiracao dos sprays gerados pela caneta de alta rotacao e seringa triplice. Este estudo avaliou a qualidade da agua usada em consultorios odontologicos da rede publica estadual de Maceio, Alagoas, Brasil, atraves da analise de contaminacao por coliformes totais e E.coli, bacterias heterotroficas e fungos filamentosos. Foram coletados 200 mL de agua, em cinco pontos de seis consultorios odontologicos pertencentes a Secretaria Estadual de Saude, localizados em diferentes bairros da cidade. Um total de 212 isolados e 16 generos de fungos filamentosos foram identificados. A presenca de coliformes totais indicou que a agua utilizada nas unidades dentais era impropria para consumo humano. O alto indice de contaminacao mostrou que as aguas estudadas eram uma fonte potencial de infeccoes cruzadas.
Palavras-chave: Biofilme; Controle de infeccao; Qualidade da agua; Servicos odontologicos.
INTRODUCTION
The quality of dental unit water is of considerable importance to patients and dental health care providers because they are exposed to water and aerosols generated from the dental unit during routine practice1,2. Microbial concentrations in dental unit waterlines were first reported by Murray and Slack in 19573. Today, the presence of high concentrations of microorganisms in the water of dental units is recog nized by the scientific community4. This contamination has been an important problem in dentistry for over 50 years5,6.
In Brazil, there is no specific standard for the microbial quality of water used in dental units, but the Ministry of Health issued Directive # 2914 in December 20117, establishing that the quality of potable water supplied to the population by the public distribution systems should be evaluated through monthly bacteriological analyses assessing total coliforms and Escherichia coli. Heterotrophic bacteria should be counted in 20% of the samples and the total should not be greater than 500 colony forming units (CFU) per milliliter of water. Similar standards are used in Japan <100 CFU/mL, Europe <200 CFU/mL and the United States <500 CFU/mL for drinking water. Coliform count is also used internationally as an indicator of unsafe drinking water5,8,9.
This study evaluated the quality of water in dental units in the public dental care system of Maceio, Alagoas, Brazil, by conducting a quantitative analysis of contamination by total coliforms, E.coli, heterotrophic bacteria and filamentous fungi.
MATERIALS AND METHODS
Water samples were collected from six dental clinics of the Department of Health located in different parts of the city, in hermetically closed, sterilized graded wide-mouth bottles containing 0.1mL of 10% sodium thiosulfate solution to neutralize residual chlorine, following the protocol recommended by Standard Methods for the Examination of Water and Wastewater10. Water was collected from the following sites: three-way syringe - SYRINGE; highspeed handpiece coupled to tubing - HANDPIECE; tubing of high-speed handpiece without handpiece coupled - TUBING; water reservoir - RESERVOIR; and the site supplying the reservoir - SOURCE.
To disinfect these sites before collection, they were wiped quickly with a piece of gauze soaked in 70% ethyl alcohol. The three-way syringe and highspeed handpiece were turned on and the water allowed to run for 10 seconds before collecting. 200mL of water from each site at the six dental units. The samples were kept cool in ice boxes and processed within four hours of collection.
Sample Inoculation and Culture Total coliforms and E. coli
An enzyme substrate test (ColilertR, IDEXX Laboratories, Westbrook, ME) was used. The water containers were cleaned with a piece of gauze soaked in 70% ethyl alcohol. Then, 100 mL of the collected water were measured with a sterile pipette and placed in a sterile Erlenmeyer flask, and the reagent was added. It was incubated at 35 ±} 0.5 °C for 24 hours. Results were read using ultraviolet light. The test was positive for total coliforms if the water was yellow, and for E.coli, if it was blue under ultraviolet light. The test was negative if there was no color.
Heterotrophic bacteria
The methodology used was adapted from the protocol suggested by Mayo et al.11. Each sample was diluted to 10-1, and 0.1 mL of the original sample and of the dilution were plated in duplicate onto Petri dishes containing plate count agar (PCA), to which 50 mg.L-1 of ketoconazole was added. The dishes were incubated at 37 °C for 24 to 72 hours.
Filamentous Fungi
The samples were diluted to 10-1, and 0.1 mL of the original sample and the dilution were plated in duplicate onto Petri dishes containing Sabouraud dextrose agar, to which 50 mg.L-1 of chloramphenicol and 50 mg.L-1 ampicillin were added. The dishes were incubated at 28o C for four to six days.
Identification of Filamentous Fungi
Filamentous fungi were identified according to genus based on macroscopic and microscopic features. Microscopic analysis was conducted using the microculture technique in Lactrimel medium (14 g wheat meal, 14 g dried milk, 7 g honey and 0.4 g chloramphenicol per liter)12.
