INTRODUCTION
Melatonin (MLT) is the main hormone synthesized and secreted by the pineal gland. Its functions include regulating various physiological processes in different parts of the body, such as circadian rhythm1, body temperature2, immune system3, sexual development4 and reproductive cycle5. MLT levels in the body are related to ambient light, reaching maximum levels at night when it is dark and lowest levels throughout the duration of daylight6.
MLT may be administered in several ways such as orally and intraperitoneally as a supplement or prescription-only, depending on the country. It is inexpensive and has few side effects compared to other drugs and can be used with a wide safety margin. Such features combined with the series of reported effects on different tissues make melatonin a potent therapeutic option for several ailments and/ or prevention thereof7.
Studies have demonstrated the influence of MLT in cell differentiation by stimulation or suppression, depending on dose and cell type8,9. MLT is believed to have the potential to play a key role in regulating bone growth since at therapeutic doses it may inhibit osteoclastic activity and induce osteoblast differentiation10.
A previous study revealed that MLT enhances mesenchymal stem cell differentiation into mature osteoblasts through the MT2 receptor11 and during osteoblastic differentiation, MLT increases the expression of osteocalcin (OC), a highly specific marker of bone formation, followed by enhanced bone mineralization12. It has also been demonstrated that MLT has the ability to stimulate the proliferation and synthesis of type I collagen, as well as other bone matrix proteins and bone markers (alkaline phosphatase, osteopontin and osteocalcin) at micromolar concentrations in human osteoblasts in vitro9, though the mechanisms by which such phenomena take place remain largely ill-understood. The oral cavity is affected by a number of conditions such as periodontitis, mucositis, neoplasms and cytotoxicity from various drugs and/ or biomaterials. MLT has been shown to down-regulate the expression of pro-inflammatory factors such as C- reactive protein, interleukin-6 and tumor necrosis factor-alpha2 as well as receptor activators of nuclear factor kappa-B ligand/osteoprotegerin ratios to reduce periodontal inflammation2,13,23,24. In addition to up-regulating salivary acid phosphatase, alkaline phosphatase, osteopontin, and osteocalcin, MLT has been associated to positive outcomes regarding gingival index and pocket probing depth, which are highly suggestive of enhanced osteoblast differentiation and bone formation14, 17,23,24.
Despite the numerous current and potential applications of MLT, further studies are needed to ascertain the effect of melatonin on bone cells to serve as the basis for in vivo studies and ultimately safe long-term clinical use. Therefore, the aim of the present study was to evaluate in vitro the effect of the hormone melatonin on the proliferation, viability, bone matrix protein expression and mineralization capacity of pre-osteoblastic cells.
MATERIALS AND METHODS
Experimental design
This was an analytical bifactorial experimental study, in which the following doses of melatonin were tested: 0.01mM (C1), 0.1mM (C2), 1mM (c3), 2.5mM (C4) and 5mM (C5) and a control group (C6) at 24h, 48h, 72h and 10 days after treatment.
MLT powder >98% (N-acetyl-5-methoxytrypta-mine) and M5250 Sigma (Sigma, St. Louis, Missouri, USA), reconstituted in 50 mg/mL absolute ethanol were used. The control group received the highest dose of ethanol (vehicle), but no MLT.
Cell culture
A mouse-derived pre-osteoblastic cell line (MC3T3-E1) obtained from the ATCC (American Type Culture Collection, ATCC, VC, USA) was used, supplemented with 15% fetal bovine serum (Nutricell Cellular Nutrients, Campinas, SP, Brazil), 100 IU/ml penicillin (Sigma) and 50 mg / ml streptomycin (Sigma).
For the mineralization assays, the cultures were supplemented with 50 pg/mL ascorbic acid, 100 pg/ mL beta-glycerophosphate (Sigma, St. Louis, MO, USA), 95% O2 and 5% CO2.
