INTRODUCTION
Colonization by Candida species begins within the first few days of life. Although the oral mucosa is a reservoir, Candida spp. are also present in the biofilm of periodontal pockets of patients with periodontitis, endodontic infection and periimplantitis1.
In 30 to 50% of subjects, Candida spp. colonize the oral cavity and the digestive tract, and are more frequent in lactating infants, elderly subjects who use a dental prosthesis, people who have received antibiotic treatment or chemotherapy, patients with poorly controlled diabetes, and hospitalized and immunosuppressed patients2.
Asymptomatic colonization of the oral mucosa is more prevalent among HIV+ subjects and considered an important predisposing factor for candidiasis.
The advent of highly active antiretroviral treatment (HAART) has markedly improved the immune system of HIV+ patients, substantially decreasing the incidence of opportunistic infections3.
The most frequently isolated yeasts form the oral cavity of HIV+ patients are Candida albicans and Candida dubliniensis. These species share a number of phenotypic features, such as the formation of green colonies in chromogenic media and the ability to produce chlamydospores and pseudofilaments4" 7. The virulence factors of these yeasts include phenotypic change, dimorphism, adherence capacity and secretion of lytic enzymes such as phospholipase, proteinase and hemolysin, among others8,9.
Production of these enzymes can therefore be an important determining factor of the potential of these microorganisms to produce invasive infection in certain groups of individuals, as is the case of immunocompromised patients10.
The aim of the present study was to describe and compare enzyme production by commensal strains of Candida albicans and Candida dubliniensis obtained from different oral ecological niches in periodontal HIV+ patients, receiving and not receiving highly active antiretroviral therapy (HAART).
MATERIALS AND METHODS
The study population comprised adults living with HIV/AIDS residents in the Buenos Aires City, who attend dental care services. The sample included 15 HIV patients (8 receiving and 7 not receiving HAART) seen at the High-Risk Patients Oral Care Unit (CLAPAR I), School of Dentistry, University of Buenos Aires, who underwent oral examination after they had voluntarily signed an informed consent form. The study was approved by the Ethics Committee of the School of Dentistry, University of Buenos Aires (Resolution 21/11/2012-33).
Patients aged 18 to 60 years, diagnosed with HIV infection (confirmed by 2 positive ELISA tests and 1 Western Blot or similar), with a minimum 6 teeth and one site per quadrant with a probing depth equal to or higher than 5mm, attachment loss, and positive bleeding on probing, were included in the study. Only HAART patients who had remained on the same regimen the whole year prior to study enrolment were included.
Patients with systemic disease unrelated to HIV infection prior to or presenting during the study, receiving antibiotic/antifungal and/or oral-antiseptic treatment within 3 months prior to microbiological testing, showing mucosal candidiasis lesions, receiving oral care at other private or public oral health care services, and/or who smoked were excluded from the study.
Under adequate lighting conditions and using standardized instruments (a Marqis color-coded probe and a dental mirror), a calibrated researcher assessed the following clinical parameters of periodontal disease at 6 different sites per tooth (3 on the buccal aspect and 3 on the palatal or lingual aspect): presence of biofilm, probing depth (PD), clinical attachment level (CAL) and bleeding on probing (BP).
The patients were then instructed to rinse their mouth with distilled water. The site showing the worst periodontal parameters in each quadrant was identified. Following removal of the supragingival plaque using a curette and tooth isolation with cotton rolls, subgingival biofilm samples were collected using 4 paper points placed one at a time into the depth of the pocket for 20 seconds. The obtained samples were placed in reduced transport fluid (RTF) medium. A sample of oral mucosa was obtained using a swab, which was placed in a tube containing RTF medium.
All the samples were submitted to the microbiology laboratory for processing.
Microbiological Procedures
The samples were homogenized by shaking in a vortex mixer; 50 pl of each suspension were seeded in CHROMagar Candida® for presumptive identification of the species7.
C. albicans and C. dubliniensis were distinguished by using conventional phenotypic methods5,6: cultures on milk-tween 80 agar for detection of germ-tubes, pseudofilaments, and chlamydospores11, cultures on Sabouraud Dextrose Agar (SDA) incubated at 45 °C12, and cultures on Staib agar for production of chlamydospores13.
Molecular identification
Genomic DNA was extracted from pure and fresh 24-h cultures in SDA. Cell lysis was reached by heating at 100 °C and centrifuging at 16000g.
