Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.30 no.3 Buenos Aires dic. 2017
ARTÍCULO ORIGINAL
The antibacterial activity and release of quaternary ammonium compounds in an orthodontic primer
La actividad antibacteriana y la liberación de compuestos de amonio cuaternario en un adhesivo imprimador ortodóncico
Mehmet B. Özel1, Tamer Tüzüner1, Zeynep A. Güplü2, Nichola J. Coleman3, Andrew P. Hurt3, Celai K. Buruk4
1 Karadeniz Technical University, Faculty of Dentistry, Trabzon, Turkey
2 Erciyes University, Faculty of Paediatric Dentistry, Kayseri, Turkey
3 University of Greenwich, Faculty of Engineering and Science, Chatham Maritime, United Kingdom
4 Technical University, Faculty of Medicine, Karadeniz, Trabzon, Turkey
ABSTRACT
The aim of this study was to evaluate the impact of 10 wt% benzalkonium chloride (TB-BAC) or 10 wt% cetylpyridinium chloride (TB-CPC) on the antimicrobial properties of the orthodontic adhesive primer, Transbond XTT (TB). Antimicrobial activity was assessed using a zone of inhibition diffusion test and the release of the antimicrobial compounds was monitored by high performance liquid chromatography (HPLC). Shear bond strength (SBS) was tested using bovine enamel. Control, TB, specimens failed to demonstrate intrinsic antibacterial activity at 1, 7 and 14 days; whereas, TB-BAC and TB-CPC showed antibacterial effects at all times. HPLC analysis indicated no significant differences in the release behaviour of TB-BAC and TB-CPC (t-test, p > 0.05), except for the 7-day release which was higher for TB-BAC (p < 0.05). By 14 days the extents of release were 27 ± 2% and 25 ± 5% of the total initial loading for TB-BAC and TB-CPC, respectively. The incorporation of 10 wt% BAC or CPC in Transbond XTT adhesive primer also resulted in superior shear bond strength at 7 and 14 days (Fisher s LSD, p < 0.05) with no significant change in the mode ofbracket failure under shear stress (Pearson's chi-squared, p > 0.05).
Key words: Antibacterial agents; Benzalkonium chloride; Cetylpyridinium chloride; Transbond self-etching primer.
RESUMEN
El objetivo de este estudio fue evaluar el impacto del cloruro de benzalconio al 10% en peso del peso (TB-BAC) o de cloruro de cetilpiridinio al 10% del peso (TB-CPC) con propiedades antimicrobianas presentes en el adhesivo acondicionador ortodóncico, Transbond XT T (TB). La actividad antimicrobiana se evaluó usando una zona de prueba de difusión de inhibición y la liberación de los compuestos antimicrobianos se controló mediante cromatografía líquida de alta resolución (HPLC). La resistencia de adhesión al corte (SBS) se probó usando esmalte bovino. Las muestras control, TB no lograron demostrar actividad antibacteriana intrínseca a 1, 7 y 14 días; mientras que TB-BAC y TB-CPC mostraron efectos antibacterianos en todo momento. El análisis por HPLC no indicó diferencias significativas en el comportamiento de liberación de TB-BAC y TB-CPC (prueba t, p> 0,05), excepto en la liberación a los 7 días que fue más alta para TB-BAC (p <0,05). A los 14 días, los grados de liberación fueron de 27 ± 2% y de 25 ± 5% de la carga inicial total para TB-BAC y TB-CPC, respectivamente. La incorporación de 10% en peso de BAC o CPC en el imprimador adhesivo Transbond XT T también dio como resultado una resistencia superior corte a los 7 y 14 días (Fisher's LSD, p <0.05) sin cambios significativos en el modo de falla del bracket bajo tensión de corte (Pearson's chi-cuadrado, p> 0.05).
Palabras clave: Agentes antibacterianos; Cloruro de banzalconio-Cloruro de Cetilpiridinio; Acondicionador autoadhesivo.