At 70% sub-confluence, the culture medium was removed and 0.25% trypsin (Nutricell Nutrientes Celulares) and 1mM EDTA (Gibco) solution were added to gather the cells in suspension. Then the cells were seeded - 3.5x103 cells / well in 96-well polystyrene plates (Sarstedt). After 24 hours, the medium was changed and the osteoblastic cells treated with MLT at 0.01, 0.1, 1, 2.5 and 5 mM.
Cell proliferation assay
The Trypan blue vital exclusion method was used at 24h, 48h and 72h of treatment with MLT at 0.01, 0.1, 1, 2.5 and 5 mM.
Cell viability assay (MTT)
Cell cultures were tested for cell viability using the MTT assay. After solubilization of the crystals, quantification was performed on an ELX800 microplate reader (Biotek Instruments, Inc.) at 590 nm. The cytotoxic dose (C50) was then determined based on the MTT assay findings. This represents cell viability below 50% in relation to the control group.
mRNA expression for collagen type I (COL-I) and osteopontin (OPN)
Real-time polymerase chain reaction (RT-qPCR) was used to evaluate the transcription levels of genes encoding COL-I and OPN proteins. Table 1 shows the sequence of primers.
At 24h, 48h and 72h, the culture medium was removed from the wells (stored at -80°C for secreted protein assays) and the cells were collected for RNA extraction using the Illustra RNASpin Mini Kit (GE Healthcare, Milwaukee, WI, USA), according to the manufacturer's instructions. Samples were quantified by spectrophotometry (NanoVue) and the quality was established at A260/A280> 1.6. The complementary DNA was synthesized using the ThermoScientific cDNA kit, according to the manufacturer's instructions.
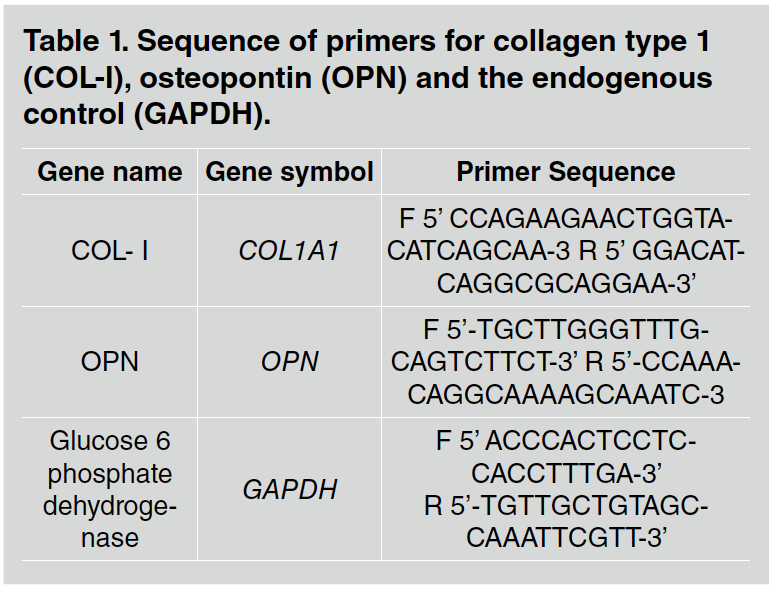
Table 1 Sequence of primers for collagen type 1 (COL-I), osteopontin (OPN) and the endogenous control (GAPDH).
The reactions of were performed using the SYBR Green system (Thermo Fisher Scientific, Waltham, MA, USA) on a Real Time PCR System (Applied Biosystems, Foster City, CA, USA).
The results were analyzed based on Ct (Cycle Threshold), which corresponds to the number of cycles at which the amplification of the samples reached a threshold between the fluorescence level and the amplification phase (determined between the fluorescence level in relation to the negative control, C6). The 2-AACT method (Livak & Schmittgen, 2001) was used to determine expression of the target genes.
