Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.28 no.1 Buenos Aires abr. 2015
ARTÍCULOS ORIGINALES
Influence of addition of 2-[3-(2H-benzotriazol-2-YL)- 4-hydroxyphenyl] ethyl methacrylate to an experimental adhesive system
Carolina C. Centenaro1, Flávia V. Rostirolla1, Vicente C.B. Leitune1, Clarissa F. Parolo2, Fabrício A. Ogliari3, Susana M.W. Samuel1, Fabrício M. Collares1
1 Dental Materials Laboratory, School of Dentistry, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil.
2 Department of Preventive and Social Dentistry, School of Dentistry, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil.
3 Materials Engineering School, Federal University of Pelotas, Pelotas, RS, Brazil
CORRESPONDENCE Dr. Fabricio M Collares Laboratorio de Materiais Dentarios, Faculdade de Odontologia Universidade Federal do Rio Grande do Sul Rua Ramiro Barcelos, 2492 - Rio Branco 90035-003 - Porto Alegre, RS, Brazil fabricio.collares@ufrgs.br
ABSTRACT
The aim of this study was to evaluate the addition of 2-[3-(2HBenzotriazol- 2-yl)-4-hydroxyphenyl]ethyl methacrylate (BTAM) to an experimental adhesive resin. An experimental base adhesive resin was formulated with BisGMA, TEGDMA and HEMA, to which BTAM was added at 1, 2.5 and 5%, in weight. One group with no addition was used as control. The experimental adhesives were evaluated for antibacterial potential (against Streptococcus mutans), degree of conversion with FTIR, softening in solvent and microRaman interface analyses. Data were analyzed by Kruskal-Wallis, paired t test and ANOVA and Tukey, considering a 5% level of significance. The results showed antibacterial activity of 5% BTAM against S. mutans (p<0.05), however, no difference was found among BTAM groups (p> 0.05). The results of degree of conversion and softening of solvent showed no statistical difference between BTAM and control groups (p>0.05). The addition of 5% BTAM showed higher antibacterial activity than the negative control, and copolymerization with comonomer blend of adhesive resin and BTAM was detected at the dentin/ adhesive interface.
Key words: Anti-Bacterial Agents; Dentin-Bonding Agents; Polymerization.
RESUMO
Influência da adição de 2-[3-(2H-benzotriazol-2-YL)- 4-hydroxiphenil]etil metacrilato em uma resina adesiva experimental
O objetivo do presente estudo foi avaliar a adicao do 2-[3-(2HBenzotriazol- 2-yl)-4-hidroxifenil]etil metacrilato (BTAM) a um adesivo experimental. Uma resina adesiva base experimental foi formulada com BisGMA, TEGDMA e HEMA e a essa resina foi adicionado o BTAM nas concentracoes de 1, 2,5 e 5%, em peso, alem de um grupo controle sem adicao. Os adesivos experi - mentais foram avaliados quanto ao potencial antimicrobiano contra Streptococos mutans, grau de conversao com FTIR, degradacao em solvente e analise da interface com microespectroscopia Raman. Os dados foram analisados considerando um nivel de significancia de 5%. Os resultados obtidos no teste antimicrobiano contra S. mutans mostrou dife renca estatisticamente significativa do grupo com 5% de BTAM em relacao aos demais grupos e ao controle negativo (p<0,05). Os resultados de grau de conversao e degradacao em solvente dos grupos com BTAM nao apresentaram diferenca quando comparado ao grupo controle (p>0,05). Foi possivel observar a penetracao do BTAM na dentina. A adicao de BTAM na concentracao de 5% mostrou atividade antimicrobiana comparado ao controle negativo, alem de ter sido capaz de copolimerizar e penetrar na dentina.
Palavras chave: Adesivos dentinarios; Antibacterianos; Polimerizacao.
INTRODUCTION
Longitudinal clinical trials show a high success rate for adhesive restorations1,2. However, new materials with improved properties need to be developed in order to further reduce the failure rate of adhesive procedures. Some of the desired features are reduction of polymerization shrinkage3 and degradation in the oral environment4, as well as the presence of antimicrobial properties5. Despite progress in monomer synthesis for low shrinkage and degradation, resin based materials with antimicrobial properties remain poorly explored. Materials with added chlorhexidine6 and triclosan7 have been tested. However, despite their antimicrobial properties, no copolymerization is observed. The absence of copolymerization could increase leaching of these agents and degradation of the polymer8. A quaternary ammonium compound with a methacrylate functional group was used for composite resin development with no decrease in the antibacterial effect over time and no leaching of compounds9. However, other methacrylate antibacterial compounds could be used for developing dental materials.
