INTRODUCTION
Due to their mechanical and aesthetic properties, composite resins have been widely used for direct restorations on anterior and posterior teeth1. However, they still have some adverse consequences as a result of polymerization contraction, which is aggravated by cavities with a high cavity configuration factor such as those occurring in posterior tooth cavities2. Polymerization contraction may lead to cusp deflection, adhesive interface cracks, postoperative sensitivity and staining at the restoration margins3,4. One way to minimize the stresses caused by polymerization contraction is by inserting conventional composite resins into cavity preparations using the incremental technique, in which small increments of composite resin are placed in the cavity, with minimal contact between opposing walls5. However, this technique takes longer and poses the risk of empty spaces forming between increments6.
In this respect, bulk-fill resins were developed with the alternative of inserting a single increment of up to 4 mm or 5 mm, and the advantages of providing reduced polymerization shrinkage and requiring less time to perform posterior tooth restorations6-9. Polymerization shrinkage was reduced by incorporating components capable of interacting with the photoinitiator and modulating polymerization kinetics, providing an additional photoinitiator, and promoting relatively slower conversion, longer pre-gel phase, and gel delay10,11. Bulk-fill resins come either in fluid consistency (flowable or low viscosity), which must be coated with a conventional composite resin except in the proximal region, or paste consistency (high-viscosity), which does not require occlusal restoration coverage12, but which may be subject to degradation due to direct interaction with the oral environment. On the other hand, in response to patients' demand for lighter-colored teeth, dental professionals recommend tooth bleaching. This is an effective conservative technique13 that can be performed in different ways. One option is the home bleaching technique in which the patient uses trays at home, supervised by the dentist. Home bleaching uses 5.5% to 10% hydrogen peroxide concentrations or 10 to 22% carbamide peroxide concentrations. Another option is the in-office technique, in which the bleaching product is applied by the professional, using 35% to 40% hydrogen peroxide concentrations14,15. During the bleaching procedure, the bleaching agents may come into contact with pre-existing oral cavity restorations, and may affect their surface roughness16,17 and surface micromorphology18. Although this problem is more serious in anterior restorations, bleaching gel is often applied in posterior regions, such as premolars, and may reach the occlusal and proximal face of these teeth and therefore, posterior restorations. Thus, considering the possibility that the monomers in bulk-fill resins may be eluted after contact with bleaching gels19, it is essential to investigate the effects of bleaching agents on the mechanical properties of conventional and bulk-fill resins, especially those of high viscosity.
In addition to dentist-supervised bleaching techniques, some over-the-counter (OTC) bleaching products can be sold to the consumer without a dentist's supervision. These products contain hydrogen peroxide concentrations of about 3%20, and include mouthwashes, toothpaste, chewing gum and whitening strips21. There is a lack of clinical evidence regarding the safety and efficacy of these products, even though they are used very frequently and without proper supervision, making them potentially harmful. Whitening rinses, in particular, may lead to an increase in the surface roughness of conventional composite resins22. However, the literature includes few studies evaluating the effects of over-the-counter bleaching products, such as mouthwashes containing hydrogen peroxide, on surface roughness and micromorphology and on color change in high- and low-viscosity conventional and bulk-fill resins.
Thus, the aim of this in vitro study was to evaluate the effect of applying bleaching protocols (office, home or OTC) at different concentrations on surface roughness and micromorphology, and on color change of high- and low-viscosity bulk-fill resins, compared to conventional nanoparticulate resin. The null hypothesis (H0) tested was that the different bleaching protocols applied would not affect the surface roughness and micromorphology or change the color of the tested composite resins.
MATERIALS AND METHODS
Ethical Aspects
The present study was exempted from ethical approvals because it did not involve animals or human subjects (Exemption protocol: 2018/0931).
Preparation of samples
Forty disk-shaped specimens were prepared in a silicone mold (5 mm in diameter and 2 mm thick) from each of three types of composite resins (see Table 1 for further details):
- Conventional composite resin (Filtek Z350 XT, 3M ESPE™ Dental Products, St. Paul, MN, USA)
- Low-viscosity bulk-fill flowable composite resin (Filtek Bulk Fill Flow, 3M ESPE™ Dental Products, St. Paul, MN, USA)
- High-viscosity bulk-fill composite resin (paste) (Filtek Bulk Fill, 3M ESPE™ Dental Products, St. Paul, MN, USA).
Table 3 Median (minimum; maximum) of the parameters of evaluation of the color change (ΔL, Δa, Δb and ΔE) according to composite resin, bleaching protocol and time.
The composite resin was inserted into the silicone mold with a #1 silicate spatula (Golgran, Sâo Caetano do Sul, SP, Brazil). The resin surface was covered with a polyester strip and a glass slide to ensure a flat surface and to achieve standard compression of the composite resin. A 500g weight was applied over the whole set for 60 s.