Total protein assay
Prior to the protein secretion assays (ELISA), the culture medium was normalized via total protein quantification following the method of Lowry et al. (1951), in a spectrophotometer (CE3021, Cecil, Cambridge, England) at 680 nm. Total protein concentration was calculated for each well in g/ mL from a standard curve based on bovine serum albumin (Sigma).
Enzyme immunoassay for collagen type I (COL-I) and osteopontin (OPN)
Quantification of COL-I and OPN secreted by the osteoblastic cells under different MLT doses was
performed using ELISA (Kit ELISA for COL-I R&D Systems, EUA; Kit ELISA for OPN Cloud-Clone Corp, Katy, TX, USA).
The reactions were terminated by adding 50 pL of 2N sulfuric acid (H2SO4) to the substrate solution in each well and the color was measured on a spectrophotometer (Epoch, Biotek, Winooski, VT, USA) at 450 nm. The total amount of COL-I and OPN were determined in picograms per milliliter (pg/mL). All experiments were performed in triplicate. The primers used are listed in Table 1.
Mineralization assays
Alizarin red
Osteoblastic cells, except those from the control group, were cultured with MLT at 0.01, 0.1 and 1 mM and analyzed at 10 days and 14 days, with the culture medium changed every 2-3 days together with supplementation of MLT. The wells were rinsed and prepared for evaluation under a light microscope (Nikon eclipse E800, Japan). The presence of calcium deposits was identified by the development of red color in the matrix and photographs were taken. The images were processed and later analyzed on Image J (NIH). The percentage of calcium nodules formed was determined based on optical density from the images taken from each sample.
After 10 days of culture with the different concentrations of MLT, 280 pl of 10% acetic acid was added to each well and the plate was shaken for 30 min. The cell layer was then scraped off and the solution transferred to 1.5 ml tubes, which were vortexed for 30 s. Samples were first heated at 85 °C for 10 min and immediately placed in ice for 5 min. Subsequently, the tubes were centrifuged at 13,000g for 20 min and 100 pl of the supernatant was transferred to a 96-well plate, where 40 pl of 10% ammonium hydroxide was added to each well. The samples were read on a spectrophotometer at 405 nm.
Alkaline phosphatase assay - Fast Red test
The cultures were treated as per the alizarin red assay until the culture medium was removed and the wells washed with Hanks' solution (Sigma) heated to 37 °C. Three hundred and twenty milligrams of the Triz reagent (Sigma) were dissolved in 20 mL of deionized water for further addition of 7 mg of the Fast Red Reagent (Sigma). Two mL of this solution were discarded and 8mg Naftol (Sigma) diluted in 2mL of dimethyl formamide (Merck), forming the working solution. One ml of this solution was added to each well and the plate was placed in a moist-air incubator at 37 °C with 5% CO2 for 30 min. The solution was then withdrawn and the plate dried for further photographic documentation. The images were processed and analyzed on Image J (NIH), which determined the percentage of nodules formed as a function of the amount of black areas within the total field.
RESULTS
Cell proliferation assay
Time and concentration factors significantly influenced cell proliferation (p<0.05), with a significant increase in proliferation over time in relation to the control group and at 0.01 mM and 0.1 mM MLT. From analysis of the data at 24h, no differences were observed between the doses evaluated (p> 0.05). At 48h and 72h, higher mean proliferation values were observed with the lower doses (0.01, 0.1 and 1 mM). Multiple comparisons showed differences, especially at 48h and 72h, in which the higher doses (2.5 and 5 mM) caused a significant decrease in cell proliferation (p<0.05). This finding guided the exclusion of the doses 2.5 mM and 5 mM from the PCR, ELISA and mineralization assays (Fig. 1).

Fig. 1 Evaluation of cell proliferation by the Trypan blue vital exclusion method in pre-osteoblastic cells at 24h, 48h and 72h, supplemented with MLT at 0.01, 0.1, 1, 2.5 and 5 mM, and the control group. Different uppercase letters represent a significant difference between the evaluation periods for each studied condition (ANOVA and Tukey, p<0.05); different lowercase letters represent a significant difference between the concentrations tested (ANOVA and Tukey, p<0.05).
