Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Odontológica Latinoamericana
On-line version ISSN 1852-4834
Acta odontol. latinoam. vol.29 no.1 Buenos Aires Apr. 2016
ARTÍCULOS ORIGINALES
Effect of angiotensin-converting enzyme inhibitors on vascular endothelial function in hypertensive patients after intensive periodontal treatment
María C. Rubio1,3, Pablo G. Lewin1, Griselda De la Cruz2, Andrea N. Sarudiansky2, Mauricio Nieto2, Osvaldo R. Costa 2, Liliana N. Nicolosi1,3
1 Department of Buccodental Pathology, School of Dentistry, University of Buenos Aires.
2 Department of Periodontics, School of Dentistry, University of Buenos Aires.
3 Spanish Hospital of Buenos Aires. Buenos Aires, Argentina.
CORRESPONDENCE Dra. Liliana N. Nicolosi Catedra de Patologia y Clinica Bucodental Facultad de Odontologia, UBA Marcelo T. De Alvear 2142, 4° "A". CP 1122AAH. Buenos Aires, Argentina. e-mail: lnicolosi@hotmail.com
ABSTRACT
There is a relation between vascular endothelial function, atherosclerotic disease, and inflammation. Deterioration of endothelial function has been observed twenty-four hours after intensive periodontal treatment. This effect may be counteracted by the action of angiotensin-converting enzyme inhibitors, which improve endothelial function. The aim of the present study was to evaluate vascular endothelial function after intensive periodontal treatment, in hypertensive patients treated with angiotensinconverting enzyme inhibitors. A prospective, longitudinal, comparative study involving repeated measurements was conducted. Fifty-two consecutive patients with severe periodontal disease were divided into two groups, one comprising hypertensive patients treated with converting enzyme inhibitors and the other comprising patients with no clinical signs of pathology and not receiving angiotensin-converting enzyme inhibitors. Endothelial function was assessed by measuring postischemic dilation of the humeral artery (baseline echocardiography Doppler), and intensive periodontal treatment was performed 24h later. Endothelial function was re-assessed 24h and 15 days after periodontal treatment. Statistical analysis: Results were analyzed using the SPSS 20 statistical software package. Student's t test and MANOVA were calculated and linear regression analysis with 95% confidence intervals and α<0.05 was performed. Arterial dilation at 24 hours was lower compared to baseline in both groups; values corresponding to the groups receiving angiotensin-converting enzyme inhibitors were 11.89 ± 4.87 vs. 7.30 ± 2.90% (p<0.01) and those corresponding to the group not receiving ACE inhibitors were 12.72 ± 4.62 vs. 3.56 ± 2.39 (p<0.001). The differences between groups were statistically significant (p<0.001). Conclusion: The increase in endothelial dysfunction after intensive periodontal treatment was significantly lower in hypertensive patients treated with angiotensin-converting enzyme inhibitors. Endothelial function improved 15 days after periodontal treatment, reaching baseline values. These results support the protective effect of angiotensin converting enzyme inhibitors on the endothelial function after intensive periodontal treatment.
Key words: Hypertension; Angiotensin-converting enzyme inhibitors; Periodontal diseases.