INTRODUCTION
It is well documented in the literature that orthodontic appliances may cause the demineralisation of enamel during fixed orthodontic treatment procedures1-3. It is difficult, particularly for young patients, to remove the plaque around the brackets which increases the risk of further cariogenic bacterial accumulation, white spot lesions and subsequent cavitated caries1-3. To address these problems, various strategies have been employed to inhibit bacterial activity in the vicinity of orthodontic brackets. These include the application of fluoride-releasing luting materials, topical fluoride therapy and the incorporation of antibacterial agents in toothpastes, gels and mouthwashes4-6.
In addition, the direct incorporation of antimicrobial compounds into orthodontic bonding materials, such as glass ionomers, zinc polycarboxylates and adhesive resins, is a subject of increasing interest7-8. A number of studies has indicated that a variety of antimicrobial compounds is effective in enhancing the antimicrobial character of these bonding materials, including chlorhexidine (CHX)9, cetylpyridinium chloride (CPC)10, cetrimide11 and benzalkonium chloride (BAC)12. Among the most popular antimicrobial agents in these applications are the quaternary ammonium salts (i.e. CPC and BAC). The aim of this study was to investigate the impact of either 10 wt% benzalkonium chloride (BAC) or 10 wt% cetylpyridinium chloride (CPC) in the light-cured acrylate-based orthodontic adhesive primer, Transbond XTT (3M, USA). The antimicrobial activities of the unmodified primer and of that admixed with BAC or CPC were compared using an inhibition zone assay with Streptococcus mutans. The in vitro release profiles of BAC and CPC from the admixed primer were monitored at 1, 7 and 14 days by high performance liquid chromatography (HPLC). The impact of lixiviation during component release on the integrity and texture of the primer was observed by scanning electron microscopy (SEM) and potential chemical interactions between the components of the primer and the quaternary ammonium compounds were investigated by Fourier transform infrared spectroscopy (FTIR). The shear bond strengths (SBS) of orthodontic brackets bonded to bovine enamel conditioned with the modified and unmodified primer, and the corresponding adhesive remnant indices (ARI), were compared at 1, 7 and 14 days.
MATERIALS AND METHODS
Sample preparation
This study employed a commercial light-cured orthodontic adhesive primer, (Transbond XTTM primer (code 712-034), 3M, CA, USA), which is principally composed of 45 - 55% triethylene glycol dimethacrylate and 45 - 55% bisphenol A diglycidyl ether dimethacrylate13. The antimicrobial compounds used were cetylpyridinium chloride (CPC) and benzalkonium chloride (BAC) powders (Sigma Aldrich, UK).
Sample discs (5 mm diameter x 1 mm depth) of the unmodified TransbondT primer were produced according to the manufacturer's instructions by placing the liquid primer in polypropylene moulds and light-curing for 10 seconds (HiLux, LEDMAX, Europe). Sample discs incorporating antimicrobial compounds were prepared by manually pre-mixing either 10 wt% of BAC powder or 10 wt% of CPC powder with the liquid primer on a polypropylene surface with a metal spatula for approximately 1 minute until the powder had dissolved. The admixed liquids were then placed in polypropylene moulds and light-cured (as described above). All light-cured samples were stored in the dark for a maximum of 2 days in hermetically sealed polypropylene tubes until required. Unmodified TransbondT primer samples were labelled 'TB' and those admixed with BAC and CPC were labelled TB-BAC and TB-CPC, respectively.
Zone of inhibition agar diffusion test
The antibacterial activities of the cured specimens (TB, TB-BAC and TB-CPC) against Streptococcus mutans were assessed by the Kirby-Bauer zone of inhibition agar diffusion test14,15. The S. mutans was initially cultured on blood agar (Merck, Darmstadt, Germany) at 37 °C for 24 hours in 5% CO2. Single colonies from the plates were transferred to BHI broth (Merck, Darmstadt, Germany) and incubated at 37 °C for 24 hours. McFarland 0.5 turbidity tubes were used to prepare a suspension of the strain in phosphate buffer solution, at approximately 1.5 x 108 cfu cm-3, which was then flood-inoculated onto the surface of BHI agar plates. The disc-shaped specimens for the TB control (n = 3), TB-BAC (n = 3) and TB-CPC (n = 3) were placed on the spread plates which were further incubated at 37 °C for 24 hours. The plates were then inspected for clear zones around the specimen discs which were measured at 3 different points. The discs were then transferred to freshly prepared test agar plates and stored at 2 - 4 °C. The inhibition zone agar diffusion procedures were then repeated at 7 and 14 days to appraise the ongoing antimicrobial activity of the specimens.