Cell viability assay (MTT)
The data relating to the MTT assay demonstrated a very similar pattern to that from the proliferation assay in terms of both dose and time, as well as the interaction between the two factors. As shown in Fig. 2, it was observed that at 24h, the lowest mean cell viability occurred at 5 mM MLT (p <0.05). At 48h, the mean cell viability was observed at 5 mM, which was significantly lower than for 0.1 mM (p <0.05). At 72h, both 5 mM and 2.5 mM showed a significantly lower mean cell viability than the remaining groups (p <0.05). At 72h, the mean viability was significantly higher than at 24h at the doses of 0.01, 0.1 and 1mM and the control. The fact that the higher concentrations (2.5 and 5 mM) caused a significant decrease in cell viability also led to their exclusion from the subsequent analyses.
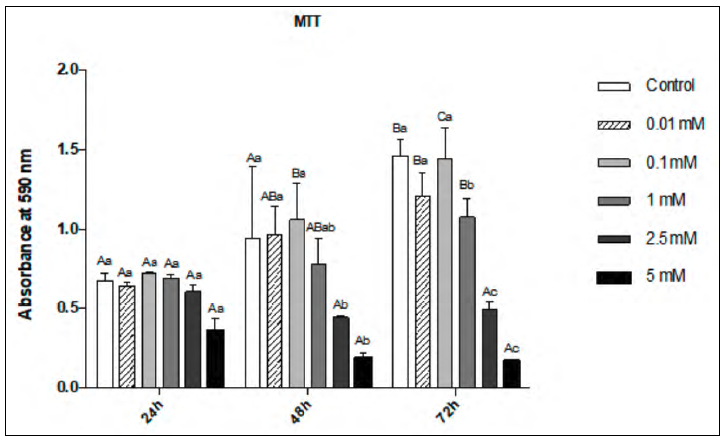
Fig. 2 Cell Viability Assay (MTT) in pre-osteoblastic cells at 24h, 48h and 72h, supplemented with MLT at 5, 2.5, 1, 0.1, 0.01 mM and the control group. Different uppercase letters represent a significant difference between the evaluation periods for each studied condition (ANOVA and Tukey, p<0.05); different lowercase letters represent a significant difference between the concentrations tested (ANOVA and Tukey, p<0.05).
Alizarin Red Assay
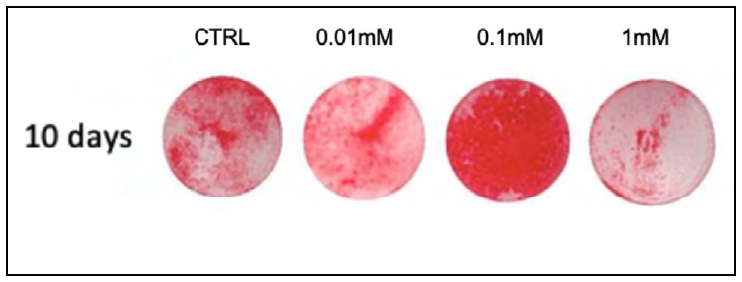
At 10 days, as shown in Table 2 and Fig. 3, all doses caused a significant increase (p <0.05) in the formation of mineral nodules when compared to the control group.
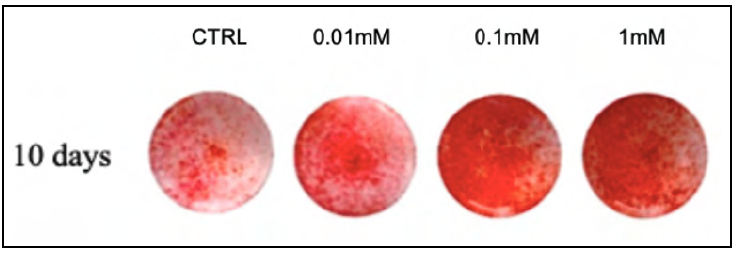
Fig. 3 Representative images from the Alizarin Red test at 10 days, for the different MLT concentrations studied.