Two species-specific primers derived from the internally transcribed spacer (ITS) region (comprising the ITS1, 5.8s rRNA and ITS2 regions) as described by Asadzadeh et al.14 were used. Amplification reactions were performed by real time PCR (qPCR) using 2X SYBR Green Supermix in a 10 pl final volume, 10 pM of each primer, and 2 pl of genomic DNA, in a thermal cycler (CFX96 C1000 Touch, BioRad, Hercules, CA, USA). PCR cycling conditions were as follows: denaturation at 95 °C for 5 minutes, followed by 39 amplification cycles at 95 °C for 15 seconds, 60 °C for 30 seconds, 65 °C for 5 minutes, and 95 °C for 5 minutes.
The amplification process was evaluated using MCA (melting curve analysis) according to Asadzadeh et al. 14.
Detection of enzyme activity
All the isolated strains were seeded in SDA and incubated at 37 °C for 18h. The cultures were used to prepare suspensions adjusted to 1 McFarland standard (1 x 106 CFU/ml).
Phospholipase, proteinase and hemolysin production was detected using malt agar supplemented with sterile egg yolk emulsion (2%)8, bovine serum albumin agar8, and SDA with sterile sheep blood (7%)15, respectively.
Sterile paper discs were placed on the surface of each dish containing the corresponding medium, inoculated with 10 pl of the suspension, and incubated under aerobic conditions at 37 °C. The assays were performed in duplicate.
Phospholipase production was assessed at 72 h and was considered positive when a zone of precipitation was observed around the colony.
The precipitation zone (Pz) value was calculated as the ratio of the diameter of the colony to the total diameter of the colony plus the precipitation zone16. Enzyme production was classified according to the Pz value as follows: Pz value = 0.35 - 0.5 (high producers), Pz value = 0.51 - 0.74 (moderate producers); Pz value = 0.75 -0.9 (low producers); and Pz value = 1 (negative)17. Hemolytic halos were measured, and the enzymatic activity Pz value was calculated according to the ranges defined above.
Proteinase production was determined seven days post-incubation; the dishes were stained with a 0.6% amido black solution and destained with acetic acid8. The presence of a hydrolysis zone around the colony showed positive proteinase activity.
Candida albicans ATCC 10231 was used as positive control for the production of all three enzymes, Candida glabrata ATCC 90030 was used as negative control for phospholipase, and Candida parapsilopsis ATCC 22019 was used as negative control for hemolysin and proteinase.
Statistical Analysis
Categorical variables were analyzed as frequencies and percentages using 95% confidence intervals, and comparisons were established using Chi square test with Yates' continuity correction. In all cases, statistical tests for independent samples were used and level of statistical significance was less than 5% to reject the null hypothesis.
All statistical analyses were performed using SPSS software version 26, MS Excel 2016, Epidat 4.0.
RESULTS
As shown by the clinical records, 7 (46.7%) of the 15 studied patients were women and 8 (53.3%) were men, and all 15 patients contracted the virus by sexual transmission.
Mean age of the patients was 41 years (SD±10 years) and age range was 28 to 58 years. Analysis of the population according to age group showed that 20.0% were below the age of 30 years, 33.0% were between the age of 30 and 40 years, and 47.0% were over the age of 40 years.
Forty-seven percent of patients had been diagnosed with HIV within 10 years prior to the study, and 53.0% had been diagnosed more than 10 years before enrolment in the study. Eight patients (53.0%) were receiving HAART and 7 (47.0%) were not receiving HAART at the time of the study; 50% of patients receiving HAART had been under treatment for more than 10 years.
A total 75 samples, including 60 samples (80.0%) of subgingival biofilm and 15 samples (20.0%) of oral mucosa, were obtained from 15 patients. A total 39 strains of Candida genus were isolated: 14 (35.9%) Candida albicans and 25 (64.1%) Candida dubliniensis strains. Distribution according to species and localization is shown in Table 1.
Both species were isolated from the same periodontal site in two patients, one receiving and one not receiving HAART.
No statistically significant differences in the distribution of Candida species or localization were observed; chi square test p=0.887.
Analysis of the isolated species according to presence or absence of treatment showed 3 C. albicans (13%) and 20 C. dubliniensis (87%) strains in the 8 patients receiving HAART, and 11 C. albicans (68.8%) and 5 C. dubliniensis (31.2%) strains in the 7 patients not receiving HAART (Fig 1).