RESULTS
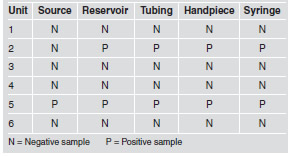
E. coli was not detected in any of the water samples analyzed. However, nine of the thirty samples (30%) showed total coliforms (Table 1).
Table 1: Total coliforms, quantitative results in 100 mL, according to collection site.
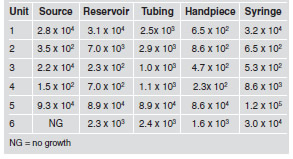
All the dental units had at least three sites at which heterotrophic bacteria exceeded the 500 CFU/mL limit (Table 2).
Table 2: Heterotrophic bacteria counts in CFU/mL according to collection site.
Filamentous fungi were isolated from 70% of the samples (21/30), totaling 212 isolates grouped in 16 genera. The most frequent genera were Acremonium (46.7%), Exophiala (14.7%), Penicillium (9.4%), Aspergillus (8.9%). Other genera had frequencies below 5%. All the genera isolated include potentially pathogenic species (Table 3 and Fig.1).
Table 3: Number of fungi according to genus isolated and identified in 30 samples from 6 dental units.
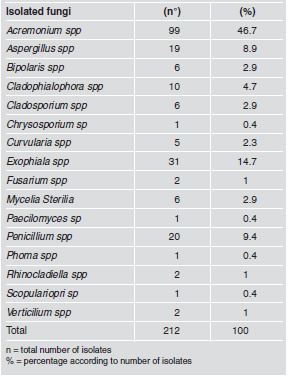

Fig. 1: Macroscopic and microscopic image of eight of the sixteen genera isolated and identified in 30 samples from 6 dental units.
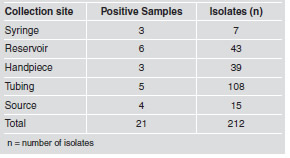
The highest number of fungi was isolated and identified from tubing, with 108 isolates (50.9%), followed by reservoir, with 43 isolates (20.3%), and handpiece, with 39 isolates (18.4%). The percentages at the remaining sites did not exceed 7% (Table 4).
Table 4: Number of fungi isolated and identified according to collection site.
DISCUSSION
Although contamination of dental unit water systems was identified over 50 years ago, many dentists nowadays are still unaware of microbiological contamination or its health risk for dental care providers and patients13. According to Standard Methods for the Examination of Water and Wastewater, sodium thiosulfate is an adequate dechlorinating agent that neutralizes any residual chlorine and prevents continuation of bactericidal action during sample transport. Thus, the exam will indicate more precisely the true microbial content of the water at the time of sampling10. The minimum contamination level of heterotrophic bacteria detected in the water samples collected from the high-speed handpiece was 2.3 x 102 CFU/mL, in agreement with Souza-Gugelmin et al.14, who found contamination levels of 1.9 x 102 CFU/mL for the same collection site.
The level of bacterial growth from all the water samples collected from the high-speed handpiece, either connected to the tubing (HANDPIECE) or not (TUBING), exceeded acceptable levels, except at dental units 3 and 4 for the HANDPIECE site, for which the results were within the acceptable limits. This finding indicates that bacterial contamination was greater in the tubing than in the handpieces. According to Watanabe et al15, water reservoirs should be cleaned regularly with mechanical and chemical methods to remove the biofilm. Our study found that the highest concentration in the reservoirs was 8.9×104 CFU/mL, which is lower than the result detected in a previous study, in which the concentration was found to be 1.1×105 CFU/mL16. The results of studies conducted by Aprea et al.17 and Watanabe et al.15 were negative for bacteria of the coliform group and E.coli. However, in our study, nine samples were contaminated with total coliforms.
Opportunistic fungal pathogens, such as Candida spp., Cryptococcus neoformans and Aspergillus spp., usually only cause infections when there are breaks in the protective skin and mucosal barriers or when immune system defects allow their penetration, colonization and reproduction in the host18. Candida yeasts mixed with traces of saliva may be present in water and aerosols produced by dental handpieces mainly because of dysfunction of antiretraction valves19. Thus, sprays contaminated with yeasts and fungi generated during routine work may be a threat to the health of patients and dental care providers20.