RESUMEN
Efecto de los inhibidores de la enzima convertidora sobre la función del endotelio vascular en pacientes hipertensos que recibieron tratamiento periodontal intensivo
Existe relación entre la disfunción del endotelio vascular, la enfermedad aterosclerótica y la inflamación. A las 24 h del tratamiento intensivo de la enfermedad periodontal se produce un deterioro de la función endotelial. Este efecto podría ser balanceado por la acción de los inhibidores de la enzima convertidora de la angiotensina que mejoran la función endotelial. El objetivo del presente estudio fue evaluar la función endotelial vascular después del tratamiento periodontal intensivo, en pacientes hipertensos tratados con inhibidores de la enzima convertidora de la angiotensina. Se realizó un estudio prospectivo, longitudinal, comparativo, con mediciones repetidas. Se incorporaron 52 pacientes consecutivos, con enfermedad periodontal severa divididos en dos grupos, uno con hipertensión arterial tratados con inhibidores de la enzima convertidora y el otro sin inhibidores ni patología clínicamente evidente. Se determinó la función endotelial cuantificando la dilatación de la arterial humeral post isquemia ecocardiografía Doppler basal. A las 24 h se efectuó el tratamiento periodontal intensivo; a 24 h y 15 días posteriores se reevaluó la función endotelial. Análisis estadístico: se empleó el paquete estadístico SPSS 20. Se realizaron: t-test de Student, MANOVA y análisis de regresión lineal con intervalos de confianza del 95% y α <0.05. Resultados: a las 24 h post tratamiento periodontal se observó una menor dilatación arterial en ambos grupos en relación a la dilatación arterial basal, siendo para el grupo con inhibidores 11.89 ±4.87 vs. 7.30 ± 2.90%, p<0.01 y para el grupo sin inhibidores 12.72 ± 4.62 vs 3.56 ± 2.39, p<0.001, con diferencias significativas entre ambos p< 0.001. En conclusión el aumento de la disfunción endotelial post tratamiento intensivo periodontal fue significativamente menor en hipertensos que recibieron inhibidores de la enzima convertidora de la angiotensina. La función endotelial mejoró a los 15 días de efectuado el tratamiento, alcanzando los valores inciales. Estos resultados permitirían relacionar a los inhibidores de la enzima convertidora con un efecto protector del endotelio posterior al tratamiento intensivo de la enfermedad periodontal.
Palabras Clave: Hipertensión arterial; Inhibidores de la enzima convertidora; Enfermedad periodontal.
INTRODUCTION
Vascular endothelial function (VEF) plays a funda - mental role in the pathogenesis of atherosclerosis1. The endothelium plays an important part in maintaining vascular homeostasis, and is involved in hemodynamics and antithrombotic activity2. It consists of a single layer of cells that separates the circulating blood from the smooth vascular muscle, and produces signaling molecules that regulate vascular tone, monocyte and neutrophil adhesion, and platelet aggregation. The endothelium produces nitric oxide (NO)3 from the amino acid L-arginine, through activation of NO synthase. NO plays a key role because of its potent vasodilator action. It inhibits leukocyte adhesion and platelet aggregation, and inhibits smooth muscle cell proliferation. Because NO acts as a free radical scavenger, it has antioxidant properties, and it is involved in the release of plasminogen activator, which has a fibrinolytic effect and antithrombotic properties. 4-6 There are certain substances that act on the vascular endothelium exerting a proinflammatory effect, and have the capacity to generate reactive oxygen species (ROS). The latter are responsible for the degradation of NO and modulate vascular tone. One of such substances with the strongest vasoconstrictor effect is angiotensin II. 2-7
A disturbance in the balance between vasodilators and vasoconstrictors causes vascular endothelial dysfunction, with decreased production and increased degradation of NO. This alteration in VEF causes vasoconstriction, platelet aggregation, leukocyte adhesion, and proliferation of smooth muscle cells8- 11. VEF is assessed by measuring brachial artery flow-mediated dilation following ischemia12. The latter is associated with NO released by the arterial endothelium in response to the shear stress in the blood flow. The decrease in NO release from dysfunctional endothelium generates a paradoxical vasoconstrictive response to acetylcholine4. Other works reported in the literature have shown vascular endothelial dysfunction to be associated with a variety of diseases and risk factors for atherosclerosis, ranging from hypertension (HTN), aging13, dyslipidemia14, diabetes15 and smoking 16-17, to specific cardiovascular diseases (CVD), including coronary, cerebral, and peripheral arteriopathy14-16 as well chronic infection and inflammation, such as periodontal disease.18-22 Case-control-cohort studies have shown that periodontitis is associated with endothelial dysfunction23, atherosclerosis24-26, and increased risk of myocardial infarction and stroke27-29. Despite these findings, the statement of the American Heart Association indicates that there is no causative relationship between periodontal and atherosclerotic vascular disease. It is of note, however, that both pathologies share risk factors, such as diabetes and smoking30. Tonetti M et al.31 found that intensive periodontal treatment caused endothelial dysfunction during the first 24 h, though dysfunction improved 60 days after treatment.