A greater proportion of Candida albicans was observed in patients not receiving HAART, and a higher proportion of Candida dubliniensis was observed in patients under HAART; Chi square test p< 0.001.
In view of the distribution of phospholipase and proteinase production, the statistical analysis was performed considering positive or negative production (presence /absence) and Pz values were not considered.
Phospholipase production was observed in 13 (92.9%) of the 14 Candida albicans strains, 11 of which were isolated from patients not receiving HAART and 2 from patients under HAART (Table 2). However, no phospholipase production was observed in any of the Candida dubliniensis strains from either group.

Table 2 Frequency and percentage distribution of phospholipase production by Candida albicans strains isolated from patients receiving and not receiving HAART.
Proteinase production was higher in both Candida albicans and Candida dubliniensis strains isolated from patients not receiving HAART; Chi square test, p< 0,001 (Fig. 2 and Table 3).

Fig. 2 Percentage distribution of proteinase production by Candida albicans and Candida dubliniensis strains isolated from patients receiving and not receiving HAART.
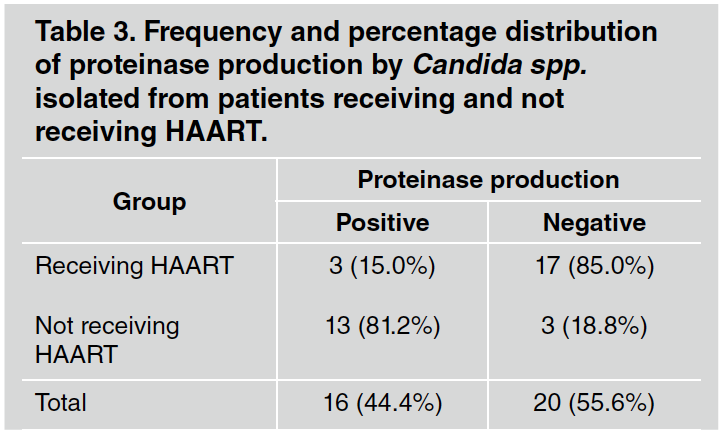
Table 3 Frequency and percentage distribution of proteinase production by Candida spp. isolated from patients receiving and not receiving HAART.
As regards hemolysin production, all the studied strains were found to produce the enzyme, and production was significantly higher (p=0.04) both in Ca and in Cd strains isolated from patients not receiving HAART (Fig. 3 and Table 4).
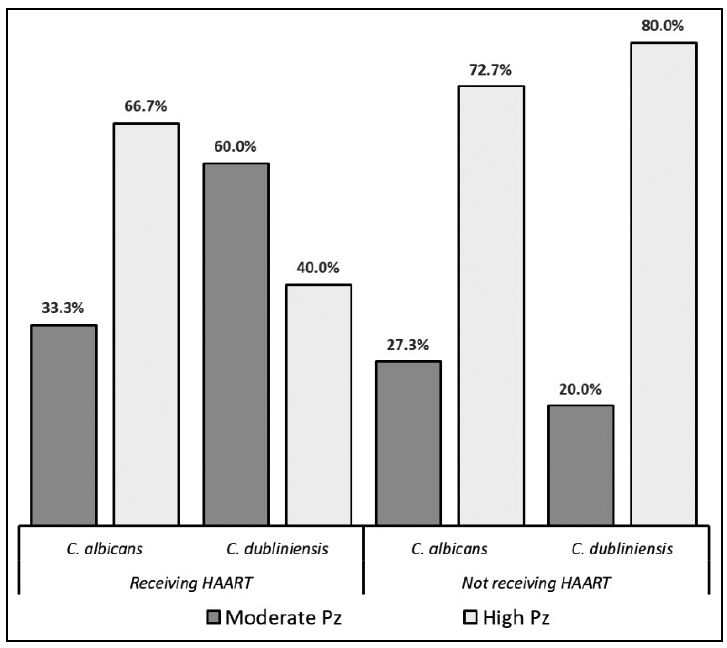
Fig. 3 Percentage distribution of hemolysin production by Candida albicans and Candida dubliniensis strains isolated from patients receiving and not receiving HAART.
DISCUSSION
The distribution of age, sex, and mode of acquisition of HIV infection was similar in both study groups of patients. Of note, all the studied yeast strains were isolated from oral mucosa and periodontal pockets of patients with no clinical manifestation of candidiasis.