Compounds with a triazole group are widely used as antifungal and antibacterial agents because they inhibit the synthesis of ergosterol – a fungal membrane constituent - preventing fungal growth10. The compound 2-[3-(2H-Benzotriazol-2-yl)-4- hydroxyphenyl]ethyl methacrylate (BTAM) has a methacrylate functional group that copolymerizes with the comonomer blend of the adhesive, preventing leaching and sustaining the antibacterial effect over time 5. Thus, the aim of this study was to evaluate the influence of the addition of different concentrations of 2-[3-(2H-Benzotriazol-2-yl)-4- hydroxyphenyl]ethyl methacrylate on the properties of experimental adhesive resins.
MATERIALS AND METHODS
Formulation
The monomers used in this study were bisphenol A glycol dimethacrylate (BisGMA), triethylene glycol dimethacrylate (TEGDMA), 2-hydroxyethyl methacrylate (HEMA) and 2-[3-(2H-Benzotriazol- 2-yl)-4-hydroxyphenyl]ethyl methacrylate (BTAM) (Fig. 1). The organic phase of the adhesive was prepared by mixing 50 wt% Bis-GMA, 25 wt% TEGDMA and 25 wt% HEMA. An antibacterial compound (BTAM) was added at four concentrations: 0, 1, 2.5 and 5 wt%. Camphoriquinone, DMAEMA and Diphenyl iodonium salt were used as initiator system. The formulations were mixed and ultrasonicated for 480 s. To perform monomer photo-activation, a light-emitting diode unit (Radii Cal, SDI LTD., Australia) was used. An irradiation value of 1200 mW/cm2 was confirmed with a digital power meter (Ophir Optronics, USA).

Fig. 1: Chemical structure of 2-[3-(2H-benzotriazol-2-yl)- 4-hydroxyphenyl] ethyl methacrylate (BTAM).
Direct Contact Inhibition (DCI)
Three cylindrical samples of adhesive (3 mm in diameter and 1 mm in height) were produced for each group. The specimens were sterilized in hydrogen peroxide plasma. S. mutans (OMZ175) was grown aerobically in Brain Heart Infusion (BHI) broth (HiMedia Laboratories Pvt.Ltd, Mumbai, India) at 37oC. Cells were harvested by centrifugation and re-suspended in fresh medium. Inocula were prepared by adjusting the cell suspension to a predetermined optical density (OD) of 0.02 at 600 nm. Using a 96-well plate, each specimen was placed in a well with 300 μl of BHI broth (HiMedia Laboratories Pvt. Ltd, Mumbai, India). Each well was inoculated with 20 μL of the S. mutans suspension. The negative control consisted of three sets of wells containing uninoculated fresh medium (300 μl). Immediately after the placement of inoculums and after a 24 hour period, 90 μl of each well content were diluted in saline to 10-8. The 10-1, 10-3, 10-6 and 10-8 dilutions were plated on BHI Agar (HiMedia Laboratories Pvt.Ltd, Mumbai, India) using 25 μl aliquots of each dilution in duplicate. Plates were incubated at 37oC, under anaerobic conditions. After 24 hours, colonies were counted visually, scaled by dilution factors and then transformed into colony forming units (CFUs) per milliliter. The groups were statistically compared to each other. The experiment was carried out under aseptic conditions.
Degree of Conversion
The degree of conversion of the experimental adhesive resins was evaluated using Fourier Transform Infrared Spectroscopy (FTIR) with a Vetrex 70 (Bruker Optics, Ettlingen, Germany) spectrometer equipped with an attenuated total reflectance device composed of a horizontal diamond crystal with a mirror angle of 45 degrees. A support was attached to the spectrometer to fix the lightcuring unit and standardize the distance between the fiber tip and sample at 5 mm. Opus software (Bruker Optics, Ettlingen, Germany) was used a Blackman- Harris 3-Term apodization in a range of 4000 to 400 cm-1 and resolution of 4 cm-1. With this setup, one spectrum was obtained prior to photocuring and one immediately after photocuring. The samples (3 μl) were directly dispensed onto the diamond crystal and light-activated for 40 s (n=3). The degree of conversion was calculated as described in a previous study10, considering the intensity of carbon-carbon double bond stretching vibration (peak height) at 1635 cm-1, and using the aromatic carbon-carbon at 1608 cm-1 from the polymerized and unpolymerized samples as an internal standard.
