INTRODUCTION
Initial stages of periodontal diseases include gingivitis and incipient chronic periodontitis. Gingivitis is defined as an inflammation of the gingival tissue, while periodontitis involves the loss of attachment of periodontal tissues from the tooth and the loss of alveolar bone. Gingivitis is reversible, whereas regeneration after the destruction caused by periodontitis is not predictably achievable in humans1. Even though most periodontal diseases are known to occur in susceptible subjects, their onset also depends on colonization by bacteria and presence of bacterial products2. Lipopolysaccharide (LPS), a toxin produced by gram-negative microorganisms, can cause an inflammatory process, with the consequent damage in the tooth-insertion tissues3. In the host tissues, LPS stimulates phagocytes to increase the production of cytokines such as TNFα, IL-ip, IL-6, IL-10, IL-12 and IL-154. Those mediators are responsible for a large number of cellular events, including the synthesis of PGE25, a potent stimulator of bone resorption associated with loss of periodontal attachment tissues6. Additionally, in animals with periodontitis, PGE2 has been found to increase in the submandibular gland, where it seems to function as an inhibitor of salivary secretion7. Thus, although colonization by pathogenic microbes is the primary origin of damage in periodontal diseases, the host immune-inflammatory response plays a critical role in the development of tissue injury resulting in the loss of connective tissue and alveolar bone8.
The cellular and humoral response deployed in bacterial processes involves the endocannabinoid system (ECS)9,10, which is an intercellular communication network that includes receptors, endogenous ligands and a series of enzymes responsible for ligand synthesis and breakdown. Cannabinoid receptors type 1 (CBlr) and type 2 (CB2r) are the main specific cannabinoid receptors, whereas transient receptor potential vanilloid type 1 is the most throughly studied unspecific receptor11. According to the classical concept and even though it has been submitted to revisions, CB1 is highly expressed in the central nervous system and to a lesser extent, in peripheral tissues. Conversely, CB2 is expressed mainly in peripheral tissues and cells such as monocytes, macrophages, lymphocytes and bone cells12. However, the stimulation of ECS has been associated with anti-inflammatory effects through both CB113,14 and CB215 receptors. In previous studies, we have shown that in a six-week LPS-induced periodontitis model in rats, daily application of a CB2r synthetic agonist, HU 308, attenuated alveolar bone loss and reduced the level of inflammatory mediators in gums16.
The aims of this study were, firstly, to identify early alveolar bone damage in periodontal disease in order to establish initial stages of the disease in an experimental model, and secondly, to evaluate the effects of the treatment with a CB2 receptor agonist, HU 308, on the oral health of rats submitted to early periodontitis, and compare these effects with those found in the pattern of long-term illness.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (350 g) from the authors' own colony were kept in group cages in an animal room with a 12-hour light photoperiod (0700 to 1900), room temperature maintained at 22°C to 25°C, and free access to rat chow and tap water. The experimental procedures performed were approved by the Animal Care Committee of the Dental School of the University of Buenos Aires, Buenos Aires, Argentina and carried out in accordance with guidelines of the National Institutes of Health.
Time range studies
Since our previous studies had been based on a six-week lipopolysaccharide-induced periodontitis model, we intended to achieve a model ofperiodontitis with emerging damage. To this end, we conducted a first experiment based on temporal studies of lipopolysaccharide-periodontitis induction at 1, 3 and 6 weeks. For each term of induction, twelve rats were divided into two groups, each group containing six rats: 1) control rats, and 2) rats submitted to experimental periodontitis. Periodontitis was induced by injecting 20 pl of lipopolysaccharide (1 mg/ml) from Escherichia coli into the vestibular and lingual gingiva of the maxillary and mandibular first molars and into the interdental space between the first and second maxillary and mandibular molars (60 pl of lipopolysaccharide per tooth and 240 pl per rat at each time of injection). Gingival injections were all performed using a 13-mm 27-gauge microfine insulin syringe. Control rats received no injection during the experiments.
CB2 receptor studies
In a second experiment, eighteen rats were distributed into three groups of six rats per group: 1) control rats; 2) rats submitted to EP; and 3) rats submitted to EP and treated with HU 308, an agonist of CB2r. Periodontitis was induced as described for the preliminary studies, while control rats received no injection during the experiment. This protocol of injections was executed for a period of 3 weeks on days 1, 3, and 5 of each week, based on a previously described method14,17. Gingival injections were applied using a 13-mm 27-gauge microfine insulin syringe.
