Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Odontológica Latinoamericana
On-line version ISSN 1852-4834
Acta odontol. latinoam. vol.32 no.2 Buenos Aires Aug. 2019
ORIGINAL ARTICLE
Coexistence of thyroid disease and oral lichen planus in a Colombian population
Coexistencia de enfermedad de la tiroides y liquen plano oral en una población colombiana
Paola A. Amato-Cuartas1, Andrés E. Tabares-Quintero1, Luis F. Vélez-Jaramillo2, Gloria Álvarez-Gómez3, Leonor V. González-Pérez4, Cecilia M. Martínez-Delgado5, Jairo Robledo-Sierra2
1 Universidad CES, Facultad de Odontología, Posgrado de Cirugía Maxilofacial, Medellín, Colombia
2 Universidad CES, Facultad de Odontología, Clínica de Medicina Oral, Medellín, Colombia
3 Universidad de Antioquia, Facultad de Odontología, Departamento de Estudios Básicos Integrados, Medellín, Colombia
4 Universidad de Antioquia, Facultad de Odontología, Departamento de Ciencias Básicas, Medellín, Colombia
5 Universidad CES, Facultad de Odontología, Departamento de Investigación, Medellín, Colombia
Received: May 2019, Accepted: July 2019
ABSTRACT
Oral lichen planus (OLP) is a chronic inflammatory mucocutaneous disease of unknown etiology. OLP has recently been linked to thyroid disease, mainly hypothyroidism. The aim of this study was to determine the prevalence of thyroid disease in Colombian patients with OLP. A total of860 clinical records of patients attending the clinics of oral medicine and oral and maxillofacial surgery at IPS CES Sabaneta, Colombia, between 2010 and 2016 were reviewed. Fourteen patients (1.6%) had a diagnosis of OLP. The prevalence of hypothyroidism in patients with OLP was 35.7%, compared to 3.95% in the entire study population (OR 15.92, 95% CI: 5.63-50.09, P = 0.0001). Patients with concomitant hypothyroidism and OLP presented with less severe oral lesions compared to those without thyroid disease. This study supports the notion that patients with OLP should be screened for thyroid disease.
Keywords: Oral lichen planus; Thyroid diseases; Hypothyroidism; Thyroxine.
RESUMEN
El liquen plano oral (LPO) es una enfermedad mucocutánea inflamatoria crónica de etiología desconocida. El LPO ha sido asociado recientemente con la enfermedad de la tiroides, especialmente con hipotiroidismo. El objetivo con este estudio fue determinar la prevalencia de la enfermedad de la tiroides en pacientes colombianos con LPO. Un total de 860 historias clínicas de pacientes que asistieron entre 2010 y 2016 a las clínicas de medicina oral y de cirugía oral y maxilofacial de la IPS CES Sabaneta, Colombia, fueron revisadas. Catorce pacientes (1.6%) habían sido diagnosticados con LPO. La prevalencia de hipotiroidismo en pacientes con LPO fue 35.7%, comparada con 3.95% en toda la población de estudio (RM 15.92, 95% IC: 5.63-50.09, P = 0.0001). Pacientes con hipotiroidismo y LPO concomitante presentaron lesiones orales menos severas comparado con aquellos sin enfermedad de la tiroides. Este estudio respalda la idea de que se debe investigar la presencia de enfermedad de la tiroides en pacientes con LPO.
Palabras clave: Liquen plano oral; Enfermedad de la tiroides; Hipotiroidismo; Tiroxina.
INTRODUCTION
Oral lichen planus (OLP) is a chronic inflammatory disease of unknown etiology. In the past decade, a growing body of evidence has accumulated suggesting that OLP is associated with thyroid disease, mainly hypothyroidism.1-6 Early studies conducted in Finnish and Swedish populations reported a significantly higher prevalence of hypothyroidism (9%-11%) in patients with OLP compared to control subjects (3%-5%).1,3,4 A more pronounced difference has been noted in Italy and Spain, where 14%-16% of the patients with OLP and 1%-5% of the controls have been diagnosed with hypothyroidism.2,5,6
A recent meta-analysis indicated a significantly high prevalence of thyroid disease among patients with OLP compared to controls (OR 2.10; 95% CI: 1.47-3.01).7 The authors suggested that routine screening for thyroid disease could be beneficial for patients with newly diagnosed OLP. However, more studies are required to elucidate the immunological mechanisms underlying the connection between autoimmune thyroid disease and OLP.