Softening in Ethanol
To determine degradation in solvent, the specimens produced during degree of conversion evaluation were used. Three specimens for each experimental adhesive (n=3) were embedded in acrylic resin and polished, after which they were stored and dried at 37°C for 24 hours. The specimens were subjected to a microhardness test in which five indentations (10 g/5 s), 100 μm apart from each other, were assessed using a digital microhardness tester (HMV 2, Shimadzu, Tokyo, Japan). The microhardness was calculated as described in a previous study12. The initial Knoop microardness number (KHN1) was recorded, and the specimens were then subjected to softening in absolute ethanol for 2 hours at 37°C, after which the hardness test was repeated, and the post-conditioning hardness value measured (KHN2). The percentage difference between KHN1 and KHN2 was calculated.
Interface Characterization
Four lower incisor bovine teeth were cleaned of organic debris and stored in distilled water at 4°C. The labial enamel was removed to expose the superficial dentin. A smear layer was produced by grinding the flat surface with a 600-grit silicon carbide (SiC) disc under water for 30 s. The dentin was etched with phosphoric acid for 15 s and washed for an additional 15 s. A commercial primer (Primer Scotch bond multi-purpose, 3M ESPE, St Paul, MN, USA) was applied, and the solvent was dried for 5 s with an air spray. Adhesive resin was applied according the experimental group and photocured for 20 seconds. A commercial composite resin (Z350XT, 3M ESPE, St Paul, MN, USA) was inserted in two increments of 2 mm and photocured for 40 seconds each to simulate tooth restoration.
The bonded specimens were stored in distilled water in a light-proof container at 37°C for 24 h. Sections (1 mm thick) were prepared by sectioning perpendicular to the flat adhesive-dentine surface. Micro-Raman spectroscopy was performed using a SENTERRA Raman Microscope (Bruker Optics, Ettlingen, Germany). The samples were analyzed using the following micro-Raman parameters: a 100 mW diode laser with 785 nm wavelength and spectral resolution of ~ 3.5 cm-1. One-dimensional mapping was performed over a 150 μm line across the adhesive-dentine interface at 1 μm intervals using a computerized XYZ stage. These areas covered the composite resin, adhesive layer, hybrid layer, partially demineralized and unaffected dentine and were viewed and focused at x500 magnification. Accumulation time per spectrum was 5 seconds with 2 co-additions. Two mappings were performed per sample at random sites. Postprocessing was performed in Opus software (Buker Optics) and consisted of analysis with modeling, which distinguished spectral components of the adhesive and dentine. One correspondent peak of each substance was used for integration. For the hydroxyapatite, 960 cm-1 was used, and for BTAM 998 cm-1 was used.
Statistical Analysis
The values of UFC were analyzed with Kruskal- Wallis. The results of the degree of conversion were evaluated with one-way ANOVA (BTAM concentration) and Tukey. For the analysis of softening in ethanol, a paired Student t-test (KHN1 and KHN2) and a one-way ANOVA for ΔKHN% were used. A level of significance of 0.05 was considered for all tests.
RESULTS
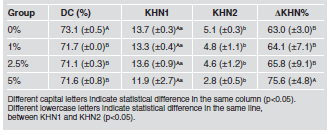
The values of direct contact inhibition are shown in Fig. 2. For the antibacterial analysis, no statistical difference was found between BTAM groups (p>0.05). However, a statistical difference was observed among negative control (uninoculated fresh medium) and groups with 5% BTAM (p<0.05). The mean values of degree of conversion ranged from 71.1 to 73.1 %. The control group presented the highest mean values of degree of conversion (p<0.05). However, none of the groups with added 2-[3-(2H-Benzotriazol-2-yl)- 4-hydroxyphenyl]ethyl methacrylate (BTAM) (1, 2.5 and 5 %) differed statistically (p>0.05). Microhardness values before (KHN1) and after (KHN2) ethanol immersion, percentage difference between KHN1 and KHN2 and degree of conversion are shown in Table 1. There was no statistical difference in initial microhardness values for any of the groups (p>0.05). After ethanol immersion, microhardness values were lower than the initial values for all groups (p<0.05). The percentage difference between KHN1 and KHN2 was higher in the group with 5 % BTAM than in the other groups (p<0.05). The spectra of pure BTAM (Fig. 3 A) and a representative image of each group from the interface characterization is shown in Figure 3 (B-H). The presence of BTAM can be observed across the hybrid layer. All groups with added BTAM exhibited the same behavior across the hybrid layer.

