Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Odontológica Latinoamericana
On-line version ISSN 1852-4834
Acta odontol. latinoam. vol.30 no.3 Buenos Aires Dec. 2017
ARTÍCULO ORIGINAL
Description of an in vivo oral mucosa HSV-1 infection model in mice
Descripción de un modelo murino de infección por HSV-1 a través de la mucosa oral
Leidy Y. Bastidas-Legarda1, Edgar O. Beltrán1, Lina M. Marín-Gallón1, Jaime E. Castellanos1,2, Sonia P. Bohórquez1
1 Universidad Nacional de Colombia, Facultad de Odontología, Grupo de Investigaciones Básicas y Aplicadas en Odontología, Bogotá D.C, Colombia.
2 Universidad El Bosque, Laboratorio de Virología, Bogotá D.C, Colombia.
ABSTRACT
In this work, we established an in vivo murine model of herpes simplex virus type 1 (HSV-1) infection involving inoculation by scarification of the oral mucosain order to study its dissemination towards the trigeminal ganglion (TG). Both viral DNA and infectious virions were detected on the third day postinfection (p.i.). Viral proteins revealed by immunohistochemistry were mainly found at seven days p.i., when the latency-associated transcript (LAT) was also detected. This model simulated the dissemination process of HSV-1, which could be used to study herpes pathogenesis starting in the oral mucosa.
Key words: Herpes Simplex Virus 1; Mouth Mucosa; Trigeminal Ganglion; Latency associated transcript.
RESUMEN
Con el propósito de estudiar la dispersión de del Herpes Simplex Virus tipo 1 (HSV-1) desde la mucosa oral hasta los ganglios trigeminales, en el presente trabajo se estableció un modelo de infección en ratones, haciendo inoculación por escarificación en la mucosa oral. Tanto ADN viral como viriones infecciosos se detectaron en los ganglios trigeminales al dia 3 postinfección (p.i.). Las proteínas virales se detectaron principalmente al día 7p.i. cuando los transcritos asociados a latencia también fueron encontrados. El modelo de infección simula adecuadamente el proceso de dispersión del HSV-1 y puede ser usado para el estudio de la patogénesis por herpes después de la infección primaria en la mucosa oral.
Palabras clave: Virus Herpes Simple; Mucosa oral; Ganglio trigeminal; Transcripción asociada a latencia.
INTRODUCTION
Herpes simplex virus type 1 (HSV-1) is a doublestranded DNA virus that belongs to the family Herpesviridae, subfamily Alphaherpesvirinae. It is transmitted by direct contact and mainly infects the skin and orofacial mucosa. After the first cycle of replication in epithelial cells, the virus travels by retrograde axonal transport towards the sensory neurons located in the trigeminal ganglia (TG), where HSV-1 establishes a latent infection characterized by shutdown of most viral proteins and continuous synthesis of the latency-associated transcript (LAT).1 After these events, the virus can be reactivated when a host is exposed to a certain stimulus that breaks the immune equilibrium in the TG, such as stress, fever or UV radiation. The recently synthesized proteins drive the assembly of virions, which travel by anterograde axonal transport, causing recurrent mucosal infections.2
In vivo studies of HSV-1 pathogenesis have mostly been performed by inoculating murine footpads or corneas; however, an animal model for studying the propagation of the virus from the oral epithelia to the TG has not been reported so far. As the oral mucosa is the main site of primary infection and subsequent reactivations, the generation of a mouse model that mirrors the most common route of infection with HSV-1 and its dissemination towards the TG would be valuable for studying the mechanisms of HSV-1 pathogenesis. The aim of the current study was therefore to establish an in vivo murine model of primary HSV-1 infection of the TG through the oral mucosa.