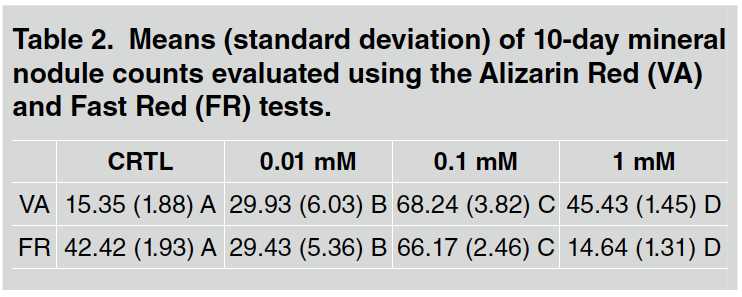
Table 2 Means (standard deviation) of 10-day mineral nodule counts evaluated using the Alizarin Red (VA) and Fast Red (FR) tests.
The 0.1 mM dose caused the highest quantity of mineral nodules (p <0.05; Fig. 4). The remaining doses did not differ significantly from the control group, with 1 mM showing the lowest quantity of mineral nodules. As shown in Table 2 and Fig. 5, a significant increase in mineral nodules (p <0.05) is observed only for the 0.1 mM group.
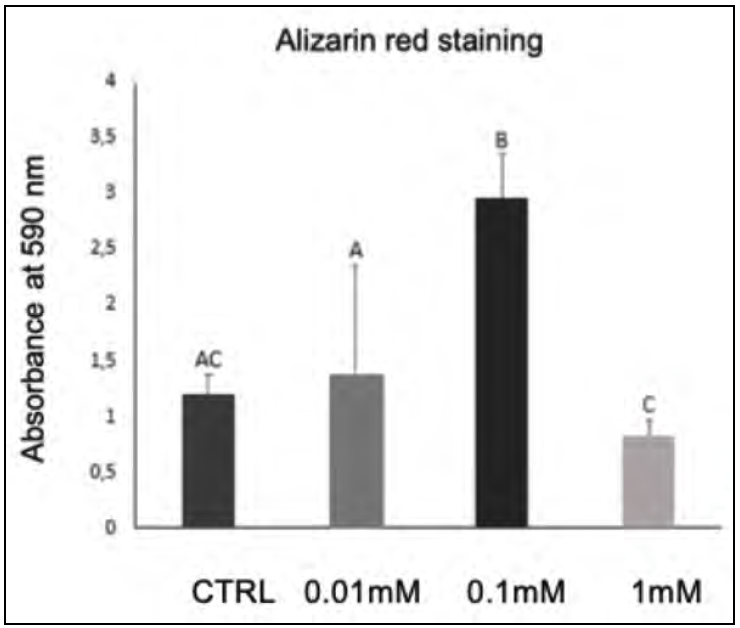
Fig. 4 Mean and standard deviation of Alizarin Red staining for the different MLT concentrations studied at 10 days. Different letters in the columns represent a significant difference between doses (ANOVA and Tukey, p<0.05).
Gene expression of COL-I and OPN
At 24h, the 1 mM dose significantly up-regulated COL-I gene expression when compared to the remaining groups (p <0.05). For OPN, a significant up-regulation occurred with the dose 0.1 mM (p <0.05). At 48h, COL-I expression was significantly up-regulated for of 0.1 and 1 mM (p<0.05), while OPN expression was significantly increased in the 0.01 mM and 1 mM groups (p <0.05).
At 72h, COL-1 expression was significantly up-regulated in 0.1 mM group (p<0.05) whereas OPN expression peaked at 1mM (p <0.05, Fig. 6).