Fig. 2: Values of median and percentile 25 and 75 of microbiological analysis in CFU (log). Different capital letters indicate significant differences (p<0.05).

Fig. 3: Micro Raman characterization of 2-[3-(2H-benzotriazol-2-yl)-4-hydroxyphenyl] ethyl methacrylate (A) and interfaces between adhesive resin and dentin (B-H). Control group (0%) is represented in Figure 3B, integrate for phosphate peak (960cm- 1). Integration of peak 998 cm-1 was not possible for control group, because of the absence of BTAM. Figures C, E and G represent the integration of phosphate (960cm-1) peak for groups with 1, 2.5 and 5% of BTAM, respectively. Figures D, F and H represent the integration of 2-[3-(2H-benzotriazol-2-yl)-4-hydroxyphenyl] ethyl methacrylate peak (998cm-1) for groups with 1, 2.5 and 5% of BTAM, respectively.
Table 1: Mean (± standard deviation) degree of conversion (DC), initial Knoop microhardness (KHN1), Knoop microhardness after solvent immersion (KHN2) and percentage difference between KHN1 and KHN2 (KHN%).
DISCUSSION
The improvement of dental materials by the addition of different compounds is ongoing12,13. Substances that copolymerize with other methacrylate compounds are desirable. In this study, 2-[3-(2HBenzotriazol- 2-yl)-4-hydroxyphenyl]ethyl methacrylate (BTAM) showed copolymerization and antibacterial activity against S. mutans compared to a negative control.
The degree of polymer conversion is directly related to mechanical properties14. For adhesive resins, a high degree of conversion is related to high values of bond strength to dental tissues15. The groups with addition of BTAM showed lower values for degree of conversion than the control groups (p<0.05). The increase in the concentration of monofunctional monomers (BTAM) may explain the reduction of reactivity and consequently the reduction of the degree of conversion in the groups with addition of BTAM (Table 1). The values of the degree of conversion shown in this study are consistent with data in the literature16,17. The increase in the degree of conversion is not necessarily directly related to an increase in crosslink density18. Polymers with low crosslink density are more prone to degradation19-21. In this study, all groups showed reduction in microhardness values after two hours of ethanol immersion. However, the change in microhardness values was significantly higher in the groups with 5% BTAM than in the other groups (p<0.05). Polymers with high degradation during ethanol immersion may absorb more fluids due to the reduction of frictional forces between polymer chains22, degrading the ester bond of methacrylate polymers and leading to a reduction of mechanical properties23. The reduction of frictional forces and degradation of ester bonds can be also detected during water immersion, although to a lesser degree than during ethanol immersion. The degradation caused by water can be detected in the oral environment and is related to color change and the indication for restoration replacement19,23.
Penetration of experimental adhesive resins into demineralized dentin was observed by micro Raman spectroscopy. It may indicate the formation of a hybrid layer. The degree of conversion of adhesive monomers is important, because unreacted monomers close to hybrid layer may leach, causing damage to pulp cells or periapical tissues24. In this study, samples with added BTAM showed a reduction in the degree of conversion compared to the control group, although the values are comparable to commercially available adhesive resins. Despite the related antibacterial activity of triazole25,26, in this study, experimental adhesive resins with 5% BTAM showed activity against S. mutans. The addition of BTAM at a higher concentration may present higher antibacterial effect, because the effect of triazole compounds is dose-dependent27. Further studies are needed at higher concentrations of triazole compound, evaluating activity against fungal contamination, since the development of adhesive systems with antimicrobial activity is desirable Based on the results of this study, the addition of 5% BTAM may have potential for the development of adhesive resins with antimicrobial activity.