After the weight was removed, photopolymerization was performed with a LED photoactivator (Valo, Ultradent Products Inc, S. Jordan, UT, USA) for 20s (standard mode, 1000 mW / cm2 power), by placing the tip of the device on the glass slide. Finishing and polishing were performed with the complete orange sequence of Soflex disks (3M ESPE™ Dental Products, St. Paul, MN, USA), according to decreasing grain. Each disk was used for 10 s and then changed.
Bleaching Protocols
The specimens made from each composite resin were divided randomly into four subgroups (n = 10), to which different surface treatments were applied, as follows:
- Home bleaching technique with 10% carbamide peroxide gel (Opalescence, Ultradent Products Inc, S. Jordan, UT, USA) - the bleaching agent was applied to the sample surface for 2 hours a day, and this protocol was repeated for 14 days23.
- Office technique with 40% hydrogen peroxide gel (Opalescence Boost, Ultradent Products Inc, S. Jordan, UT, USA). Three bleaching sessions were performed at one-week intervals. In each session, the bleaching agent was applied to the sample surface for 15 min and this procedure was repeated 3 times. The timing and frequency of application were as recommended by the manufacturer.
- Immersion in a hydrogen peroxide-containing whitening rinse (Listerine Whitening Extreme, Johnson & Johnson, New Jersey, USA). In this group, the resin samples were immersed in 2 mL of bleaching solution for 2 min, once a day, and then placed on a seesaw agitator (Model: K40-3012, Kasvi Import and Distribution of Laboratory Products, Curitiba, Paraná, Brazil). This protocol was repeated for 14 days. The application time for each cycle was as recommended by the manufacturer.
- Control group - immersion in distilled water for 14 days, with daily water renewal.
Between the bleaching protocol cycles, all samples were immersed in distilled water and stored in an oven at 37°C (Bacteriological oven - Odontobrás Ind. e Com. Med. Odont.; Model: ECB 1.3 Ribeirao Preto, SP, Brazil).
Surface roughness, color change and surface micromorphology were evaluated before and after the bleaching protocols.
Surface roughness test
A roughness meter (SurfTest SJ-210, Mitutoyo Corporation, Kanagawa, Japan) was used to measure surface roughness. Three random measurements were made on the surface of each sample, with a 0.25 mm cut off, at a speed of 0.1mm/s16. An average value was assigned to each sample for each timepoint.
Color evaluation
Color was measured with a spectrophotometer (Vita Easyshade, Vita-Zahnfabrik, Germany), using the CIELAB color system. The detector was positioned perpendicular to the surface of the specimens to measure the parameters of luminosity (L*), red-green coordinate (a*), and yellow-blue coordinate (b*). The analysis was performed in a box, so that there would be no influence of the external environment, over a white background.
After completion of the experimental periods, the *L, *a, *b parameters were measured again, and the values of ΔL, Δa and Δb color change were calculated. The total color change expressed in ΔE is represented by the following formula:
ΔE = [(ΔL) 2 + (Δa) 2 + (Δb) 2] 1/2 24
Surface micromorphology evaluation
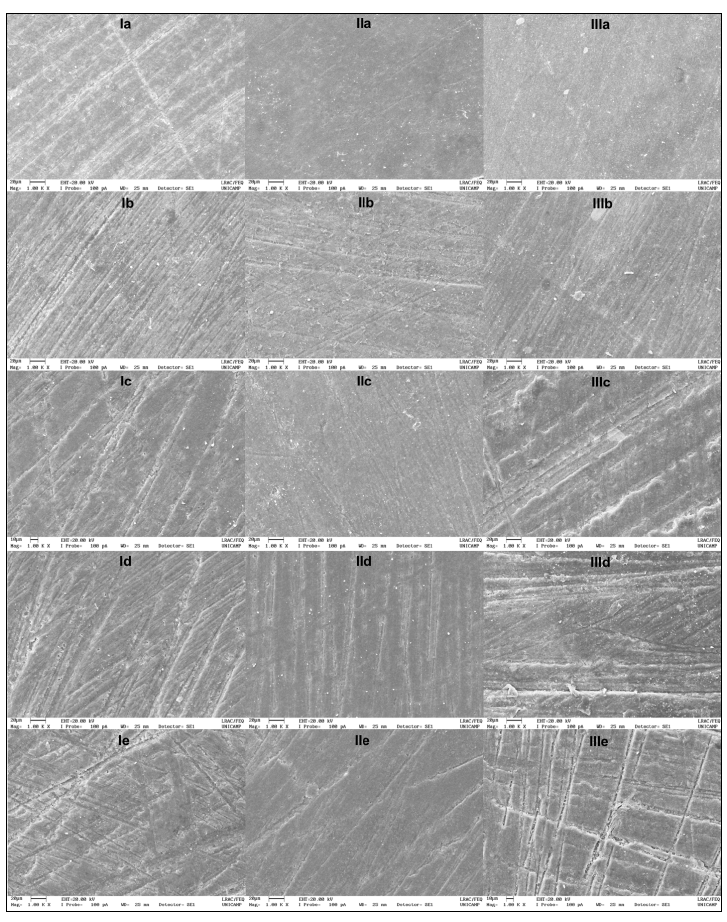
Three samples from each group were prepared for surface micromorphology analysis. The samples were gold-plated (Sputter Coater EMITECH, K450, Kent, UK) and examined under scanning electron microscopy (Scanning Electron Microscope, SEM: Leo 440i, EDS: 6070, LEO Electron Microscopy / Oxford, Cambridge, England) to obtain the images. Each sample showed the full extent of the surface run at 500x magnification, performed by a single, previously calibrated operator. A representative area was selected to characterize what occurred in most of the sample surface and photographed at 1000x magnification.