Deterioration of VEF leads to an increase in pro-inflammatory and thrombogenic potential, an increase in the likelihood of ischemic episodes, and a higher incidence of cardiovascular events in periods immediately after invasive periodontal treatment. Hypertensive patients have vascular endothelial dysfunction32-34 and administration of angiotensinconverting enzyme inhibitors (ACEI) 35-38 is a therapeutic pillar in the treatment of HTN. In addition to lowering blood pressure, renin-angiotensin system blockade with ACEI provides a rational approach to reverse endothelial dysfunction. The beneficial effects of ACEI on VEF in hypertensive patients may go beyond HTN. The aim of the present study was to evaluate vascular endothelial function after intensive periodontal treatment, in hypertensive patients treated with ACEI.
MATERIALS AND METHODS
A prospective longitudinal study involving repeated measures was conducted at the Departments of Periodontics and of Buccodental Pathology of the School of Dentistry, University of Buenos Aires, and the Department of Cardiology of the Spanish Hospital of Buenos Aires, between September 2010 and August 2014. The study design was similar to that used in controlled clinical trials. The study protocol was approved by the ethics committees of both institutions. Consecutive patients with severe periodontal disease (SPD) were divided into two groups: one group comprised patients with HTN treated with enalapril (ACEI) in doses according to blood pressure (cases), and the other group included non hypertensive patients (controls). The subjects were male and female outpatients over the age of 18 years. Patients receiving vasodilators, transplant patients, renal failure patients on dialysis, patients with concomitant infection or inflammation, pregnant and lactating patients, patients receiving antibiotics within three months and/or periodontal treatment within 6 months prior to the study, patients with fewer than 10 teeth, inadequately controlled hypertensive patients or with blood pressure values above 140/90 mmHg at the onset of the study, and patients who failed to sign the informed consent form, were excluded from the study. The following covariables were recorded: sex, age, CVD, smoking, HTN, dyslipidemia and diabetes (DBT). The patients who met the inclusion criteria underwent VEF assessment (baseline), and received intensive periodontal treatment 24 h later. VEF assessment was repeated twenty-four hours and 15 days after periodontal treatment. The study design is shown in Fig. 1.
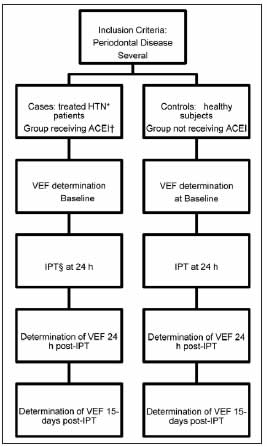
Fig. 1: Study Design. *HTN: Arterial hypertension; †ACEI: Angiotensin-converting enzyme inhibitors; ‡VEF: Endothelial Vascular Function; §IPT: Intensive Periodontal Treatment.
All enrolled patients underwent clinical and radiographic dental examination. The radiographic study included a standardized periapical radiographs using a CS2200 - intraoral x-ray system (Carestream Health, Inc). All permanent teeth, except for the third molars, were evaluated. Patients with fewer than 10 teeth were excluded from the study in order to ensure that periodontal diagnosis was representative of the clinical dental status of the patient and avoid overestimation of diagnosis of periodontal disease. Clinical periodontal examination was performed by a single calibrated operator. Periodontal measurements were performed at six sites per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual/ palatal, mid-lingual/palatal and disto-lingual/ palatal) on all present teeth, using a manual Marquis Type periodontal probe (Hu-FriedyR). The studied periodontal parameters included Probing depth (PD), Clinical attachment level (CAL), Bleeding on probing (BOP), and radiographic alveolar bone loss (ABL), which were assessed following a standardized protocol. Intra-examiner reproducibility of PD and CAL measurements was assessed before the study. The weighted K coefficients for PD and CAL were 0.96 and 0.91 respectively. Diagnosis of periodontal disease was performed based on CAL values, in keeping with the 1999 International Workshop for a Classification of Periodontal Disease accepted by the American Academy of Periodontology (AAP)39. Severe generalized periodontal disease (SPD) was established when:
• Average CAL values were ≥ 5 mm. This value was obtained by averaging all six determinations performed on each of the teeth. This method allows avoiding overestimation of periodontal disease diagnosis, since many patients have a high CAL value at only one periodontal site as a result of trauma (for example, trauma associated with tooth brushing, ill-fitting removable dentures, or harmful oral habits, among other causes).