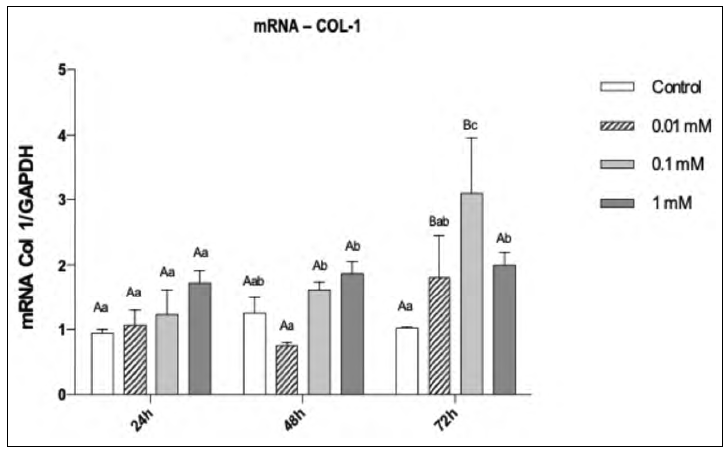
Fig. 6 mRNA expression for COL-1 in pre-osteoblastic cells as a function of MLT dose and time. Different uppercase letters represent a significant difference between the evaluation periods for each studied condition (ANOVA and Tukey, p<0.05); different lowercase letters represent a significant difference between the concentrations tested (ANOVA and Tukey, p<0.05).
For the factor "time", COL-I expression was highest at 72h for the doses 0.1 mM and 1 mM. This also occurred for OPN expression at 1 mM (p<0.05, Fig. 7).
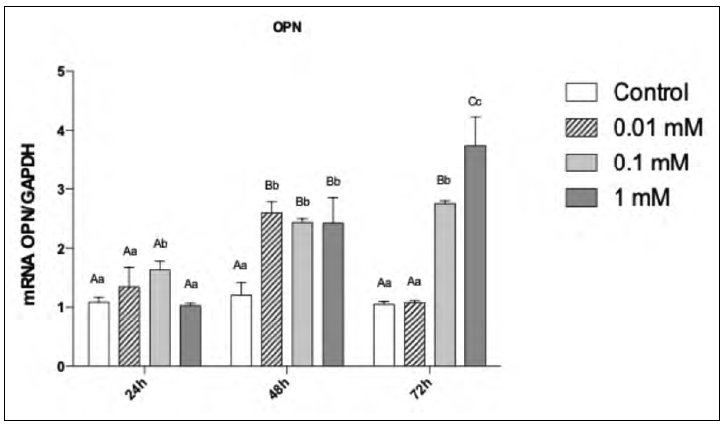
Fig. 7 mRNA expression for OPN in pre osteoblastic cells as a function of MLT dose and time. Different uppercase letters represent a significant difference between the evaluation periods for each studied condition (ANOVA and Tukey, p<0.05); different lowercase letters represent a significant difference between the concentrations tested (ANOVA and Tukey, p<0.05).
COL-I and OPN secretion (ELISA)
The 1 mM dose of MLT significantly increased COL-I secretion when compared to the remaining groups at 24h, 48h and 72h (p<0.05, Fig. 8). A very similar pattern was observed for OPN secretion (p<0.05, Fig. 9).