Statistical Analysis
Surface roughness data and color variables (ΔL, Δa, Δb and ΔE) did not meet the assumptions of a parametric analysis. Thus, the surface roughness data were analyzed by generalized linear models for repeated measures over time, with the factorial resin vs. bleaching protocol. The delta values (A) were analyzed by generalized linear models, considering the resins vs. bleaching protocols as the factorial scheme. The analyses were performed on R programs (Core Team, R Foundation for Statistical Computing, Vienna, Austria) and SAS programs (SAS Institute Inc. SAS® Studio 3.5: User's Guide, Cary, NC: SAS Institute Inc) with a significance level of 5%.
RESULTS
Surface roughness was found to be higher in high-viscosity bulk-fill resin, and lower in low-viscosity bulk-fill resin, regardless of the bleaching protocol (Table 2). All the bleaching protocols promoted increased surface roughness, regardless of the composite resin.

Table 2 Median (minimum and maximum values) of surface roughness (Ra) according to composite resin, bleaching protocol and time.
Color analysis showed that the low-viscosity bulk-fill resin had a statistically lower ΔL and a negative value when 10% carbamide peroxide gel was applied, compared with the other bleaching protocols (p <0.05) (Table 3). As for the high-viscosity bulk-fill resins and conventional composite resins, there was no significant difference among the bleaching protocols regarding ΔL (p> 0.05).

Table 3 Median (minimum; maximum) of the parameters of evaluation of the color change (ΔL, Δa, Δb and ΔE) according to composite resin, bleaching protocol and time.
The group of low-viscosity bulk-fill resins submitted to the 10% carbamide peroxide gel protocol showed a statistically lower Δa (p <0.05) than the group receiving the 40% hydrogen peroxide gel application, or the group immersed in whitening rinse, but was statistically similar to the group immersed in distilled water (Table 3). For the high-viscosity bulk-fill composite resin, there was no significant difference among the bleaching protocols for Δa (p> 0.05) and for the conventional resin; statistically higher Δa was observed in the group immersed in whitening rinse than in the other bleaching protocols (p <0.05).
For the low-viscosity bulk-fill resin, a statistically higher Δb value was observed for the group immersed in distilled water, in which 10% carbamide peroxide gel was applied, than for the other bleaching protocols (Table 3). For the high-viscosity bulk-fill resin, Δb did not differ significantly among the bleaching protocols (p> 0.05). For the conventional resin, Δb was significantly higher when the resin was immersed in whitening rinse than with the other bleaching protocols (p <0.05).
The low-viscosity bulk-fill resin showed significantly greater variation in total color (ΔE) when 10% carbamide peroxide gel was applied. The high-viscosity bulk-fill resin presented higher ΔE in water than when subjected to the 40% hydrogen peroxide gel protocol (p <0.05). The conventional composite resin showed significantly higher ΔE after bleaching with 10% carbamide peroxide gel, and when in contact with the whitening rinse (p <0.05) (Table 3). For illustrative purposes, the surface morphology of the sample was analyzed by scanning electron microscope, and the influence of bleaching protocols on the resins tested was observed. All composite resins tested showed altered surface morphology, with deepening of furrows and grooves, regardless of the bleaching protocol used (Fig. 1).
DISCUSSION
According to the results of the present study, the null hypothesis was rejected, since there were significant differences in roughness, surface micromorphology and color among the composite resins after being subjected to the bleaching protocols.