• Average CAL values were < 5 mm but with values ≥ 5 mm at more than 30% of studied sites, in addition to horizontal alveolar bone loss > 1/3 of the root length in at least 30% of teeth, as measured on the radiographic images.
Periodontal treatment
One hour before intensive periodontal treatment, all patients underwent a protocol of antibiotic prophylaxis, and intra and extra oral antisepsis was carried out. Intensive periodontal treatment was performed in a single session40. Under local infiltration anesthesia (lidocaine), root scaling and planing was performed using rigid Gracey-type curettes (Hu-FriedyR), numbers 5/6, 7/8, 11/12 and 13/14 according to the surface, and an ultrasound cavitation machine (cavitron plus scaler, DentsplyR).
Endothelial function assessment
Endothelial vascular function was assessed in the morning after 12 h fasting, before smoking and after 15 minutes rest under controlled temperature conditions (22 - 26o C). An ECG lead and a blood pressure cuff were placed on the patient's forearm. Endothelial-dependent dilation was assessed with the FMD (Flow mediated dilation) of the brachial artery using Doppler ultrasonography, according to the Guidelines of the International Brachial Artery Reactivity Task Force41. The forearm was occluded by cuff inflation to at least 50 mmHg above systolic pressure for 5 min, resulting in a reactive hyperemia after the release of the cuff, and the increased shear stress led to endothelial-mediated vasodilatation. FMD was measured with an ultrasound scanner with a 7.5 MHz linear transducer (ATL HDI 3000); five consecutive discrete measurements were obtained and averaged into the final value at each time point. The coefficient of variation of brachial artery hyperemic flow values was calculated by dividing peak hyperemic flow by peak baseline flow. After 15 minutes, smooth muscle response to an exogenous stimulus was measured. Brachial artery diameter was determined at baseline, and three times consecutively 3 minutes after sublingual administration of 0.4mg; the obtained values were averaged41.
Statistical Analysis
The data were entered on an Excel spreadsheet and analyzed using the SPSS 20 software package. Frequency distribution and percentages of categorical variables were calculated. Number of cases, minimum value, maximum value, arithmetic mean, standard deviation of each variable measured on ordinal scale were calculated. Data were analyzed using Student's t test, MANOVA, and linear regression analysis, with α<0.05 and 95% confidence intervals (CI). Sample size calculation: Because the statistical analysis was based on regression procedures, given an effect size of 0.35, a power of 0.8, and an alpha value of 0.05, and considering a maximum of 5 predictors, minimum sample size was calculated to be 43 patients.
RESULTS
Out of a total of 86 patients studied initially, 52 patients with SPD were included in the study. The ACEI group comprised 51.9% (n=27) of the study population, and the remaining 48.14% (n=25) were included in the control group. The patients in the ACEI group received 14.20 ±4.25 mg of enalapril twice daily (IC 95% 12.51-15.88) for HTN treatment. Baseline values of both groups are shown in Table 1.
Table 1: Baseline characteristics of the population.