Topical treatment with a CB2 receptor agonist
HU 308 ([(1R,2R,5R)-2-[2,6-dimethoxy-4-(2-
methyloctan-2-yl) phenyl]-7,7-dimethyl-4-bicyclo [3.1.1] hept-3-enyl] methanol) was prepared by dissolving the powdered drug in 100% ethanol and diluting it in saline, containing approximately 1% ethanol, to obtain a final concentration of 500 ng/ml with which to treat animals.
The volume of each topical application was 200 pl per tooth, resulting in 800 pl per animal when treatment was performed. As mentioned above, the second experiment consisted of a 3-week study in which group 3 animals received a daily topical application of HU 308 at sites of LPS during the 3 weeks of the experiment. The optimal dose of HU 308 was based on previous reports using anandamide, but mainly using methanandamide, a selective synthetic agonist of CB1 receptor14,18, on oral tissues in vivo concomitantly with the dose response curves in these preliminary studies.
Macroscopic examination of periodontal bone loss: distance and width methods
Immediately after euthanasia of the rats, hemimandibles were resected, defleshed, and stained with 1% aqueous methylene blue to delineate the cementoenamel junction (CEJ) and the alveolar crest (AC)19. A stereomicroscope and a digital calliper were used to measure three buccal and three lingual/palatal distances (mesial, central, and distal), from the CEJ to the AC14. The sum of the three distances on each side of molars was used as a measure of alveolar bone loss (ABL) in millimetres. Thus, ABL was assessed on the buccal side, palatal/ lingual side and also as whole tooth of the first upper and lower molars. Mandibular alveolar process width was measured in the mandibular first molar area. Distance between a point located at the central root level of the buccal surface and another equally located on the lingual surface was measured in the mandibles in millimetres using a digital calliper. This examination was performed for all the groups in the first and second experiments.
Histological analysis
Hemimandibles on the opposite side to those used for macroscopic examinations were fixed in formalin buffer. After 3 days, they were decalcified in 10% EDTA pH 7 for 45 days. Then hemimandibles were dehydrated with ethyl alcohol and clarified with xylene. Finally, the sector containing the first molar of each decalcified hemimandible was embedded in paraffin at 56°C to 58°C. Under the stereomicroscope, sections oriented mesial-distally of each mandibular first molar were cut from the paraffin blocks using a microtome. Sections 5 pm thick were stained with haematoxylin and eosin (H&E), and histomorphometric evaluation was performed on digitized microphotographs using imaging software. Interradicular bone loss was evaluated by measuring the periodontal space height, plotting 10 equidistant lines between the alveolar crest and the cementum of the furcation zone. The length of the lines was measured and the mean value was calculated. Additionally, ABL was assessed by the following parameter: bone volume (B.Ar)/total volume (T.Ar) (%) = fraction of TV corresponding to bone tissue. T.Ar was taken as bone tissue plus bone marrow and periodontal ligament20. The remaining histomorphometric parameters were evaluated in the bone of the interdental septum located between the first and second lower molars. These parameters were: whole perimeter of the interdental septum, in pm; bone formation areas, in % as Ob.S/BS (osteoblast surface related to total bone surface); bone resorption areas, in % as ES/ BS (eroded surface related to total bone surface), and number of osteoclasts21,22, as N.Oc/mm, in the cervical third of the mentioned septum. Histological analysis was performed for all the groups in the second experiment.
Radioimmunoassay of PGE2
To determine PGE2 content, gingival tissue surrounding first molar was homogenized in 500 ml absolute ethanol and after centrifugation, supernatants were dried in a centrifugal vacuum concentrator at room temperature. Residues were then resuspended with buffer, and antiserum was used as described in Mohn et al.23. Sensitivity of the assay was 12.5 pg per tube. The cross-reactivity of PGE2 and PGE1 was 100%, but the cross-reactivity of other prostaglandins was 0.1%. The intra- and inter-assay coefficients of variation for PGE2 were 8.2% and 12.0%, respectively. The results were expressed in picograms of PGE per milligram of wet weight, since the protocol of PGE extraction from the tissue includes homogenization in ethanol, which interferes with protein determination. This evaluation was performed for all the groups in the first and second experiments.
Statistical analysis
Data were expressed as mean ± standard error of the mean. Results were evaluated by one-way analysis of variance followed by the Tukey multiple comparisons test for unequal replicates. Only the number of osteoclasts was analysed using the Kruskal-Wallis nonparametric test. All analyses were conducted with Prism software and differences with P values <0.05 were considered statistically significant.