Acremonium spp., Bipolares spp., Cladosporium spp., Penicillium spp., Paecilomyces spp. and Verticillium spp. may cause corneal infections and be a problem for contact lens wearers20-26. In our study the most frequent genus was Acremonium spp., which differed from the study conducted by Szymanska27, in which the most frequent filamentous fungus was Aspergillus spp. Aspergillus spp. and some other species of fungi, such as Curvularia spp., Mycelia Sterilia, Phoma spp. and Scopulariopsis spp., have a pathogenic potential to cause allergic reactions and hypersensitivity27-29. Infections resulting from inhaling Aspergillus spp. spores may cause asthma in patients who are allergic to it20. This study showed rates of 8.9%, 2.9%, 2.3%, 0.4% and 0.4% of Aspergillus spp., Mycelia Sterilia, Curvularia spp., Phoma spp. and Scopulariopsis spp., respectively. Exophiala spp. and Chrysosporium spp. may cause skin lesions and endocarditis30. Porteus et al.31 isolated Exophiala sp, which they claim was not often found in dental unit waterlines. Exophiala spp. was also found in this study, being the second most frequent genus.
Our study also found Cladophialophora spp. (4.7%), Rhinocladiella spp. (1.0%) and Fusarium spp. (1.0%). The two former are an additional source of concern because they may cause chromoblastomycosis, a chronic, granulomatous infection characterized by verrucous, occasionally ulcerated nodules32,33. The latter has been associated to infections in patients after trauma and surgeries, because some of these fungal species may cause ocular and systemic infections, sinusitis, and skin and nail infections20,34. A
ccording to Mungara et al35, to maintain the sterility of dental unit waterlines it is essential to have a good water source and an effective disinfectant. In this study, the water delivered to most patients was poor of quality and was considered a potential source of cross infection. Regular microbiological evaluation of the water used in dental units is extremely important to prevent infections in patients and dental care providers. Standardized procedures to evaluate the water used in dental units should therefore be established. Water should be monitored not only for number of total coliforms, E.coli and heterotrophic bacteria, but also for the presence of filamentous fungi.
ACKNOWLEDGEMENTS
We thank Elaine Santos, Mirella Vieira, Rosana Santos and Iara Fonseca from UFAL for their valuable assistance in identifying fungi.
1. Pareek S, Nagaraj A, Sharma P, Atri M, Walia S, Naidu S, Yousuf A. Disinfection of dental unit water line using aloe vera: in vitro study. Int J Dent 2013;2013:618962 DOI: 10.1155/2013/618962. [ Links ]
2. Kumar S, Atray D, Paiwal D, Balasubramanyam G, Duraiswamy P, Kulkarni S. Dental unit waterlines: source of contamination and cross-infection. J Hosp Infect 2010;74:99-111. [ Links ]
3. Murray JP, Slack GL. Bacterial colonization of dental units. Br Dent J 1957;102-172. [ Links ]
4. Barbeau J. Lawsuit against a dentist related to serious ocular infection possibly linked to water from a dental handpiece. J Can Dent Assoc 2007;73:618-622. [ Links ]
5. O'Donnell MJ, Boyle MA, Russell RJ, Coleman DC. Management of dental unit waterline biofilms in the 21st century. Future Microbiol 2011;6:1209-1226. [ Links ]
6. Lin SM, Svoboda KK, Giletto A, Seibert J, Puttaiah R. Effects of hydrogen peroxide on dental unit biofilms and treatment water contamination. Eur J Dent 2011;5:47-59. [ Links ]
7. Brasil. Ministerio da Saude. Portaria n° 2914 de 12 de dezembro de 2011. Brasilia: Ministerio da Saude. 2011 URL: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt2914_12_12_2011.html. [ Links ]
8. Pankhurst CL, Johnson NW, Woods RG. Microbial contamination of dental unit waterlines: the scientific argument. Int Dent J 1998;48:359-368. [ Links ]
9. Coleman DC, O'Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol 2009;106:1424-1437. [ Links ]
10. American Public Health Association. Standard Methods for the Examination of Water and Wastewater. 20 ed. Washington: APHA 1998;1728p. [ Links ]
11. Mayo JA, Oertling KM, Andrieu SC. Bacterial biofilm: a source of contamination in dental air-water syringes. Clin Prev Dent 1990;12: 13-20. [ Links ]
