Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Odontológica Latinoamericana
On-line version ISSN 1852-4834
Acta odontol. latinoam. vol.28 no.1 Buenos Aires Apr. 2015
ARTÍCULOS ORIGINALES
Efficiency in bracket bonding with the use of pretreatment methods to tooth enamel before acid etching: sodium hypochlorite vs. hydrogen peroxide techniques
Hermann Rivera-Prado1, Ángeles Moyaho-Bernal1, Alejandro Andrade-Torres1, Guillermo Franco-Romero1, Álvaro Montiel-Jarquín2, Claudia Mendoza-Pinto2, Eugenio García-Cano2, Ana K Hernández-Ruíz2
1 Departament of Stomatological Sciences at Benemeritus Autonomous University of Puebla. 2 Division of Health Research, High Speciality Medical Unit, Hospital of Traumatology and Orthopedics, Mexican Institute of Social Security in Puebla, Mexico
CORRESPONDENCE Dra. Angeles Moyaho-Bernal Diagonal Defensores de la Republica esquina 6 poniente S/N, Col. Amor, Puebla, Mexico. C.P. 72140. moyaho3@gmail.com
ABSTRACT
Bond failures are produced by the existence of biofilm on the tooth surface. Because biofilm is impermeable, it prevents contact in many areas, reducing the etching effect which selectively dissolves calcified tissues but does not seem to eliminate biofilm from the tooth surface, and thus the bond between the tooth and the bracket is not strong enough. The aim of this study is to compare bracket bonding efficiency with two dental surface pretreatments: sodium hypochlorite vs. hydrogen peroxide techniques. This was a cross-sectional, comparative, in vitro study. Seventy-five premolars extracted for orthodontic purposes were evaluated. They were divided into three groups of 25 teeth and assigned randomly toone of the pretreatment techniques (5.25%sodium hypochlorite or 3.5% hydrogen peroxide) or to a control group. The most efficient pretreatment technique for bonding to brackets was sodium hypochlorite, with an average of 17.15 (kg/F). Significant differences were observed between groups (p=0.0001). The post hoc bond strength test showed statistically significant differences between the sodium hypochlorite technique and the control group (p=0.0001). The sodium hypochlorite technique improves bracket adhesion to tooth enamel.
Key words: Dental enamel; Dental etching; Sodium hypochlorite.
RESUMEN
Eficiencia en la adhesión de brackets con el empleo de métodos de pre tratamiento al esmalte antes del grabado ácido: técnica hipoclorito de sodio versus técnica peróxido de hidrógeno
Las fallas de adhesion se producen por la existencia de la biopelicula en la superficie del organo dental, ya que es impermeable y no permite el contacto en muchas areas, de manera que disminuye el efecto del grabado acido; el cual tiene la capacidad de disolver selectivamente los tejidos calcificados, pero no parece eliminar la biopelicula en la superficie dental, por lo tanto, no se lleva a cabo la suficiente fuerza de adhesion en la interfase diente-bracket. El objetivo es comparar la eficiencia en la adhesion de los brackets con el empleo de dos metodos de pre-tratamientos de la superficie del esmalte, el hipoclorito de sodio vs. peroxido de hidrogeno. Estudio comparativo, transversal, in vitro. Se evaluaron 75 premolares extraidos con fines ortodoncicos, tres grupos de 25 dientes, asignados aleatoriamente con alguna de las dos tecnicas de pre-tratamiento al esmalte, hipoclorito de sodio al 5.25%, peroxido de hidrogeno al 3.5% y un grupo control. La tecnica de pre-tratamiento al esmalte mas eficiente para la fuerza de adhesion a los brackets fue el hipoclorito de sodio, con una media de 17.15 (Kg/F), se observaron diferencias significativas inter-grupos (p= 0.001). Las pruebas post hoc para las fuerzas de adhesion mostraron diferencia estadistica - mente significativa para la tecnica de hipoclorito de sodio/ grupo control (p=.001). La utilizacion de hipoclorito de sodio ayuda a mejorar la adhesion de los brackets en la superficie del esmalte.