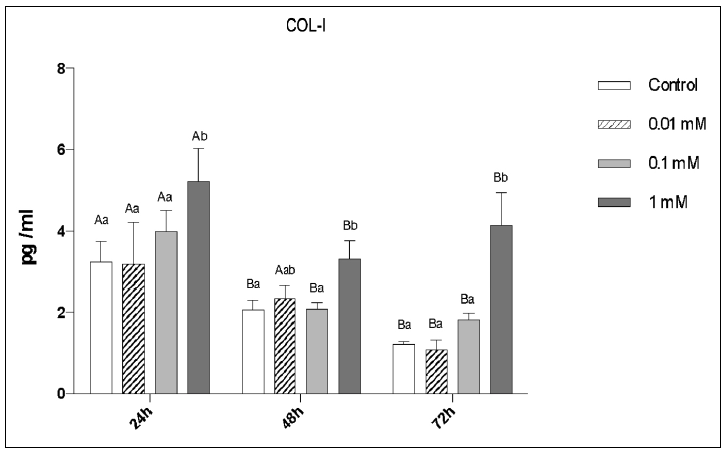
Fig. 8 ELISA for COL-I secretion by pre-osteoblastic cells as a function ofMLT dose and time- Different uppercase letters represent a significant difference between the evaluation periods for each studied condition (ANOVA and Tukey, p<0.05); different lowercase letters represent a significant difference between the concentrations tested (ANOVA and Tukey, p<0.05).
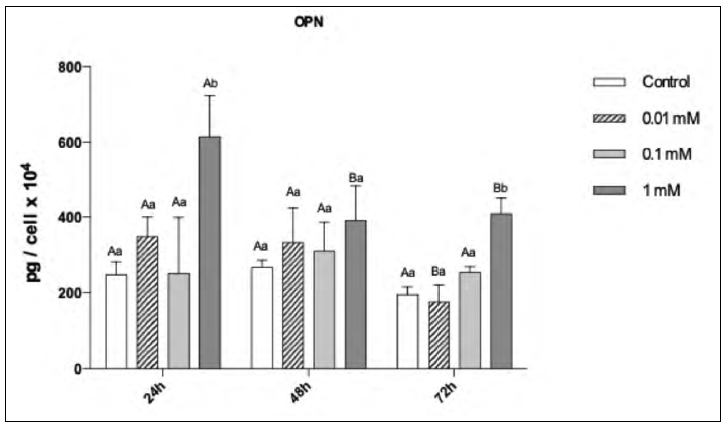
Fig. 9 ELISA for OPN secretion by pre-osteoblastic cells as a function ofMLT dose and time. Different uppercase letters represent a significant difference between the evaluation periods for each studied condition (ANOVA and Tukey, p<0.05); different lowercase letters represent a significant difference between the concentrations tested (ANOVA and Tukey, p<0.05).
DISCUSSION
This study investigated the role of MLT in bone metabolism using an in vitro experimental model based on osteoblast cultures for gene expression as well as secretion of key proteins involved in bone physiology, namely COL-I and OPN, over time17,10,12,24,25. MLT has been shown to stimulate proliferation and differentiation of human osteoblasts in vitro, as well as the synthesis of COL-I and other proteins of the bone matrix (OPN, OC). Additionally, it has been demonstrated that MLT has the potential to reduce the time of osteoblast differentiation and proliferation9,23,24 as a result of the increased expression of bone sialoproteins and enhanced ALP, OPN and osteocalcin activity10,18.