12. Riddell RW. Permanent stained mycological preparations obtained by slide culture. Mycol 1950;42:265-270. [ Links ]
13. Szymańska J, Sitkowska J. Evaluation of activities aimed at preventing microbiological risks in dental practice. Med Pr 2013;64:11-17. [ Links ]
14. Souza-Gugelmin MC, Lima CD, Lima SN, Mian H, Ito IY. Microbial contamination in dental unit waterlines. Braz Dent J 2003;14:55-57. [ Links ]
15. Watanabe E, Agostinho AM, Matsumoto W, Ito I. Dental unit: bacterial decontamination of old and new dental units by flushing water. Int J Dent Hyg 2008;6:56-62. [ Links ]
16. SzymaDska J, Sitkowska J. Bacterial contamination of dental unit waterlines. Environ Monit Assess 2013;185:3603-3611. [ Links ]
17. Aprea L, Cannova L, Firenze A, Bivona MS, Amodio E, Romano N. Can technical, functional and structural characteristics of dental units predict Legionella pneumophila and Pseudomonas aeruginosa contamination? J Oral Sci 2010;52:641-646. [ Links ]
18. Murray P, Rosenthal K, Pfaller M. Microbiologia Medica. Rio de Janeiro: Elsevier 2006;693-694 [ Links ]
19. Barbot V, Migeot V, Rodier MH, Deborde M, Imbert C. Saliva promotes survival and even proliferation of Candida species in tap water. FEMS Microbiology Lett 2011; 324:17-20. [ Links ]
20. Szymańska J. Evaluation of mycological contamination of dental unit waterlines. Ann Agric Environ Med 2005;12: 153-155. [ Links ]
21. Haddad RS, El-Mollayess GM. Combination of intracameral and intrastromal voriconazole in the treatment of recalcitrant Acremonium fungal keratitis. Middle East Afr J Ophthalmol 2012;19:265-268. [ Links ]
22. Patil S, Kulkarni S, Gadgil S, Joshi A. Corneal abscess caused by Bipolaris picifera. Indian J Pathol Microbiol 2011;54:408-410. [ Links ]
23. Zhang X, Sun X, Wang Z, Zhang Y, Hou W. Keratitis-associated fungi form biofilms with reduced antifungal drug susceptibility. Invest Ophthalmol Vis Sci 2012;53:7774- 7778. [ Links ]
24. Nath R, Baruah S, Saikia L, Devi B, Borthakur A K, Mahanta J. Mycotic corneal ulcers in upper Assam. Indian J Ophthalmol 2011;59:367-371. [ Links ]
25. Monden Y, Sugita M, Yamakawa R, Nishimura K. Clinical experience treating Paecilomyces lilacinus keratitis in four patients. Clin Ophthalmol 2012; 6:949-953. [ Links ]
26. Shin JY, Kim HM, Hong JW. Keratitis caused by Verticillium species. Cornea 2002;21:240-242. [ Links ]
27. Szymańska J. Exposure to airborne fungi during conservative dental treatment. Ann Agric Environ Med 2006; 13:177-179. [ Links ]
28. Metzger F,Haccuria A, Reboux G, Nolard N, Dalphin JC, De Vuyst P. Hypersensitivity pneumonitis due to molds in a saxophone player. Chest 2010;138:724-726. [ Links ]
29. Menezes EA, Trindade EC, Costa MM, Freire CC, Cavalcante Mde S, Cunha FA. Airborne fungi isolated from Fortaleza city, State of Ceara, Brazil. Rev Inst Med Trop Sao Paulo 2004;46:133-137. [ Links ]
30. Murray PR. Microbiologia Clinica. Rio de Janeiro: MEDSI 2003;60-71. [ Links ]
31. Porteous NB, Redding SW, Thompson EH, Grooters AM, De Hoog S, Sutton DA. Isolation of an unusual fungus in treated dental unit waterlines. J Am Dent Assoc 2003; 134:853-858. [ Links ]
32. Deng S, de Hoog GS, Badali H, Yang L, Najafzadeh MJ, Pan B, Curfs-Breuker I, Meis JF, Liao W. In vitro antifungal susceptibility of Cladophialophora carrionii, an agent of human chromoblastomycosis. Antimicrob Agents Chemother 2013;57:1974-1977. [ Links ]
33. Badali H, Bonifaz A, Barron-Tapia T, Vazquez-Gonzalez D, Estrada-Aguilar L, Oliveira NM, Sobral Filho JF, Guarro J, Meis JF, De Hoog GS. Rhinocladiella aquaspersa, proven agent of verrucous skin infection and a novel type of chromoblastomycosis. Med Mycol 2010;48:696-703. [ Links ]
34. Khan S, Pillai GS, Vivek V, Dinesh K, Karim PM. Post-operative endophthalmitis due to Fusarium dimerum. Southeast Asian J Trop Med Public Health 2012; 43:1484- 1488. [ Links ]
35. Mungara J, Dilna NC, Joseph E, Reddy N. Evaluation of microbial profile in dental unit waterlines and assessment of antimicrobial efficacy of two treating agents. J Clin Pediatr Dent 2013;37:367-371. [ Links ]