1. da Rosa RPA, Cenci MS, Donassollo TA, Loguercio AD, Demarco FF. A clinical evaluation of posterior composite restorations: 17-year findings. J Dent 2006;34:427-435. [ Links ]
2. van Dijken JW, Kieri C, Carlen M. Longevity of extensive class II open-sandwich restorations with a resin-modified glass-ionomer cement. J Dent Res 1999;78:1319-1325. [ Links ]
3. Ilie N, Hickel R. Resin composite restorative materials. Aust Dent J 2011;56:59-66. [ Links ]
4. Moszner N, Fischer UK, Angermann J, Rheinberger V. Bis-(acrylamide)s as new cross-linkers for resin-based composite restoratives. Dent Mater 2006;22:1157-1162. [ Links ]
5. Imazato S, Russell RR, McCabe JF. Antibacterial activity of MDPB polymer incorporated in dental resin. J Dent 1995;23:177-181. [ Links ]
6. Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young AM. Chlorhexidine-releasing metacrylate dental composite materials. Biomaterials 2005;26:7145 -7153. [ Links ]
7. Rathke A, Staude R, Muche R, Haller B. Antibacterial activity of a triclosan-containing resin composite matrix against three common oral bacteria. J Mater Sci Mater Med. 2010;21:2971-2977. [ Links ]
8. Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater 2003;19:449-457. [ Links ]
9. Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of Bacterial Inhibitor into Resin Composite. J Dent Res 1994;73:1437-1443. [ Links ]
10. Shelke S, Mhaske G, Gadakh S, Gill C Green synthesis and biological evaluation of some novel azoles as antimicrobial agents. Bioorg Med Chem Lett 2010;20:7200-7204. [ Links ]
11. Collares FM, Ogliari FA, Zanchi CH, Petzhold CL, Piva E, Samuel SM. Influence of 2-hydroxyethyl methacrylate concentration on polymer network of adhesive resin. J Adhes Dent 2011;13:125-129. [ Links ]
12. Leitune VC, Collares FM, Trommer RM, Andrioli DG, Bergmann CP, Samuel SM. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J Dent 2013;41:321-327. [ Links ]
13. Leitune VC, Collares FM, Takimi A, de Lima GB, Petzhold CL, Bergmann CP, Samuel SM. Niobium pentoxide as a novel filler for dental adhesive resin. J Dent 2013;41:106-113. [ Links ]
14. Bae JH, Cho BH, Kim JS, Kim MS, Lee IB, Son HH, Um CM, Kim CK, et al. Adhesive Layer Properties as a Determinant of Dentin Bond Strength. J Biomed Mater Res B Appl Biomater 2005;74:822-828. [ Links ]
15. Loguercio AD, Stanislawczuk R, Mittelstadt FG, Meier MM, Reis A. Effects of diphenyliodonium salt addition on the adhesive and mechanical properties of an experimental adhesive. J Dent 2013;41:653-658. [ Links ]
16. Czasch P, Ilie N. In vitro comparison of mechanical properties and degree of cure of a self-adhesive and fournovel flowable composites. J Adhes Dent 2013; 15:229-236. [ Links ]
17. Alshali RZ, Silikas N, Satterthawait JD. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent Mater 2013; 29:e213-217. [ Links ]
18. Andrezejewska E. Photopolymerization kinetics of multifunctional monomers. Prog Polym Sci 2001;26:605-665. [ Links ]
19. Ferracane JL. Elution of leachable components from composites. J Oral Rehabil 1994; 21:441-452. [ Links ]
20. Pfeifer CS, Shelton ZR, Braga RR,Windmoller D, Machado JC, Stansbury JW. Characterization of dimethacrylate polymeric networks: a study of the crosslinked structureformed by monomers used in dental composites. Eur Polym J 2011; 47:162-170. [ Links ]
21. Baroudi K, Saleh AM, Silikas N, Watts DC. Shrinkage behaviour of flowable resin-composites related to conversion and filler-fraction. J Dent 2007;35:651-655. [ Links ]
22. Benetti AR, Peutzfeldt A, Asmussen E, Pallesen U, Franco EB. Influence of curing rate on softening in ethanol, degree of conversion, and wear of resin composite. Am J Dent 2011;24:115-118. [ Links ]
23. Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med 2001;12:136-151. [ Links ]
24. Koulaouzidou EA, Papazisis KT, Yiannaki E, Palaghias G, Helvatjoglu-Antoniades M. Effects of dentin bonding agents on the cell cycle of fibroblasts. J Endod 2009;35:275-279. [ Links ]
25. Ezabadi IR, Camoutsis C, Zoumpoulakis P, Geronikaki A, Soković M, Glamocilija J, Cirić A. Sulfonamide-1,2,4- triazole derivatives as antifungal and antibacterial agents: synthesis, biological evaluation, lipophilicity and conformational studies. Bioorg Med 2008;16:1150-1161. [ Links ]
26. Gaikwad ND, Patil SV, Bobade VD. Synthesis and biological evaluation of some novel thiazole substituted benzotriazole derivatives. Bioorg Med Chem Lett 2012;22: 3449-3454. [ Links ]
27. Warn PA, Sharp A, Parmar A, Majithiya J, Denning DW, Hope WW. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob Agents Chemother 2009;53:3453-3461. [ Links ]