RESULTS
Time range studies
• Alveolar bone loss: distance and width methods
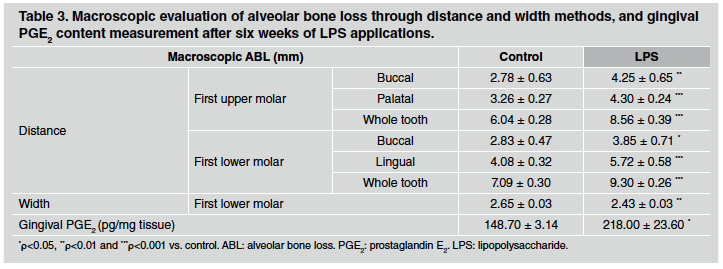
After 1 week of LPS injections, ABL measured by the distance method did not differ between control and LPS groups in either upper or lower first molars (Table 1). However, a significant alveolar bone loss was noticeable after 3 weeks of LPS induction, especially when whole teeth where assessed (Table 2). Furthermore, the differences described were stronger after 6 weeks of LPS induction, when both sides of upper and lower first molars as well as the whole tooth showed an increase in alveolar bone loss in LPS compared to control group (Table 3). ABL measured by the width method in the mandible did not reveal changes between control and EP group after 1 and 3 weeks of procedure. In contrast, the width of the lower alveolar process was significantly lower in the LPS than in control rats after 6 weeks of induction (Table 3).
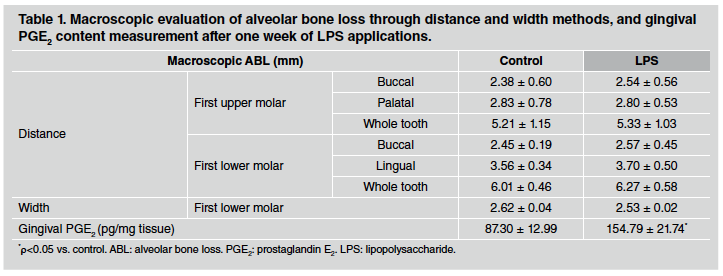
Table 1 Macroscopic evaluation of alveolar bone loss through distance and width methods, and gingival PGE2 content measurement after one week of LPS applications.
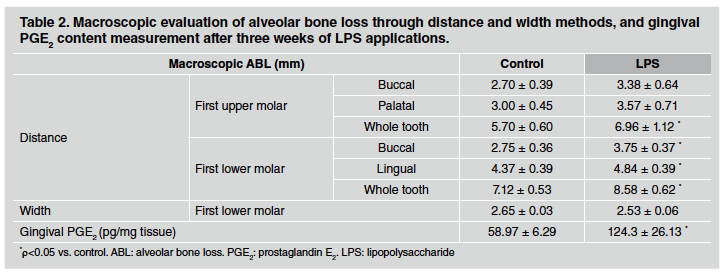
Table 2 Macroscopic evaluation of alveolar bone loss through distance and width methods, and gingival PGE2 content measurement after three weeks of LPS applications.
• Radioimmunoassay of prostaglandin E2
PGE2 content was evaluated as a marker of inflammatory response in gingival tissue surrounding the first molar. PGE2 content measured by RIA was markedly higher in gingival tissue after 1, 3 and 6 weeks of LPS injections in comparison to the control group. Results for 1, 3 and 6 weeks are shown at the bottom of Tables 1, 2 and 3, respectively.
CB2 receptor stimulation in early stageexperimental periodontitis rats - Three-week studies
• Alveolar bone loss: distance and width methods
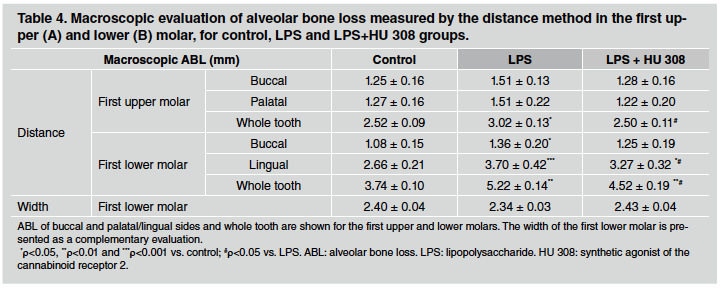
In early stage-experimental periodontitis rats, alveolar bone loss measured through the distance method did not show major changes among groups in the first upper molar when buccal and palatal sides were analysed separately. In contrast, the early-stage experimental periodontitis group experienced higher alveolar bone loss than control group at the lingual side of the first lower molar, whereas this increase was significantly prevented in HU 308-treated group (Table 4). The differences mentioned for the lingual side were not detectable on the buccal side of the mandibles, except for the difference between the control and LPS groups. In turn, when the whole tooth was evaluated, both upper and lower first molars evidenced the preventive effect of HU 308 treatment on alveolar bone loss, increased by lipopoly saccharide. In the same 3-week study, alveolar bone loss measured by the width method did not exhibit significant differences among control, early stage-experimental periodontitis and HU 308 early-stage experimental periodontitis groups in the lower first molar (Table 4).