Release of antimicrobial compounds
One cured sample disc of either TB, TB-BAC or TB-CPC was placed in 5 cm3 of deionised water in a 15 cm3 screw-capped polypropylene centrifuge tube and stored at 37 °C. Each sample type was prepared in triplicate. After 1, 7 and 14 days, 0.1 cm3 of the supernatant liquor was removed for HPLC analysis.
Concentrations of released cetylpyridinium ions were determined by HPLC using an Agilent 1200 series chromatograph fitted with a reverse-phase C-18 Primsep column of 150 mm in length and 4.60 mm in diameter. A total volume of 20 pl were injected into the instrument at a flow rate of 1.0 cm3 min-1, with an isochratic mobile phase consisting of 55:45 acetonitrile: water with an added drop of concentrated sulphuric acid. Detection was by means of a variable wavelength detector set to 259 nm. Concentrations of released benzalkonium ions were determined by HPLC using an Agilent 1200 series chromatograph fitted with a reverse-phase C18 Kromasil column of 150 mm in length and 4.60 mm in diameter. A total volume of 20 pl were injected into the instrument at a flow rate of 1.0 cm3 min-1, with an isochratic mobile phase consisting of 55:45 acetonitrile:water with an added drop of glacial acetic acid. Detection was by means of a variable wavelength detector set to 254 nm.
Scanning electron microscopy
Secondary electron images of the surfaces of the TB, TB-BAC and TB-CPC discs prior to and following immersion in 5 cm3 of distilled water for 14 days at 37 °C were obtained from uncoated samples attached to carbon tabs on a JEOL (Welwyn Garden City, UK) JSM-5410 LV electron microscope with an Oxford Instruments (Oxford, UK) X-MaxN EDX detector in low vacuum mode. All images were obtained with an accelerating voltage of 1.0 kV at a working distance of 21 mm and a magnification of x100.
FTIR spectroscopy
FTIR spectra of the unset Transbond XTT adhesive primer solution and of the cured TB, TB-BAC and TB-CPC discs were obtained in triplicate using a Perkin Elmer (MA, USA) Spectrum Two spectrometer with a Universal Diamond attenuated total reflectance attachment. Spectra were recorded with 16 accumulated scans between 4000 cm-1 and 450 cm-1 wavenumbers at a resolution of 4 cm-1. The degree of conversion (DC) was estimated by comparing the ratios of the intensities of the FTIR peaks for the reactive polymerising C=C bond (at 1639 cm-1) and the unreactive C=O bond (at 1717 cm-1) in the cured polymer and monomer using the following equation16:
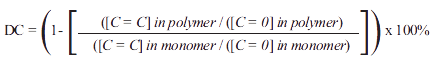
Shear bond strength analysis
Ninety freshly extracted permanent mandibular bovine incisors with no visible imperfections were used for shear bond strength analysis. The root portion of each tooth was embedded in a 3 cm diameter cylinder of dental acrylic (Orthocryl, Dentarium, Ispringen, Germany) such that the buccal surfaces could be positioned parallel to the applied shear force. The buccal surfaces were polished with pumice, rinsed with water spray, air-dried, etched with 37% phosphoric acid (Super Etch, SDI, Australia) for 30 seconds, and again, rinsed and dried.