Surface roughness (Ra) was statistically lowest for the low-viscosity bulk-fill resin and highest for the high-viscosity bulk-filled resin, with an intermediate value for conventional nanoparticulate resin, whether at the initial timepoint (baseline) or after application of the bleaching protocols. This may be attributed to the polishing system used for the samples, as reported by Rigo et al. 25, who compared the same bulk-fill resins as those in the present study. They reported that the smoothness of the Ra of the resins was promoted by the polyester tape, and was statistically similar among the resins. However, when using the Soflex system (3M ESPE), they showed that Ra was higher for the high-viscosity bulk-fill resin than for the low-viscosity bulk-fill resin. This can be explained by the composition of the high-viscosity bulk-fill resin, in which the new monomers that were introduced (such as AUDMA and DDDMA) may have been more eluted, resulting in greater abrasion of the organic matrix during polishing, and consequent exposure of the surface loads and increased Ra25. It can be assumed that alternative polishing systems may promote different results.
Ra increased significantly in all resins after bleaching with any of the protocols. SEM showed that irregularities and grooves in the micromorphology increased after the bleaching protocols, possibly due to structural changes in the composite resins caused by the action of peroxides18,26-28 and water sorption. Water sorption may soften the matrix and degrade the composite resin surface, with consequent exposure of the inorganic load and increased Ra29-31. Color analysis found that the low-viscosity bulk-fill resin had statistically lower ΔL and Δb values than the other resins when submitted to the 10% carbamide peroxide bleaching agent and to the other bleaching protocols. This may be associated with the effect of 10% carbamide peroxide on the surface, since it is associated with higher translucency of low-viscosity bulk-fill resin. Thus, constant daily application may have led to chromatic change of the resin, represented clinically by a darkening and blueness of the material. It is worth noting that low-viscosity bulk-fill resin is not exposed to the buccal environment except on the proximal face, since the manufacturer recommends that it be coated with conventional composite resin. Thus, from a clinical standpoint, color change in this type of resin may be less relevant than in high-viscosity bulk-fill resins and conventional resins.
ΔE was high in the conventional nanoparticulate composite resin, especially after immersion in whitening rinse and 10% carbamide peroxide. This may be associated with the significantly higher Δb and the positive value of this nanoparticulate composite resin under these conditions, demonstrating that there was yellowing of conventional composite resin samples. These changes may be related to the structural composition of the organic matrix of the conventional resin, which has a higher hydrophobic monomer content, but which has hydrophilic characteristics, in the case of BisGMA and TEGDMA. Both monomers have water sorption capacity when in contact with aqueous solutions32, which may have some effect on color change. These results were more significant when the composite resin was subjected to the home bleaching protocol with 10% carbamide peroxide, and especially with the whitening rinse protocol. Thus, it is believed that the daily contact of these agents with the resin surface may have increased the degradation of the organic matrix and exposure or detachment of the inorganic load33, which may have altered and contributed to greater color change. It should also be emphasized that whitening rinses contain phosphoric acid and alcohol in their composition, as well as having acidic pH. These substances may have had deleterious effects on the surface of the conventional composite resin, promoting the color change.
The color change was also high for low-viscosity bulk-fill resins. This may be explained by the similarity of the composition of these resins to the conventional composite resin, which includes BisGMA and TEGDMA monomers. It was observed that the high-viscosity bulk-fill composite resin had less color change than the conventional composite resin, as reported by Mansouri and Zidan34. This result may be related to the composition of the high-viscosity bulk-fill resin, because of the presence of UDMA and AUDMA monomers, as well as the absence of Bis-GMA and TEGDMA. UDMA is a monomer that has higher hydrophobia35 and lower sorption and water solubility30 than the Bis-GMA and TEGDMA monomers present in Z350 and low-viscosity bulk fillers.
Several authors consider 3.3 a critical value for ΔE, considering it is clinically unacceptable, because the color modification at this rate becomes perceptible to the human eye31,36-38. In this study, the low-viscosity bulk-fill resin presented values greater than 3.3, regardless of the protocol tested, whereas the high-viscosity bulk-fill resin presented values lower than 3.3, regardless of the protocol tested. It was concluded that the high-viscosity bulk-fill resin material provides greater color stability.
Within the limits of this study, it was observed that the tested agents influenced the color of the restorative materials. Dentists should be aware that pre-existing restorations may change significantly after tooth bleaching, and replacement may be necessary. Moreover, it is believed that phenomena such as greater Ra and alterations in color and in surface micromorphology occur more markedly in the oral cavity, because of the ionic composition of saliva, the ingestion of acidic foods and beverages, and the presence of acids from bacterial metabolism39, and should be considered. Furthermore, brushing exerts an effect on optical properties of bulk fill composites,40 and this was not simulated in the present study. Thus, further research and clinical trials are needed to prove the effects of different bleaching protocols applied to high- and low-viscosity bulk-fill resins.
It was concluded that the characteristics of each composite resin influenced the surface roughness more significantly than the bleaching protocol. After the resins came into contact with the bleaching protocols and water, roughness increased and surface morphology changed. In general, the greatest color change in the low-viscosity bulk-fill resin was observed after contact with 10% carbamide peroxide gel, whereas the greatest color