The prevalence of subclinical hypothyroidism in a population of nearly 6000 adults from Medellin, Colombia, has been shown to be 5.9%.8 However, to the best of our knowledge, no studies investigating the association between thyroid disease and OLP have been conducted in Colombian or Latin American populations. The aims of this crosssectional study were to 1) determine the prevalence of hypothyroidism or levothyroxine supplementation in Colombian patients with OLP, and 2) describe the clinical characteristics of patients with concomitant OLP and thyroid disease.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of CES University (Act 77, Project Code 370 of 2015). We reviewed the clinical records of all patients (n=860) attending the clinics of oral medicine, and oral and maxillofacial surgery at Institución Prestadora de Salud (IPS) CES Sabaneta, Colombia, between 2010 and 2016. The WHO clinical and histopathological criteria9 were used for the diagnosis of OLP. However, biopsies were only performed when the disease did not present with typical clinical manifestations, as has been previously suggested.10 All lesions had to present with reticular or papular features with or without plaque, erythema or ulcerations. Gingival OLP with erythema but without reticulum or papules, which is sometimes referred to as an oral lichenoid lesion,11 was also included. Lichenoid contact reactions observed in close contact with amalgam restorations were excluded. Fisher’s exact test was used to analyze the difference in the prevalence of hypothyroidism/levothyroxine supplementation in patients with OLP compared to the entire study population.
RESULTS
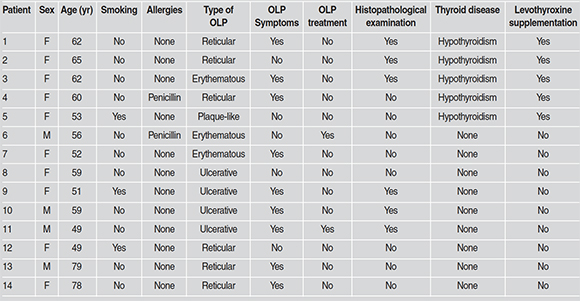
Fourteen (1.6%; mean age 59.6 years; females n=11) patients were diagnosed with OLP. The lesions in 6 of these patients did not present with typical clinical characteristics and thus the diagnoses were confirmed with histopathologic examination. Only one patient was on systemic steroids for treatment of OLP. Five patients with OLP (35.7%) had been previously diagnosed with hypothyroidism and were taking levothyroxine supplementation. The prevalence of hypothyroidism/ levothyroxine supplementation in the study population was 3.9% (34/860). The difference in the prevalence of hypothyroidism/levothyroxine supplementation in patients with OLP compared to the entire study population was found to be statistically significant (OR 15.9, 95% CI: 5.6350.09, P = 0.0001). In addition, patients with concomitant OLP and hypothyroidism presented with less severe lesions, i.e. reticular and plaquelike, and symptoms compared to those without thyroid disease (Table 1).
Table 1: Clinical characteristics of patients with oral lichen planus (OLP) (n=14).
DISCUSSION
We found a significantly higher prevalence of thyroid disease, specifically hypothyroidism, in patients with OLP (35.7%) compared to the entire study population (3.9%). This finding is consistent with previous studies conducted in Scandinavia and Mediterranean countries.1,2,4, 5 In addition, the prevalence of hypothyroidism registered in the entire study population corresponds with the results of a recent investigation conducted in Medellin, Colombia.8
The biological mechanism underlying the association between hypothyroidism and OLP is unknown. A number of studies have shown that in most cases of concomitant hypothyroidism and OLP, the diagnosis of thyroid disease and the initiation of thyroxine supplementation therapy have preceded the onset of oral lesions.24 Considering that Hashimoto’s thyroiditis is the most common cause of hypothyroidism in iodine-replete regions, it has been hypothesized that serum levels of thyroid antibodies may be involved in the pathogenesis of OLP. Chang et al.12 reported significantly elevated levels of antithyroid-peroxidase (TPOAb) and antithyroglobulin (TgAb) antibodies in Chinese patients with OLP compared to healthy controls. TPOAb have also been associated with an increased risk of erosive OLP in an Iranian population (OR = 4.02, 95% CI 1.21-13.4; P = 0.023).13 In contrast, Robledo-Sierra et al.14 did not find an association between OLP and antithyroid antibodies, i.e. TPOAb, TgAb, and antithyroid-stimulating hormone receptor antibody (TRAb). However, the same study showed a significantly higher expression of thyroid-stimulating hormone receptor in OLP lesions. Previous studies have shown the presence of thyroid-specific antigens, including thyroid-stimulating hormone receptor and thyroglobulin, in the skin of patients with autoimmune thyroid diseases. A similar organ-specific autoimmune response may occur in the oral mucosa and influence the development of OLP in a subgroup of patients, in whom basal keratinocytes expressing thyroid or thyroid-like proteins become a target of cytotoxic T cells.4,14 Another aim was to describe the clinical characteristics of patients with concomitant thyroid disease and OLP. Despite the limited number of patients (n=5), it was found that they presented with less severe OLP, namely, more reticular and less erythematous/ ulcerative lesions compared to those without thyroid disease. Similar results were shown in a Swedish study, where OLP patients with thyroid disease presented with more reticular and less erythematous lesions compared to those without thyroid disease. The reason for this is yet to be determined.