Severity of periodontal disease was similar in ACEI - treated patients and controls, as shown by the following results: present teeth 19.11 ±5.6 vs. 16.14 ±6.18 (p=0.258), CAL 3.49 ±1.49 vs. 4.39 ±1.72 mm (p=0.21), PD 3.45 ± 0.63 vs. 3.59 ±0.64 mm (p=0.61), sites with BOP 43.56 ±20.58 vs. 42.5 ±30.34 (p=0.92), and ABL 36.87 ±14.64 vs. 38.7 ±10.99 % (p=0.76). Baseline values of end-diastolic humeral artery diameter and nitroglycerin-mediated humeral artery dilation were 4.3 ± 0.50 vs. 4.7± 0.3 mm and 19.32 ± 8.62 vs. 20.64 ±6.43% respectively. A significantly lower percentage of humeral artery flow mediated dilation was observed in both groups 24 h after intensive periodontal treatment, as compared to baseline values, showing deterioration of VEF (ACEI p<0.01; Controls p<0.001) (Table 2).
Table 2: Endothelial function in the studied groups at the studied time points.

Deterioration of VEF was significantly greater 24 after intensive periodontal treatment in patients not receiving ACEI (p<0.001). VEF improved 15 days after treatment, and was not significantly different from baseline values (Fig. 2). ACEI treatment was a significant variable in the differences between groups, having an average impact of 26.7% (95% CI 1-49.2).
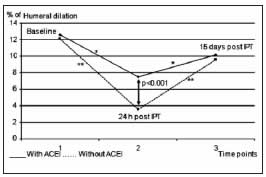
Fig. 2: Endothelial function at the different post-treatment time points. 1) Baseline determination: Humeral artery flow-mediated dilation; 2) Determination 24 h after intensive periodontal treatment; 3) Determination 15 days post-treatment.
* Level of significance p<0.01
** Level of significance p<0.001
DISCUSSION
A number of studies have demonstrated association between periodontal disease and CVD. The link between both pathologies remains to be elucidated in order to determine the causal, contributing or coincidental role of periodontitis in CVD, irrespective of shared risk factors. Periodontal disease is caused by an array of microorganisms, such as Porphyromonas gingivalis, which have the ability to invade endo thelial cells42. In addition, periodontal pathogens have been identified in atherosclerotic plaque specimens obtained during carotid endarterectomy43. These microorganisms can act directly on the endothelium or via a systemic inflammatory response, thus contributing to arterial damage44. Atherosclerosis is a complex process involving a number of different cell types (monocytes, macrophages, T lymphocytes, smooth muscle cells, endothelial cells) and inflammatory molecules, such as C Reactive Protein (CRP), interleukin 1β, interleukin 6, and tumoral necrosis factor (TNF), among others19, 45-49.
Its pathogenesis involves a variety of immune mechanisms which interact with multiple causal factors capable of triggering, developing, perpetuating, and activating arterial lesions50. The aforementioned factors include metabolic disorders such as DBT, hypercholesterolemia, obesity, and other independent risk factors like hypertension, smoking, and infection51-56. Elevated inflammatory reactants have been found in patients with periodontitis and vascular endothelial dysfunction57-58. Underlying acute cardiovascular events, there is an inflammatory phenomenon that triggers plaque instability. Endothelial dysfunction is the initial pathophysiological step in a progression of vascular damage, and it is considered an early event in the development of atherosclerosis59. Periodontal disease treatment would lower the inflammatory component and improve endothelial function (6 months after intensive periodontal treatment), as shown by Tonietti et al 31, providing an additional benefit to VEF. It is well documented that HTN results in endothelial dysfunction60. Nevertheless, no significant differences in baseline VEF indices were observed between HTN patients and controls in the present study. This finding is most likely the result of treatment with ACEIs, which have been shown to improve endothelial dysfunction. 61-62
The decrease in inflammatory reactants and improvement in VEF observed 6 months after intensive periodontal treatment31 is preceded by a period of endothelial function deterioration and increase in inflammatory response immediately after treatment. The increase in inflammatory reactants is shown by the increase in serum levels of RCP, IL-6, E-selectin, tissue plasminogen activator (tPA), plasminogen activator inhibitor type 1 (PAI-1), von Willebrand factor, and neutrophils. As a result, during that period, the patient is at higher risk for a cardiovascular event. Moreover, a higher incidence of stroke and acute myocardial infarction has been observed in the first 4 weeks after periodontal disease treatment.32. It must be pointed out that around 40% of the patients studied here had coronary disease and an even higher percentage also had other risk factors for CVD. In agreement with findings reported by Tonetti et al, deterioration of VEF was observed immediately after intensive periodontal treatment (24h) in both groups, though the impact in the group receiving ACEI was lower. Our results showed no significant differences between VEF determinations obtained at baseline and 15 days after intensive periodontal treatment.