Unmodified Transbond XTT adhesive primer (TB, n = 30) and the primer modified with either 10 wt% of BAC (TB-BAC, n = 30) or 10 wt% of CPC (TB-CPC, n = 30) were individually applied to the etched buccal surfaces of the teeth in accordance with the manufacturer's instructions. An Ormco mini 2000 metallic orthodontic premolar bracket (Ormco Corp., CA, USA) with 9.63 mm2 base area was bonded to each tooth with TransbondT adhesive (3M, CA, USA), via light-curing for 20 s (HiLux, LEDMAX, Europe). The brackets were positioned with orthodontic tweezers and excess adhesive was removed with a sharp scaler prior to curing. All samples were then stored in distilled water at room temperature until required for shear bond analysis. Ten of each sample-type (TB, TB-BAC and TB-CPC) were subjected to shear bond testing at 1, 7 and 14 days using a universal testing machine (Instrom Corp., MA, USA) with a shear force blade at a cross-head speed of 1 mm min-1 in the occluso-gingival direction. The results of each test were obtained as a force (N) which was then converted to a pressure (MPa).
The mode of bond failure was determined by observation using a stereomicroscope (Nikon, Tokyo, Japan) at x20 magnification. The failure sites on the tooth surface were classified according to their adhesive remnant index (ARI) score: 0 = no remaining adhesive; 1 = less than half of the adhesive remaining; 2 = more than half of the adhesive remaining; and 3 = all of the adhesive remaining on the enamel with a distinct impression of the bracket mesh17.
Statistical analysis
Statistical analysis was performed with SPSS v17.0 for Windows (SPSS Inc., Chicago, USA) software. The comparison of the experimental groups for the agar diffusion and HPLC analyses at each time interval were carried out using the Mann Whitney U test and the Student's ¿-test, respectively, at a significance level of p < 0.05. The normal distribution of the shear bond strength data was confirmed using the Shapiro Wilk test, and these data for each group were compared using a twoway ANOVA and Fisher's LSD test at a significance level of p < 0.05. The Pearson's chi-squared test was used to compare the ARI scores among the groups at each time period.
RESULTS
During manual mixing with a metal spatula on a polypropylene surface, both 10 wt% BAC and 10 wt% CPC were visually observed to dissolve in the TransbondT primer within one minute. The incorporation of these antimicrobial compounds did not visibly appear to adversely affect the lightcuring of the primer.
The zone of inhibition data for the control TB and experimental TB-BAC and TB-CPC specimens are listed in Table 1. The control TB specimens failed to demonstrate any antimicrobial activity against S. mutans throughout the duration of the study. Both BAC and CPC were observed to confer sustained antimicrobial character to the TransbondT primer throughout the 14-day period. The incorporation of BAC into the adhesive primer resulted in significantly higher antibacterial activity (p < 0.05) compared with that of CPC at all times. In both cases, the extent of the antimicrobial activity was generally seen to diminish as a function of time. The concentrations of the antimicrobial compounds released from the admixed TransbondT primer samples into deionised water at 1, 7 and 14 days are listed in Table 2. Within the 14-day period, the total release of the antimicrobial compounds from TB-BAC and TB-CPC were, respectively, 27 ± 2% and 25 ± 5% of the total initial loading (as shown in Fig. 1). HPLC analysis indicated no statistically significant differences in the cumulative release behaviour of TB-BAC and TB-CPC (p > 0.05), with the exception of the release at 7 days (p < 0.05) which was higher for sample TB-BAC.
Table 1: Median (and range) zone of inhibition data for TB, TB-BAC and TB-CPC

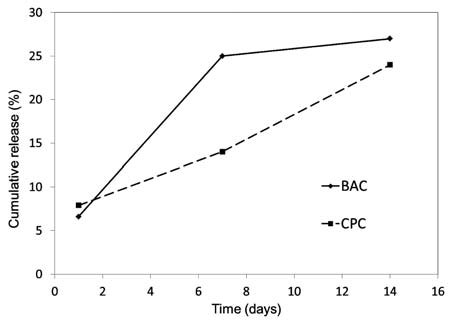
Fig. 1: Cumulative release of the antimicrobial quaternary ammonium compounds.