MATERIALS AND METHODS
HSV-1 in vivo inoculation and TG processing. This study was approved by the Ethics Committee of the School of Dentistry at Universidad Nacional de Colombia, through Resolution 005-2008. Four-week-old female ICR-strain mice were anesthetized intraperitoneally with ketamine (90 mg/kg) and xylazine (15 mg/kg) prior to two-spot mucosal scarification on each side of the medial line of the vestibular mucosa using a 21G needle. A total 10 gl of viral suspension (4X105 PFU) was placed on each lesion for 10 minutes, and mice were allowed to recover from anesthesia. Two mice were evaluated at each time point post-infection (p.i.) (3, 5 and 7 days), and two mice were mock inoculated (culture medium) (n=8). Mice were euthanized by cervical dislocation, and the TGs were obtained (two mice, four ganglia). The first TG was formalin fixed, and the second and third TGs were used to isolate DNA and RNA, respectively, while the last TG was mechanically dissociated in a glass mortar to quantify infectious virions. This procedure was performed in triplicate (n=24). The virus was recovered from infected Vero cells and titrated using a protocol for plaque formation, as previously described.3
Trigeminal ganglia processing To detect viral antigens in the formalin-fixed TG, 10 gm sections were permeabilized and incubated with an anti-HSV-1 rabbit antibody (DAKO, N1562) for 1 hour and then washed. Slides were incubated with a biotinylated anti-rabbit IgG and streptavidin peroxidase. Finally, the infected cells were visualized with H2O2 0.02% and diaminobenzidine 0.1%. A PCR was performed on the DNA isolated from the second TG, to detect viral DNA by amplifying a fragment of the viral polymerase gene using the primers 5'-CTG CCG GAC ACC CAG GGG CG-3' and 5'-CCC GCC CTC CTC GCG TTC GT-3', as reported previously.4 RT-PCR was conducted on the RNA isolated from the third TG, to amplify the LAT mRNA using the primers 5'-CAC CAG CGG GTC TTT AGT GT-3' and 5'-CGA GAT CCA TCC AAC ACA GA-3'. The housekeeping gene P-actin was used as control for PCR and RT-PCR using the primers BM-1 5' ATC CTC TTC CTC CCT GGA GA and BM-2 5' TGC CTG GGT ACA TGG TGG TA. Infectious virus recovered from the lysate of the fourth TG lysate was quantified by inoculating serial dilutions on Vero cells for five days at 37 °C in a 5% CO2 atmosphere. Monolayers were stained with 1% crystal violet, and lytic plaques were counted.
RESULTS
At three days p.i., few neurons immunopositive for viral antigens grouped in the center or at the periphery of the TG were observed (Fig. 1A). At day five p.i., the neuronal immunostaining was more intense (Fig. 1B), and at seven days p.i., 10 or more intensely positive neurons per field were found (Fig. 1C). In addition, a pattern of staining associated with nerve fibers was observed from the fifth day p.i. (Fig. 1B, thin arrows). The mock-infected mice did not show any stained cells (Fig. 1D). Viral DNA was detected at all three time points (Fig. 2A). The LAT transcript was detected only at seven days p.i. (Fig. 2B), whereas the infectious virus was detected only at day 3 (4,5x104 ± 7,7x103 PFU/ganglion) and 5 p.i. (6,8x107± 1,8x103 PFU/ ganglion).

Fig. 1: Immunoperoxidase HSV1 antigen detection in mouse trigeminal ganglia (TG).A. TG at 3 days p.i. with few neuronal cells stained. B. Staining in TG slides from mice infected at 5 days. Arrows show the positive cells, Double thin arrows identify positive nerve fibers. C. Numerous and intensely stained cells at seven days p.i. D. Appearance of mockinfected and immuno peroxidase processed ganglia. Scale bars correspond to 50 μm.
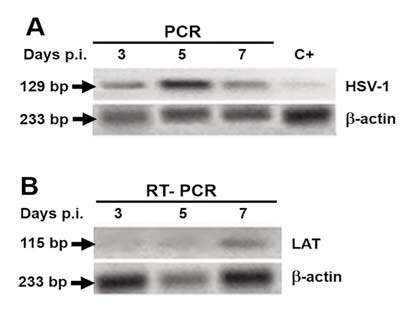
Fig. 2: Viral DNA and RNA detection using TG from infected mice. A. Viral DNA was detected at all time points. B. Note that the latency associated transcript appeared at seven days p.i.
DISCUSSION
Infection with HSV-1 is a major cause of morbidity, with seropositivity rates estimated at ~90%. Clinical manifestations include ulcerations that primarily affect oral mucosa and, depending on their severity, can lead to potentially life-threatening neurological consequences in susceptible individuals5. Different aspects of HSV-1 biology and pathogenesis have been studied in animal models6,7; however, there is no information about the kinetics of HSV-1 infection when inoculated in the oral epithelium. Given that orolabial herpes is the most prevalent viral infection of the mouth worldwide; our aim was to establish an in vivo model to evaluate the dissemination of the virus from the oral epithelium to the TG. The number and intensity of HSV-1-immunopositive neurons increased over time, reflecting a productive infection after HSV-1 entry into the TG. Studies with mouse models that involve ocular inoculation have detected viral antigen in different TG cell types at two days p.i., with a peak at day three p.i. and a decrease over the following days (days 4 and 5), until staining disappeared at day seven p.i.8 In our mucosal inoculation model, the infection peak occurred at 5 days, presumably after an intense and massive local replication that was partially controlled by the immune system in order to prevent viral entry into the nerve endings. This phenomenon may not occur in the corneal tissue because of its poor irrigation. Labetoulle et al (2003)9 found that only TG neurons were LAT positive, and they increased in number over time. However HSV inoculation by ocular abrasion is more efficient to reach TG than intravenous or intranasal inoculation10, demonstrating the main involvement of sensory fibers, as we demonstrated here. Additionally, we found that LAT expression began at 7 days p.i., suggesting that at this point, viral gene transcription stops and viral latency is established.