In the present study, a larger number of strains of both Candida albicans and Candida dubliniensis was isolated from subgingival biofilm (Table 1). Although the role of yeasts in the pathogenesis of periodontitis has not been fully elucidated to date, a number of authors have reported the presence of yeasts in periodontal pockets of subjects with chronic periodontitis, including both immunocompetent and immunocompromised patients18,19.
There are reports in the literature of Candida dubliniensis isolates from non-HIV patients20-22. Ursua B. et al. found that C. albicans and C. dubliniensis were capable of colonizing periodontal pockets, regardless of pocket depth, in non-HIV patients with chronic periodontitis23.
According to Vieira Colombo AP et al., subgingival biofilm can be a source of development and dissemination of systemic infections24. Periodontal scaling could cause fungemia, which in turn could lead to serious complications, depending on the immunological status of the patient.
In the present study, a larger number of Candida dubliniensis strains were isolated from HIV+ patients receiving HAART (Fig. 1), suggesting that compliance with treatment would result in homeostasis between the immunological status of the patient and the yeasts, decreasing the development of opportunistic infections, particularly candidiasis. Vilela MM et al. showed that Candida dubliniensis is less virulent than Candida albicans25.
Paula SB et al. recovered fewer Candida dubliniensis isolates from HIV+ patients receiving HAART26.
The detection of Candida species in periodontal pockets may be due to the ability of these yeasts to adapt to changes in the environment by altering pH, oxygen concentration, and nutrient availability27. This enables them to coexist in the subgingival biofilm and coaggregate with periodontopathic bacteria, as shown in a paper recently published by our research team28.
According to Lourenqo et al, the discrepancies among studies regarding the prevalence of Candida spp. could be due to the method used to collect the study samples, such as swabbing, collecting total saliva or oral rinses.29 In line with Samaranayake et al, the authors found that the oral rinse technique was the most sensitive for detecting Candida spp.30 We consider that collecting subgingival biofilm samples is an adequate method to recover Candida spp.Jewtuchowicz et al. isolated Candida spp. from subgingival samples obtained using a curette from immunocompromised patients22.
Several authors have reported coinfection by Candida dubliniensis and other Candida species, particularly Candida albicans.31 In the present study, we observed coinfection of a single site by both species in two cases: one patient under HAART and the other patient not receiving HAART.
All the strains were identified using phenotypic methods, and then confirmed using molecular biology techniques. Pineda G. et al. suggested that the combined use of at least three phenotypic differentiation techniques might be a valid alternative for laboratories that do not have access to molecular diagnostic methods6.
The transition of yeasts from commensal to opportunistic pathogens is the result of a number of mechanisms to overcome the host's defense barriers, including adhesion, biofilm formation and production of hydrolytic extracellular enzymes such as phospholipase and proteinase; all these properties are considered virulence determinants.
Aspartic proteases play an important role in the degradation of the mucosal (collagen, keratin, and mucin) as well as the immune components (cytokines, antibodies, and complement), facilitating invasion of the host's tissues8.
Phospholipase can damage the host's cell membrane by degrading its constitutive lipids. Phospholipase production can therefore be an important factor in determining the ability of Candida spp. to invade tissues10.
Hemolysin production was observed in all the strains isolated in the present study. Manns et al.showed that iron is found intracellularly as ferritin or contained in the heme group, and that small amounts of extracellular iron are found bound to transport proteins such as transferrin and lactoferrin. Microorganisms also require iron to grow, and their ability to acquire iron is essential to their survival and to establishment of infection9. We understand that hemolysin production is a survival rather than a virulence factor.
The results of the present study showed that both species isolated from patients not receiving HAART produced proteinase (Fig. 2), whereas strains recovered from patients under HAART were low proteinase producers. As suggested by Thomas et al.32 and Ribeiro et al33, this difference could be explained by the use of protease inhibitors in HAART. Nevertheless, other authors posit that the presence of protease inhibitors in HAART does not affect the production of this enzyme34-36.
In the present study, phospholipase production was observed in all the Candida albicans strains isolated from patients not receiving HAART, but in none of the Candida dubliniensis strains from either group of patients. Our findings are in line with studies testing different quantities of Candida dubliniensis strains20,3,21.

