Table 2: Cumulative release of the antimicrobial quaternary ammonium compounds

SEM analysis (not shown) of samples TB, TB-BAC and TB-CPC prior to and following exposure to distilled water indicates that the flat featureless surfaces of the TransbondT primer remain unchanged after immersion in water for 14 days. The absence of voids or changes in texture confirms that the release of the added BAC and CPC from the primer does not impair the physical integrity of the resin.
The FTIR spectra of the monomeric TransbondT primer solution, the antimicrobial agents (BAC and CPC), and the light-cured polymerised resins (TB, TB-BAC and TB-CPC) are shown in Fig. 2. The corresponding functional group assignments are given in Table 318. According to the manufacturer's safety data sheet (13), TransbondT primer comprises a mixture of triethylene glycol dimethacrylate, bisphenol A diglycidyl ether dimethacrylate, triphenylantimony, 4-(dimethylamino) benzeneethanol, DL-camphorquinone and hydroquinone. Present in these monomers are carboxylic acid, amine, carbonyl, alkane, alkene, aromatic and ether groups. All of these functional groups appear in the FTIR spectra of both the unset TransbondT solution and light-cured polymerised discs (as indicated in Fig. 2 and Table 3).
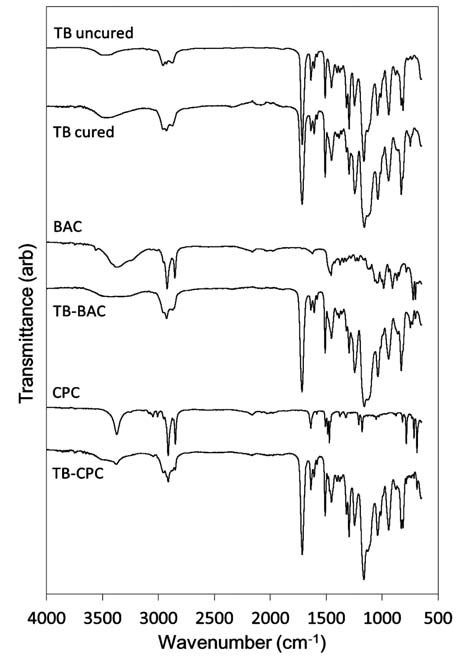
Fig. 2: FTIR spectra of cured and uncured TB, BAC, TBBAC, CPC and TBCPC.
Table 3: FTIR assignments of major bands present in TB, BAC and CPC18

The degree of conversion of light-cured acrylate-based resins is defined as the percentage of acrylate C=C groups from the various monomers which have undergone polymerisation16. Analysis of the FTIR spectra of the unset and light-cured TransbondT primer indicated that this material achieved a degree of conversion of 52 ± 2% under the selected experimental conditions. It is not possible to estimate the extent of polymerisation of the primer in the presence of the antimicrobial agents, as their vibrational bands are concurrent with the carbonyl and acrylate bands of the primer. No new bands appear and none of the original bands is shifted by the incorporation of BAC or CPC in the primer, indicating that there is no direct chemical interaction between the antimicrobial agents and the components of the TransbondT primer.
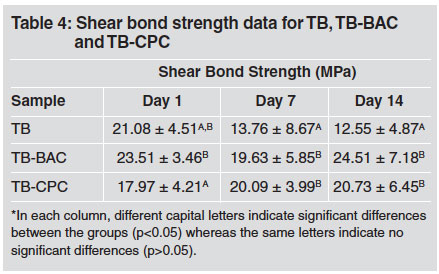
Shear bond strength (SBS) data for samples TB, TB-BAC and TB-CPC as functions of storage time in distilled water are given in Table 4. The initial 1-day SBS of the unmodified TB control sample was found to be significantly higher than its subsequent values at 7 and 14 days. The incorporation of either BAC or CPC in the primer did not have an impact on the 1-day SBS values relative to that of the unmodified control; although, after 7 and 14 days, both modified samples exhibited significantly higher bond strengths than those of the control. The initial 1-day SBS of TB-BAC was higher than that of TB-CPC; and there was found to be no difference between the two modified materials at the later time points.