Palabras clave: Esmalte dental; Grabado dental; Hipoclorito de sodio.
INTRODUCTION
Problems with bonding, such as bracket detachment, are common in clinical practice, delaying the treatment and ultimately causing enamel demineralization1. Bonding quality is diminished by the presence of biofilm on the tooth surface, therefore it is important to use mechanical or chemical prophylaxis on teeth before etching the enamel, in order to remove the biofilm and thus increase the surface energy of the substrate2,3.
There are different opinions regarding whether enamel should be pre-treated, and many different preferences regarding the agent to be used for conditioning the enamel before any treatment4,5. The conventional technique for bracket placement consists exclusively of enamel etching, which can be achieved by demineralization with acid. Nowadays, pretreatment is recommended using physical abrasive methods such as pumice stone to eliminate biofilm and prevent continuous bracket detachment, or the use of sodium hypochlorite by depolarization or hydrogen peroxide to prepare the enamel surface6-10. The solvent and antimicrobial activity of sodium hypochlorite is principally due to its ability to oxidize and hydrolyze cell proteins, to release chlorine to form hypochlorous acid in the long term, and its osmotic ability to draw fluids out of cells1,11-14.
Deproteinization is the removal of collagen from the previously conditioned surface by the use of substances capable of dissolving the protein content (NaOCI). It has been demonstrated as a way to minimize the sensitivity of the hybridization technique, consequently fostering adequate marginal seal without altering bond strength2-4,13-16. NaOCl is a non-specific proteolytic agent which removes organic components from the dentin, such as superficial destabilized collagen and the remnant smear layer from the etching, changing the chemical composition and leaving many exposed hydroxyapatite crystals in this deproteinized substrate1,17,18.
Another enamel pretreatment method is hydrogen peroxide application, as a result of which oxygen and bleaching agents are retained in the enamel. Little is known about the effects of one application on the bonding to enamel 1, 19,20.
MATERIAL AND METHODS
This study was approved by the Master's Program in Stomatological Science in Orthodontics at the Faculty of Stomatology of the Benemeritus Autonomous University of Puebla and the Ethics Committee. The study was conducted at the dental biomaterials laboratory of the Faculty of Stomatology of the Autonomous University of Puebla, Mexico, in February 2012. It was a cross-sectional, comparative, in vitro study. Seventy-five premolars extracted for orthodontic purposes were evaluated. They were divided into three groups of 25 teeth, which were randomly assigned toone of the pretreatment techniques (5.25%sodium hypochlorite or 3.5% hydrogen peroxide)orto a control group.
A pilot test was performed before the definitive procedure, in order to adjust the shear test technique. The teeth indicated for orthodontic extraction were collected, kept in plastic containers of bidistilled water in a 41x35x30cmculture oven at 36°C, in order to replicateoral moisture and temperature conditions. The teeth were placed in transparent acrylic cubes (Nictone) with parallel walls, leaving the cervical third of the root free. Three groups of 25 teeth each were formed, by assigning them randomly to one of the pretreatment techniques: 5.25%sodium hypochlorite, 3.5% hydrogen peroxide, or to a control group with37% phosphoric acid, each for 15 seconds. A thin layer of primer (3M McMark) was applied to the pretreated surface with a microbrushand spread with air from a triple syringe for about 3 seconds.
A metallic MBT Gemini prescription bracket (3M Unitekal) was used and Transbond Xt resin (3M Unitek) applied on its mesh. A bracket was placed on the vestibular surface of each tooth using forceps (Ormco), and excess resin carefully removed. Theresin was immediately photopolymerized with a CuringLight XL 300 lamp (3M) for 20 seconds (10 on the mesial side and 10 on the distal side of the bracket). Thetreated teeth were kept in the 41x35x30cm culture oven at 36°C inplastic containers with bidistilled water for 72 hours, after which the shear test was performed using a universal testing machine (Instron model 4465, InstronCorp.; Canton MA, USA) (Fig. 1) at a speed of 2.5mm per minute. The results were recorded and plotted in Kg/cm2 by the machine software.