It has been demonstrated that the cytoprotective effects of MLT on osteoblasts are noted mainly at low concentrations and shorter times, using concentrations of 1, 10, 50, 100 and 200 mM for incubation periods of 24, 48 and 72h, showing the cytoprotective effect promoted by MLT in cells treated with bisfosfonate26,27,28,29. There is, however, great variability in MLT concentrations across the literature, ranging from 0.001 to 2.5mM in osteoblastic cells of the MC3T3 lineage9,10,18. In the present study, the higher concentrations used, 2.5mM and 5mM, proved cytotoxic, so they were excluded from the subsequent experiments. The 0.01, 0.1 and 1 mM concentrations were maintained. The time of 21 days is required to allow bone cells to proliferate, differentiate and mineralize in vitro,though considering the potential of melatonin to accelerate this process9, the times used in the present investigation were reduced to 10 days. MLT had a substantial effect on COL-I and OPN expression, including decreasing cell differentiation time to 5 -7 days15, which has guided the design of the present study to evaluate phenotypical features at earlier stages of bone neoformation (10 days as opposed to 14 days). The findings presented herein therefore demonstrate that MLT enhances the process of osteogenesis. This may be attributed to an increase in the expression of bone sialoproteins and alkaline phosphatase activity, activated by the presence of MLT10,24. The data obtained in the present study demonstrate that the metabolism of osteoblastic cells was positively affected by the MLT, mainly in relation to the calcium nodule count. On the 10th day, the alizarin red assay showed that all MLT concentrations were able to promote greater formation of mineral nodules than the control group, especially at the therapeutic dose of 0.1mM. Previous studies10,12,24 investigated the direct impact of MLT on pre-osteoblastic cells using increasing doses from 0.001mM to 2.5mM to determine its effects on metabolic activity, cell viability and gene expression of markers relating to osteoclastic activation. Subsequently, the best dose (0.1mM) was applied to assess mineralization, demonstrating that when evaluated using the alizarin red assay at 14 days, a greater formation of mineral nodules was observed in the group treated with MLT, in agreement with the results of the present study.
The alizarin red assay demonstrated the largest quantities of calcium nodules in the presence of 0.1mM MLT. The higher rate of mineralization in the group treated with melatonin at 0.1mM may indicate this as most beneficial in an in vitro context. In addition, higher doses can negatively interfere with cell mineralization, demonstrating that there is probably an ideal dose, in which the effect is enhanced, without being cytotoxic10,12,24.
In the present study, alkaline phosphatase activity was verified at day 10 and an increase in mineral nodules was observed for the concentration of 0.1mM MLT, in agreement with studies that suggest that the hormone may have a regulatory effect on bone growth26,27. Previous studies13,14,28 demonstrated that melatonin was able to regulate osteoblastic function via the MT2 receptor, which showed greater expression in bone during the experiments. In addition, inactivation of this receptor, leaving only MT1 receptor active, negatively affected cell differentiation, proliferation and deposition of the mineralized matrix, thus reiterating the promising role of melatonin as a potential strategy to stimulate bone neoformation.
In an in vivo study performed on a rodent experimental model of induced periodontitis19, treatment with MLT reduced serum COL-I and increased ALP levels, demonstrating that expression of essential proteins occurred early in the cell differentiation process, as a result of MLT treatment. MLT has an anabolic effect on bone tissue, enhancing mineralization of MC3T3 cells12. The MLT-treated cells showed a significant increase in COL-1 and OPN secretion20-22. COL-1 and OPN have important roles in organic matrix formation and structural integrity, and are considered key to the mineralization of bone tissue10,18,19. These findings were also observed herein for COL-I expression, which increased at 72h at 0.1 mM and 1mM of MLT, and for OPN expression at 1 mM, with concomitant increased ALP at 0.1 mM. All such findings are corroborated by studies performed elsewhere12,25, though a considerable variation in therapeutic doses is observed across such studies.
The present study has demonstrated a potentially beneficial effect of MLT on MC3T3 cells in the early stages of osteogenesis, both in terms of cell proliferation and viability using doses of 0.01 mM and 0.1 mM, respectively, in addition to up-regulating the expression of COL-I and OPN, especially at 72h of treatment and doses of 0.1 mM and 1 mM, respectively. Increased secretion of COL-I and OPN followed suit at 1 mM and 24 h of treatment, thus corroborating previous studies on the regulatory effect of melatonin on bone growth10,13,15,21,22,24,25. Meanwhile, several important issues remain to be addressed regarding the use of melatonin as a bone augmentation strategy. One of the most important is the lack of consensus regarding the most effective delivery mode, route of administration and dose30,25. According to the findings in the present in vitro study, MLT at doses ranging from 0.01mM to 1 mM had a stimulatory effect on pre-osteoblasts by increasing the synthesis and secretion of both OPN and COL-I, as well as mineralization, thus favoring the process of osteogenesis.