Table 4: Shear bond strength data for TB, TB-BAC and TB-CPC
The frequency distribution data for the ARI scores for samples TB, TB-BAC and TB-CPC as functions of storage time in distilled water are listed in Table 5. Differences in the median scores were observed among the groups, but, statistically, these differences were not found to be significant. These ARI data indicate that the incorporation of either BAC or CPC in TransbondT primer has no impact on the mode of bracket failure under applied shear force.
Table 5: Distribution of adhesive remnant index scores for TB, TB-BAC and TB-CPC

DISCUSSION
Plaque accumulation around fixed orthodontic appliances is not easily removed by the patient and may cause enamel demineralisation, gingival inflammation and bracket debonding1-3. Currently, the majority of commercial orthodontic primers, adhesives and luting agents exhibit limited or no intrinsic defence against bacterial accumula-tion8-101415. One strategy to address this problem is the direct incorporation of antimicrobial compounds into these bonding agents8-10,14,15. In this respect, broad-spectrum antimicrobial cationic quaternary ammonium compounds (QACs) are popular candidates. These compounds can be introduced either as a polymerisable monomer (e.g. methacryloyloxydodecylpyridinium bromide (MDPB)) which is permanently incorporated into the polymer matrix of the bonding agent19, or as an admixed component which is able to be released during service (e.g. benzalkonium chloride (BAC) and cetylpyridinium chloride (CPC))10,12.
A number of studies has indicated that the addition of between 1 and 10 wt% BAC or CPC to orthodontic bonding agents such as glass ionomers, zinc polycarboxylates and methacrylate-based resins can confer enhanced broad-spectrum antimicrobial characteristics against cariogenic bacteria1012. However, there are also reports that these compounds can compromise the mechanical properties and bond strengths of these systems2021. In this current study, the antimicrobial compounds, BAC and CPC, were added to the adhesive primer in order to circumvent potential problems arising from the diminished mechanical integrity of the luting material. In addition, it is considered that the sustained release of antimicrobial compounds from the primer which is in direct contact with the enamel surface may be advantageous against bacterial accumulation in the immediate vicinity of the contacting surfaces.
During this investigation TransbondT primer exhibited no intrinsic microbial resistance to S. mutans under the selected experimental conditions during the zone of inhibition agar diffusion test (Table 1). The literature contains few, yet conflicting, reports of the intrinsic antimicrobial properties of Transbond XTT primer in vitro. Research by Çatalbaş et al.9 suggests that Transbond XTT affords microbial resistance to S. mutans; although, the common consensus is that it possesses negligible inherent antimicrobial activity against this microorganism10,22. The findings of the current study concur with this latter viewpoint. It should be noted that, the semi-quantitative zone of inhibition agar diffusion tests are not standardised, and that different experimental parameters (e.g. concentration of colony forming units, bacterial strain, incubation time, sample dimensions, type of growth medium and agar) impact upon the apparent antimicrobial activities of materials in vitro.
In a previous study, Al-Musallam et al.10 demonstrated that the addition of 10 wt% CPC to the Transbond XTT primer effected sustained release and antimicrobial activity against S. mutans throughout a 196-day observation period. The 14-day CPC release behaviour and antimicrobial activity of the TB-CPC system observed in the present study confirm these findings. The current study also indicates that BAC may be a more potent antimicrobial agent than CPC in the inhibition of S. mutans when incorporated into the Transbond XTT primer, since the clear zones around TB-BAC were significantly greater than those around TB-CPC even though there was little difference between the release profiles of the two antimicrobial agents. The ongoing integrity and adhesive strength of modified bonding systems is of paramount clinical importance to the successful long-term functionality of fixed orthodontic appliances. In vitro shear bond strength analysis using bovine enamel is a convenient and commonly employed method to compare orthodontic adhesive systems23. A number of studies has indicated significant differences between the SBS of human and bovine enamel, which appear to depend predominantly upon the method of enamel preparation, the nature of the adhesive system and the storage regime23-25. However, in many cases, for a given experimental procedure, a proportional relationship is reported between the SBS of human and bovine enamel which supports the use of bovine teeth as a relative proxy for human teeth in orthodontic adhesive bonding studies24,25. It should be noted that there is some evidence to indicate a relationship between in vitro bond strength and the longevity of orthodontic restorations; although, at present, in vitro SBS tests cannot be used for direct predictions of clinical outcomes2627.