CONCLUSION
The prevalence of hypothyroidism in Colombian patients with OLP is remarkably higher than in the general population. This finding is in accordance with what has been reported in other populations, which supports the notion that patients with OLP should be screened for thyroid disease.
FUNDING
None
CORRESPONDENCE
Dr.Jairo Robledo-Sierra
Faculty of Dentistry, CES University
Calle 10A # 22 - 04, Medellin 050021, Colombia
Tel: +57 4 4440555 ext. 1603
Fax: +57 4 3113505
jrobledo@ces.edu.co
1. Siponen M, Huuskonen L, Laara E, Salo T. Association of oral lichen planus with thyroid disease in a Finnish population: a retrospective case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:319-324. [ Links ]
2. Lo Muzio L, Santarelli A, Campisi G, Lacaita M, Favia G. Possible link between Hashimoto’s thyroiditis and oral lichen planus: a novel association found. Clin Oral Investig. 2013;17:333-336.
3. Robledo-Sierra J, Mattsson U, Jontell M. Use of systemic medication in patients with oral lichen planus - a possible association with hypothyroidism. Oral Dis. 2013;19:313-319. [ Links ]
4. Robledo-Sierra J, Landin-Wilhelmsen K, Nystrom HF, Mattsson U, Jontell M. Clinical characteristics of patients with concomitant oral lichen planus and thyroid disease. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:602-608. [ Links ]
5. Garcia-Pola MJ, Llorente-Pendas S, Seoane-Romero JM, Berasaluce MJ, Garcia-Martin JM. Thyroid Disease and Oral Lichen Planus as Comorbidity: A Prospective Case-Control Study. Dermatology. 2016;232:214-219. [ Links ]
6. Arduino PG, Karimi D, Tirone F, Sciannameo V, Ricceri F, Cabras M, Gambino A, Conrotto D, et al. Evidence of earlier thyroid dysfunction in newly diagnosed oral lichen planus patients: a hint for endocrinologists. Endocr Connect. 2017;6:726-730. [ Links ]
7. Li D, Li J, Li C, Chen Q, Hua H. The Association of Thyroid Disease and Oral Lichen Planus: A Literature Review and Meta-analysis. Front Endocrinol (Lausanne). 2017;8:310. [ Links ]
8. Carmona Carmona C, Bedoya P, Acevedo J, Cardona Arias J. Prevalence of Thyroid Disorders in an Institution Providing Health Services in Medellin-Colombia. Transl Biomed. 2018;9:1-6. [ Links ]
9. Kramer IR, Lucas RB, Pindborg JJ, Sobin LH. Definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46:518-539. [ Links ]
10. Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, Migliorati CA, Axell T, Bruce AJ, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103 Suppl:S25 el-12. [ Links ]
11. van der Waal I. Oral lichen planus and oral lichenoid lesions; a critical appraisal with emphasis on the diagnostic aspects. Med Oral Patol Oral Cir Bucal. 2009;14:E310-14. [ Links ]
12. Chang JY, Chiang CP, Hsiao CK, Sun A. Significantly higher frequencies of presence of serum autoantibodies in Chinese patients with oral lichen planus. J Oral Pathol Med. 2009;38:48-54. [ Links ]
13. Alikhani M, Ghalaiani P, Askariyan E, Khunsaraki ZA, Tavangar A, Naderi A. Association between the clinical severity of oral lichen planus and anti-TPO level in thyroid patients. Braz Oral Res. 2017;31:e10. [ Links ]
14. Robledo-Sierra J, Landin-Wilhelmsen K, Filipsson Nystrom H, Eggertsen R, Larsson L, Dafar A, Warfvinge G, Mattsson U, et al. A mechanistic linkage between oral lichen planus and autoimmune thyroid disease. Oral Dis. 2018;24:1001-1011. [ Links ]