Fig. 1: Shear test using the Instron universal machine.
RESULTS
The results were analyzed by the statistical software SPSS version 20. Descriptive statistics, average, standard deviation of the numeric variables, percentages, proportions of the ordinal variables and inferential statistics ANOVA were performed. Average bond strengths according to placement technique are shown in Table 1.
Table 1: Average bond strength for both placement techniques.
The models with sodium hypochlorite, hydrogen peroxide and control group are shown in Figs. 1 and 2. The results of the analysis of variance (ANOVA) are shown in Table 2.
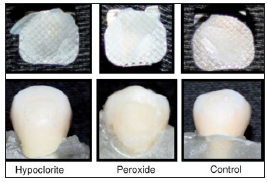
Fig. 2: Sample of pre-treatment according to the different enamel conditioning techniques.
Table 2: Average differences between groups using ANOVA (analysis of variance).

Statistically significant differences are observed between groups. Results of the post hoc test for bond strength showed that the 5.25% sodium hypochlorite pretreatment differs significantly from the other groups. Tables 3 and 4.
Table 3: Results of post hoc tests for membership.

Table 4: Results of post hoc tests for membership.

DISCUSSION
The group treated with sodium hypochlorite had the highest bond strength. Previous studies1,3,18,20- 23,have concluded that the use of 5.25% sodium hypochlorite for 30 seconds to eliminate the superficial collagen layer from the enamel surface as a pretreatment method improves bond strength24. Espinosa R et al.23 ,showed that the use of 5% sodium hypochlorite for one minute followed by phosphoric acid, improves bond strength, a result which is consistent with this research. The sodium hypochlorite technique for one minute was found to be the most efficient1.
Our study observed lower bonding efficiency with peroxide; however, little is known of the effects on bonding to enamel when it is applied once, even when the amount is minimum in quantity, which turns out to be dependent on the time elapsed. Peroxide was used for its antiseptic and biofilm stripping action at a 3.5% concentration, which is why no article about the use of peroxide as pretreatment method for the bracket cementation was found. Based on the results of this study, it may be concluded that 5.25% sodium hypochlorite as a pretreatment agent for the enamel significantly increases the bond strength for brackets and any type of resins on the surface of the enamel. Therefore, this method is recommended for better etching outcomes. Further studies are required to assess the use of hydrogen peroxide accurately before it is used as an alternative pretreatment method.
1. Moyaho-Bernal A, Vaillard-Jimenez E, Soberanes-De la Fuente E, Franco-Romero G, Montiel-Jarquin AJ, Martinez- Fernandez RG. Dos tecnicas para la retencion de selladores dentales. Rev Med Inst Mex Seguro Soc 2011;49:13-16. [ Links ]
2. GomezDe Ferraris ME, Campos Munoz A. Histopatologia y Embriologia e Ingenieria Tisular Bucodental. Mexico Ed. Medico Panamericana, 2000;235-315. [ Links ]
3. Tormo J, Bolaos R, Miranda Z. Ultraestructura superficial del esmalte dental humano observado al microscopio electronico de rastreo. Rev Cost Cienc Med 1986;7:23-28. [ Links ] URL: http://www.binasss.sa.cr/revistas/rccm/v7n1/art4.pdf
4. Bhaskar SN. Histologia y embriologia bucal de Orban. Mexico, Ed. Prado, 2000;39-90. [ Links ]
5. Marcanti M. Caries dental. Antimicrobianos y vacunas para su control. In: Negroni M. Microbiologia estomatologica. Mexico Ed. Panamericana, 2001;220-247. [ Links ]