As previously mentioned, the incorporation of antimicrobial QACs can have a deleterious impact on the mechanical properties and bonding strengths of orthodontic cements and adhesives20,21. Furthermore, the incorporation of additives and modifications to orthodontic adhesives that increase the ARI score are generally regarded as unfavourable with respect to the ease and safety of removal of the residual resin remnants after debonding28. The shear bond strength analysis carried out in this study indicated that after 7 and 14 days, both modified Transbond XTT primer samples exhibited significantly higher SBS values than those of the control, despite the sustained release of the QACs from the resin matrix (Table 4). In this case, the improvement in SBS on addition of 10 wt% of either BAC or CPC was accompanied by no significant change in the mode of bracket failure under shear force (Table 5).
It is argued that polymerisable QAC monomers that are permanently immobilised within the adhesive resin matrix generally exhibit lower bactericidal efficacy than their 'free' QAC antimicrobial counterparts29. In addition, permanently incorporated QACs may provide defence against biofilm formation on the resin surface, but they do not afford any sustained release of antimicrobial agents in the vicinity of the enamel. Hence, the direct incorporation of cheap 'over the counter' QAC compounds, such as BAC and CPC, offers clinicians a cost-effective, convenient method for enhancing the antimicrobial characteristics of orthodontic adhesives. In this respect, the incorporation of 10 wt% BAC or CPC in Transbond XTT adhesive primer has been shown to confer significant antibacterial activity, sustained release and superior shear bond strength with no significant change in the mode of bracket failure under shear stress.
CORRESPONDENCE
Dr. Nichola J. Coleman
Faculty of Engineering and Science, University of Greenwich, Chatham Maritime, Kent, ME4 4TB, UK
n.coleman@gre.ac.uk
1. Chapman JA, Roberts WE, Eckert GJ, Kula KS, Gonzalez-Cabezas C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop 2010; 138:188-194. [ Links ]
2. Millett DT, Nunn JH, Welbury RR, Gordon PH. Decalcification in relation to brackets bonded with glass ionomer cement or a resin adhesive. Angle Orthod 1999; 69:65-70. [ Links ]
3. Sukontapatipark W, el-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod 2001; 23:475-484. [ Links ]
4. Berg JH, Croll TP. Glass ionomer restorative cement systems: an update. Pediatr Dent 2015; 37:116-124. [ Links ]
5. De Rossi A, Ferreira DC, da Silva RA, de Queiroz AM, da Silva LA, Nelson-Filho P Antimicrobial activity of toothpastes containing natural extracts, chlorhexidine or triclosan. Braz Dent J 2014; 25:186-190. [ Links ]
6. Haps S, Slot DE, Berchier CE, Van der Weijden GA. The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to toothbrushing on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg 2008; 6:290-303. [ Links ]
7. Mulla Z, Edwards M, Nicholson JW.Release of sodium fusidate from glass-ionomer dental cement. J Mater Sci Mater Med 2010; 21:1997-2000. [ Links ]
8. Ali MN, Edwards M, Nicholson JW. Zinc polycarboxylate dental cement for the controlled release of an active organic substance: proof of concept. J Mater Sci Mater Med 2010; 21:1249-1253. [ Links ]
9. Çatalbas B, Ercan E, Erdemir A, Gelgor IE, Zorba YO. Effects of different chlorhexidine formulations on shear bond strengths of orthodontic brackets. Angle Orthod 2009; 79:312-316. [ Links ]