No cardiovascular events occurred during the study, likely due to treatment with ACEI, in view of the beneficial effect of these drugs on the endothelium63. One of the limitations of the present study is the small number of patients. However, the differences in VEF observed after intensive periodontal treatment are so significant that they render the sample sufficient to demonstrate the protective effect of ACEI. Follow-up at 15 days does not allow establishing the incidence of cardiovascular events beyond that period. Further studies using longer follow-up periods are necessary to establish the incidence of events beyond the time point established in the protocol used herein.
CONCLUSIONS
Endothelial dysfunction after intensive treatment of severe periodontal disease was significantly lower in patients receiving angiotensin converting enzyme inhibitors. Endothelial function improved 15 days after treatment, returning to baseline values. There are data that would seem to indicate a protective effect of ACEI on vascular endothelium after intensive treatment for severe periodontal disease.
ACKNOWLEDGMENT
This study was supported by a grant from School of Dentistry, University of Buenos Aires, Prof. Rodolfo Erausquin Program (CD) 284/10
1. Gokce N, Keaney Jr JF, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for longterm cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 2003; 41:1769-1775. [ Links ]
2. Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF et al. Hypertension Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens 2005; 23:233-246. [ Links ]
3. Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988; 333:664-666. [ Links ]
4. Furchgott RF, Zawadski JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980; 228:373-376. [ Links ]
5. Loscazo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis 1995; 38:87-104. [ Links ]
6. Cohen RA. The role of nitric oxide and others endothelium derived vasoactive substances in vascular disease. Prog Cardiovasc Dis 1995; 38:105-128. [ Links ]
7. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994;74:1141-1148. [ Links ]
8. Wennmalm A. Endothelial nitric oxide and cardiovascular disease. J Intern Med 1994; 235:317-327. [ Links ]
9. Furchgott RF. The discovery of endothelium- derived relaxing factor and its importance in the identification of nitric oxide. JAMA 1996; 276:1186-1188. [ Links ]
10. Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet 1994; 343:1199-1206. [ Links ]
11. Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant 2001; 16 (Suppl 1): 60-62. [ Links ]
12. Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S , Lerman A, Mancia G et al . Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005; 23:7-17. [ Links ]
13. Bottino DA, Lopes FG , de Oliveira FJ, Mecenas Ade S, Clapauch R, Bouskela E. Relationship between biomarkers of inflammation, oxidative stress and endothelial/microcirculatory function in successful aging versus healthy youth: a transversal study. BMC Geriatr. 2015 Apr 8; 15:41 DOI:: 10.1186/s12877-015-0044-x. [ Links ]
14. Creager MA, Cooke JP, Mendelsohn ME Gallagher SJ, Coleman SM, Loscalzo J, Dzau VJ. Impaired vasodilatation of forearm resistance vessels in hypercholesterolemia humans. J Clin Invest 1990; 86:228-234 [ Links ]
15. Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA.Impaired endothelium dependent vasodilatation in patients with insulin-dependent diabetes mellitus. Circulation 1993; 88:2510-2516. [ Links ]
16. Celermajer DS, Sorensen KE, Georgakopuolos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose- related and potentially reversible impairment of endothelium dependent dilation in healthy young adults. Circulation 1993;88: 2149-2155. [ Links ]
17. Zeiher A, Schachinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary vasodilator function. Circulation 1995; 92:1094-1100. [ Links ]
18. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilatation dysfunction on adverse longterm outcome of coronary heart disease. Circulation 2000; 101:1899-1906. [ Links ]
19. Fuster V, Moreno PR, Fayad ZA, Corti R, Badinon JJ. Atherothrombosis and high- risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005;46:937-954. [ Links ]
20. Sanada H, Higashi Y, Goto C, Chayama K, Yoshizumi M, Sueda T. Vascular function in patients with lower extremity peripheral arterial disease: a comparison of function in upper and lower extremities. Atherosclerosis 2005; 178: 179-185. [ Links ]
21. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after infection or vaccination. N Engl J Med 2004;351:2611- 2618 [ Links ]
22. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135-1143. [ Links ]
23. Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T, Takemoto H, Nakamura S, et al. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension 2008 Feb; 51(2):446-453. [ Links ]
24. Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol 2003; 23:1245-1249. [ Links ]
25. Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D'Aiuto F, Kastelein JJ, Loos BG. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 2014 Jan; 41:70-79. [ Links ]
26. Bartova J, Sommerova P, Lyuya-Mi Y, Mysak J, Prochazkova J, Duskova J, Janatova T, Podzimek S. Periodontitis as a risk factor of atherosclerosis. J Immunol Res. 2014; 2014:636893. [ Links ]
27. Kodovazenitis G, Pitsavos C, Papadimitriou L, Vrotsos IA, Stefanadis C, Madianos PN. Association between periodontitis and acute myocardial infarction: a case-control study of a nondiabetic population. J Periodontal Res. 2014 Apr; 49:246-252. [ Links ]
28. Marfil-Alvarez R, Mesa F, Arrebola-Moreno A, Ramirez- Hernandez JA, Magan -Fernandez A, O'Valle F, Galindo- Moreno P, Catena A. Acute myocardial infarct size is related to periodontitis extent and severity. J Dent Res. 2014; 93:993-998. [ Links ]
29. Soder B, Meurman JH, Soder PO. Gingival Inflammation Associates with Stroke—A Role for Oral Health Personnel in Prevention: A Database Study. PLoS One. 2015. 25; 10(9):e0137142.
30. Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012; 125:2520-2544. [ Links ]
31. Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, M Phil, Suvan J. et al. Treatment of periodontitis and endothelial function. N Engl. J Med 2007; 356: 911-920. [ Links ]
32. Minassian C , D'Aiuto F , Hingorani AD , Smeeth L. Invasive dental treatment and risk for vascular events: a self-controlled case series. Ann Intern Med 2010; 153(8):499-506. [ Links ]
33. Olsen MH, Wachtell K, Aalkjaer C, Dige-Petersen H, Rokkedal J, Ibsen H. Vasodilatory capacity and vascular structure in long-standing hypertension: a LIFE substudy. Losartan Intervention For Endpoint-Reduction in Hypertension. Am J Hypertens 2002; 15:398-404. [ Links ]
34. Quyyumi AA, Patel RS. Endothelial Dysfunction and Hypertension. Cause or effect? Hypertension 2010; 55: 1092-1094. [ Links ]
35. On YK, Kim CH, Oh BH, Lee MM, Park YB. Effects of angiotensin converting enzyme inhibitor and calcium antagonist on endothelial function in patients with essential hypertension. Hypertens Res. 2002; 25:365-371. [ Links ]
36. Enseleit F, Hurlimann D, Luscher TF. Vascular protective effects of angiotensin converting enzyme inhibitors and their relation to clinical events. J Cardiovasc Pharmacol. 2001; 37 Suppl 1:S21-30. [ Links ]
37. Schmieder RE. Mechanisms for the clinical benefits of Angiotensin II receptor blockers. Am J Hypertens 2005; 18:720-730. [ Links ]
38. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015; 116:960-975. [ Links ]
39. Armitage G.C. Development of classification system for periodontal diseases and condition. Ann Periodontal 1999; 4:1-6. [ Links ]
40. Lang NP, Tan WC, Krahenmann MA, Zwahlen M.A Systematic review of the effects of full-mouth debridement with and without antiseptics in patients with chronic periodontitis.J Clin Periodontol. 2008;35:8-21. [ Links ]