A study conducted by Labetoulle et al (2000) described the pathways that HSV-1 may use from oral mucosa to cornea that can lead to herpetic ocular disease by inoculating the virus into the upper lip of BALB/c mice11. This and other animal models of HSV-1 infection that include intravenous or intranasal inoculation have been useful tools for evaluating viral pathogenesis and latency5,6,12.
However, a murine model for examining the propagation of HSV-1 to the TG when inoculated at the main site of primary infection in humans, which is the oral epithelium, had not been generated. The model described here, which accurately mimics primary herpetic infection beginning in the oral cavity, may be considered as a first step in the development of further studies on latency and reactivation mechanisms, the specific trigeminal immune response and antiviral drugs.
CONCLUSION
Despite the high prevalence of herpes virus infection in oral mucosa, there is little knowledge of the kinetics of infection and transport to the trigeminal ganglion. We established an in vivo mouse model of HSV-1 infection through scarification of the oral mucosa and were able to detect DNA, RNA and antigen and to recover infectious virus from TG. This model could be used in both pathogenesis and pharmacological studies on how to control herpes infection.
CORRESPONDENCE
Dr.Jaime E. Castellanos
Facultad de Odontología, Universidad Nacional de Colombia Av. Carrera 30 No. 45-03, Edificio 210, Oficina 301 Bogotá, Colombia jecastellanosp@unal.edu.co
ACKNOWLEDGMENTS
This project was funded by Facultad de Odontología, Universidad Nacional de Colombia, Research Fund 2008. We are very grateful to Myriam Velandia-Romero, Head of the Virology Laboratory, Universidad El Bosque, Bogotá, Colombia.
1. Roizman B, Pellett PE. The Herpesviridae family: a brief introduction. In: Knipe D, Howley P, Griffin D, Lamb RA, Martin M, Roizman B, Straus S. Fields Virology. 5thEd. Philadelphia, USA. Lippincott Williams & Wilkins, 2007: 2501-2602. [ Links ]
2. Imai Y, Apakupakul K, Krause PR, Halford WP, Margolis T. Investigation of the mechanism by which herpes simplex virus type 1 LAT sequences modulate preferential establishment of latent infection in mouse trigeminal ganglia. J Virol 2009;83: 7873-7882. [ Links ]
3. Low-Calle AM, Prada-Arismendy J, Castellanos JE. Study of interferon-P antiviral activity against Herpes simplex virus type 1 in neuron-enriched trigeminal ganglia cultures. Virus Res 2014;80:49-58. [ Links ]
4. Sun Y, Chan RK, Tan SH, Ng PP. Detection and genotyping of human herpes simplex viruses in cutaneous lesions of erythema multiforme by nested PCR. J Med Virol 2003; 71:423-428. [ Links ]
5. Wald A, Corey L. Persistence in the population: epidemiology and transmisson. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore P, Roizman B, Whitley R, Yamanishi K. Human Herpesviruses: Biology, Therapy and Immunoprophylaxis. Cambridge University Press; 2007: Chapter 36. [ Links ]
6. Kollias CM, Huneke RB, Wigdahl B, Jennings SR. Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol. 2015; 21:8-23 [ Links ]
7. BenMohamed L, Osorio N, Khan AA, Srivastava R, et al. Prior Corneal Scarification and Injection of Immune Serum are Not Required Before Ocular HSV-1 Infection for UV-B-Induced Virus Reactivation and Recurrent Herpetic Corneal Disease in Latently Infected Mice. Curr Eye Res 2016;41: 747-756. [ Links ]
8. Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol 1996;70:264-271. [ Links ]
9. Labetoulle M, Maillet S, Efstathiou S, Dezelee S, Frau E, Lafay F. HSV1 Latency sites after inoculation in the lip: assessment of their localization and connections to the eye. Invest Ophthalmol Vis Sci 2003;44:217-225. [ Links ]
10. Huang W, Zhao P, Chen X, Li P, et al.. Comparative Study of Different Latent Infections of Herpes Simplex Virus Type I in a Murine Model. Cell Biochem Biophys. 2014; 68:159-162. [ Links ]
11. Labetoulle M, Kucera P, Ugolini G Lafay F, Frau E, Offret H, Flamand A. Neuronal propagation of HSV1 from the oral mucosa to the eye. Invest Ophthalmol Vis Sci. 2000;41:2600-2606. [ Links ]
12. Park NH, Pavan-Langston D, McLean SL. Acyclovir in oral and ganglionic herpes simplex virus infections. J Infect Dis 1979;140:802-806. [ Links ]