10. Al-Musallam T, Evans CA, Drummond JL, Matasa C, Wu CD. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am J Orthod Dentofacial Orthop 2006; 129:245-251. [ Links ]
11. Botelho MG. Compressive strength of glass ionomer cement with dental antibacterial agents. SADJ 2004; 59:51-53. [ Links ]
12. Saito K, Hayakawa T, Kawabata R, Meguro D. Kasai K. In vitro antibacterial and cytotoxicity assessments of an orthodontic bonding agent containing benzalkonium chloride. Angle Orthod 2009; 79:331-337. [ Links ]
13. Transbond XTT Materials Safety Data Sheet URL: http://solutions.3m.com/wps/portal/3M/en_US/orthodontics/Unitek/resources/MSDS/ [ Links ]
14. Tüzüner T, Kuçgoz A, Er K, Taçdemir T, Buruk K, Kemer B. Antibacterial activity and physical properties of conventional glass-ionomer cements containing chlorhexidine diacetate/cetrimide mixtures. J Esthet Restor Dent 2011; 23:46-56. [ Links ]
15. Korkmaz FM, Tüzüner T, Baygin O, Buruk CK, Durkan R, Bagis B. Antibacterial activity, surface roughness, flexural strength, and solubility of conventional luting cements containing chlorhexidine diacetate/cetrimide mixtures. J Prosthet Dent 2013; 110; 107-115. [ Links ]
16. Gu9lu ZA, Donmez N, AP Hurt, Coleman NJ. Characterisation and microleakage of a new hydrophilic fissure sealant-UltraSeal XT® hydroT. J Appl Oral Sci. 2016; 24:344-351.
17. Artun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod 1984; 85:333-340. [ Links ]
18. Hurt A, Coleman NJ, Tuzuner T, Bagis B, Korkmaz FM, Nicholson JW. Release of cetyl pyridinium chloride from fatty acid chelate temporary dental cement. Acta Biomater Odontol Scand 2016; 2:1-6. [ Links ]
19. Zhang K, Cheng L, Imazato S, Antonucci JM, et al.Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentin bond. J Dent 2013; 41:464-474. [ Links ]
20. Ahmet SO, Mutluay MM, Polat ZS, Dirihan RS, Bek B, Tezvergil-Mutluay A. Addition of benzalkonium chloride to self-adhesive resin-cements: some clinically relevant properties. Acta Odont Scand 2014; 72:831-838. [ Links ]
21. Chen L, Shen H, Suh BI. Antibacterial dental restorative materials: A state-of-the-art review. Am J Dent 2012; 25:337-346. [ Links ]
22. Jacobo C, Torrella F, Bravo-Gonzalez LA, Ortiz AJ, Vicente A. In vitro study of the antibacterial properties and microbial colonization susceptibility of four self-etching adhesives used in orthodontics. Eur J Orthodont 2014; 36:200-206. [ Links ]
23. Ruttermann S, Braun A, Janda R. Shear bond strength and fracture analysis of human vs. bovine teeth. PLoS ONE 2013; 8; [ Links ]e59181.
24. Saieh F, Taymour N. Validity of using bovine teeth as a substitute for human counterparts in adhesive tests. East Mediterr Health J 2003; 9:201-207. [ Links ]
25. Reis AF, Giannini M, Kavaguchi A, Soares CJ, Line SR. Comparison of microtensile bond strength to enamel and dentin of human, bovine, and porcine teeth. J Adhes Dent 2004; 6:117-121. [ Links ]
26. Van Meerbeek B, Peumans M, Poitevin A, Mine A, et al. Relationship between bond strength tests and clinical outcomes. Dent Mater 2010; 26:100-121. [ Links ]
27. Altmann AS, Collares FM, Leitune VC, Samuel SM. The effect of antimicrobial agents on bond strength of orthodontic adhesives: a meta-analysis of in vitro studies. Orthod Craniofac Res 2016; 19:1-9. [ Links ]
28. Montassera MA, Drummond JL. Reliability of the adhesive remnant index score system with different magnifications. Angle Orthod 2009; 79:773-776. [ Links ]
29. Namba N, Yoshida Y, Nagaoka N, Takashima S, et al. Antibacterial effect of bactericide immobilized in resin matrix. Dent Mater 2009; 25:424-430. [ Links ]