6. Perez-Luyo AG. La Biopelicula: una nueva vision de la placa dental. Rev Estomatol Herediana 2005;15:82-85. URL: http://revistas.concytec.gob.pe/scielo.php?script=sci_arttext& pid=S1019- [ Links ]
7. Levine M, Goldman GC. Saliva y cuticula dentales en periodoncia. Mexico Ed Mc Graw Hill, 1994;125-129. [ Links ]
8. Brown MR, Foreman FJ, Burgess JO, Summitt JB. Penetration of gel and solution etchants in occlusal fissures. ASDC J Dent Child 1988;55:265-268. [ Links ]
9. Bogert TR, Garcia-Godoy F. Effect of prophylaxis agents on the shear bond strength of a fissure sealant. Pediatr Dent 1992;14:50-51. [ Links ]
10. Cua-Benward GB, Luna-Naim JJ, Kapala J. A comparative study of pumice versus hydrogen peroxide as pretreatments for acid etching for resin bonding. Pediatr Dent 1993; 15: 353-354. [ Links ]
11. Ellis RW, Latta MA, Westerman GH. Effect of air abrasion and acid etching on sealant retention: an in vitro study. Pediatrics Dent 1999;21:316-319. [ Links ]
12. Caspersen IVAR. Residual acrylic adhesive after removal of plastic orthodontic brackets: A scanning electron microscopic study. Am J Orthod 1977;71:637-650. [ Links ]
13. Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res 1955;34:849-853. [ Links ]
14. Johnson BR, Remeikis NA. Effective shelf-life of prepared sodium hypochlorite solution. J Endod 1993;19:40-43. [ Links ]
15. Cunningham WT, Balekjian AY.. Effect of temperature on collagen-dissolving ability of sodium hypochlorite endodontic irrigant. Oral Surg Oral Med Oral Pathol. 1980; 49:175-177. [ Links ]
16. Pişkin B, Turkun M. Stability of various sodium hypochlorite solutions. J Endod 1995;21:253-255. [ Links ]
17. Badilla A, Tijerino S. Adhesion a esmalte despues del blanqueamiento. Odontologia Vital 2009;1:41-45. URL: http://es.scribd.com/doc/176652525/Curva-Tura#scribd [ Links ]
18. Cohen MA, Burns RC. Vias de la pulpa. Madrid Ed. Harcourt, 1999;650-666. [ Links ]
19. Patusco VC, Montenegro G, Lenza MA, Alves de Carvalho A. Bond strength of Metallic Brackets After Dental Bleaching. Angle Orthod 2009;79:122-26. [ Links ]
20. Turkkahraman H, Adanir N, Gungor AY. Bleaching and desensitizer application effects on shear bond strengths of othodontic brackets. Angle Orthod 2007;77:489-493. [ Links ]
21. Bernal-Quintana JL, Palma-Calero JM, Guerrero-Ibarra J, Espinosa-Fernandez R. Valoracion de la resistencia al desprendimiento de brackets cementados con ionomero de vidrio a esmalte con y sin grabado previo. Rev Odontol Mex 2010; 14:145-150. URL: http://www.medigraphic.com/pdfs/odon/uo-2010/uo103b.pdf [ Links ]
22. Ballesteros-Pinzon C, Bermudez-Lozano JA, Coronel- Corzo N, De Leon-Goenaga E, Delgado LP, Baez-Quintero L. Comparacion de la fuerza de adhesion de brackets utilizando dos metodos de acondicionamiento para porcelana. Rev Nac Odontol 2011;7:12-19. URL: http://revistas.ucc.edu.co/index.php/od/article/view/288 [ Links ]
23. Espinosa R, Valencia R, Uribe M, Ceja I, Saadia M. Enamel deproteinization and its effect on acid etching: an in vitro study. J Clin Pediatr Dent 2008;33:13-19. [ Links ]
24. Bayona-Marin AE, Fonseca-Cano M, Macias-Leguizamon CM. Comparacion de la resistencia adhesiva de brackets cementados, efectuando o no un pretratamiento al esmalte dental con hipoclorito de sodio al 5.25%. Especial ortodoncia. Odontos 2010;12:10-17. [ Links ]




![Influence of addition of 2-[3-(2H-benzotriazol-2-YL)- 4-hydroxyphenyl] ethyl methacrylate to an experimental adhesive system](/img/en/prev.gif)