41. Guidelines for the Ultrasound Assessment of Endothelial- Dependent Flow-Mediated Vasodilation of the brachial Artery. A Report of the International Brachial Artery Task Force. J Am CollCardiol 2002;39:257-267. [ Links ]
42. Dorn BR, Burks JN, Seifert KN, Progulske-Fox A. Invasion of endothelial and epithelial cells by strains of Porphyromonas gingivalis. FEMS Microbiol Lett 2000;187:139-144. [ Links ]
43. Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol 2000; 71:1554- 1560. [ Links ]
44. Miyajima S, Naruse K, Kobayashi Y, Nakamura N, Nishikawa T, Adachi K, Suzuki Y, Kikuchi T et al. Periodontitis-activated monocytes/macrophages cause aortic inflammation. Sci Rep. 2014; 4;4:5171. [ Links ]
45. Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 2011; 31:1506-1516. [ Links ]
46. Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, et al. Interleukin- 18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:41-45. [ Links ]
47. Fenyo IM, Gafencu AV. The involvement of the monocytes/ macrophages in chronic inflammation associated with atherosclerosis. Immunobiology. 2013; 218:1376-1384. [ Links ]
48. Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest 2004; 114:427-437. [ Links ]
49. Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V , Pinto A , Licata G. Atherosclerosis as an inflammatory disease. Curr Pharm Des 2012; 18:4266-4288. [ Links ]
50. Gregersen I, Holm S, Dahl TB, Halvorsen B, Aukrust P. A focus on inflammation as a major risk factor for atherosclerotic cardiovascular diseases. Expert Rev Cardiovasc Ther. 2015; 28:1-13. [ Links ]
51. Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol 2006; 47:1093-1100. [ Links ]
52. Lemieux I, Pascot A, Prud'homme D, Almeras N, Bogaty P, Nadeau A, Bergeron J, Despres JP.. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 2001;21:961-967. [ Links ]
53. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005; 96:939-949. [ Links ]
54. Kanter, JE, Bornfeldt KE. Inflammation and Diabetes- Accelerated Atherosclerosis: Myeloid Cell Mediators. Trends Endocrinol Metab. 2013; 2: 137-144. [ Links ]
55. Hsich E, Zhou YF, Paigen B, Johnson TM, Burnett MS, Epstein SE. Cytomegalovirus infection increases development of atherosclerosis in apolipoprotein-E knockout mice. Atherosclerosis 2001;156:23-28. [ Links ]
56. Grundy SM. Inflammation, hypertension, and the metabolic syndrome. JAMA 2003; 290:3000- 3002. [ Links ]
57. Pink C, Kocher T, Meisel P, Dorr M, Markus MR, Jablonowski L, Grotevendt A, Nauck M et al. Longitudinal effects of systemic inflammation markers on periodontitis. J Clin Periodontol. 2015; 42:988-997. [ Links ]
58. Schwahn C, Volzke H, Robinson DM, Luedemann J, Bernhardt O, Gesch D, John U, Kocher T. Periodontal disease, but not edentulism, is independently associated with increased plasma fibrinogen levels: results from a population-based study. Thromb Haemost 2004; 92:244-252. [ Links ]
59. Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015; 209:13-22. [ Links ]
60. Shimbo D, Muntner P, Mann D, Viera AJ, Homma S, Polak JF, Barr RG, Herrington D, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension 2010;55:1210-1216. [ Links ]
61. Wallace SM, Yasmin, McEniery CM, Maki-Petaja KM, Booth AD, Cockcroft JR, Wilkinson IB. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension 2007; 50: 228-233. [ Links ]
62. Ceconi C, Fox KM, Remme WJ, Simoons ML, Bertrand M, Parrinello G, Kluft C, Blann A et al. ACE inhibition with perindopril and endothelial function. Results of a substudy of the EUROPA study: PERTINENT. Cardiovasc Res 2007; 73(1):237-246. [ Links ]
63. Ferrari R, Guardigli G, Ceconi C. Secondary prevention of CAD with ACE inhibitors: a struggle between life and death of the endothelium. Cardiovasc Drugs Ther 2010; 24:331-339. [ Links ]