• Histological analysis
In the analysis of the interradicular bone of the first lower molar, the early-stage experimental periodontitis and HU 308 early-stage experimental periodontitis groups exhibited an increase in the periodontal space compared to the control group, but did not differ significantly from each other after the 3 week-treatment (Fig. 1A and B). On the other hand, the evaluation of the alveolar bone area, represented as B.Ar/T.Ar (%), revealed that early-stage experimental periodontitis rats had a lower value than control rats, while that reduction was prevented, at least partially, by the HU 308 treatment (Fig. 1C).
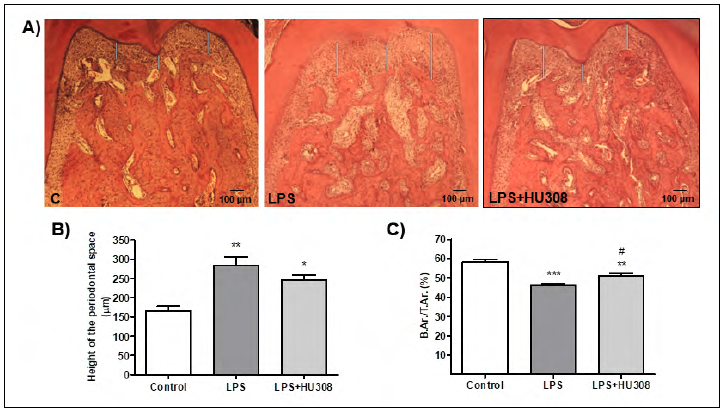
Fig. 1 A) Photomicrographs (H&E stain; original magnification x40) showing histologic features of the mandibular first molar interradicular area of rats subjected to different experimental conditions (control, LPS and LPS+HU 308). B) Periodontal space height evaluation. C) Interradicular bone measured as B.Ar/TAr (%). Results are presented as mean ± SEM. *p<0.05, **p<0.01 and ***p<0.001 vs. control; #p<0.05 vs. LPS.
In the analysis of the interdental septum bone, the early-stage experimental periodontitis group revealed lower whole perimeter of the interdental septum, less bone formation areas, more bone resorption areas and higher quantity of osteoclasts than the control group. However, HU 308 treatment reversed all altered records, showing longer perimeter of whole septum, more formation areas, fewer resorption areas and fewer osteoclasts than the early stage-experimental periodontitis group (Fig. 2).
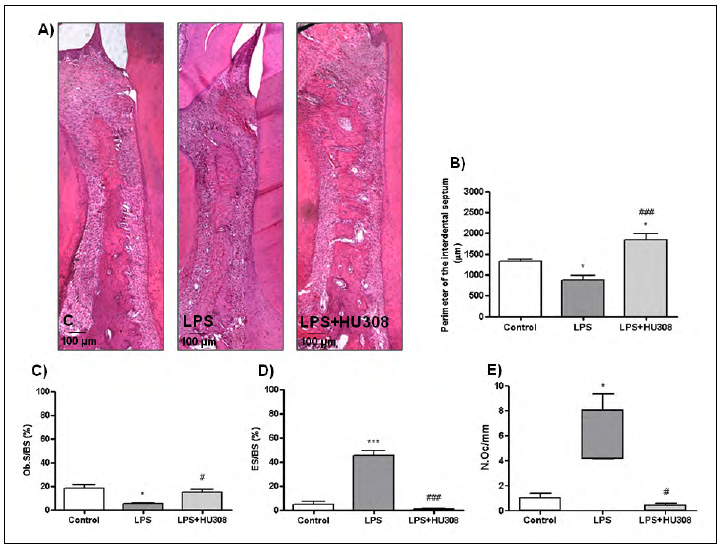
Fig. 2 A) Photomicrographs (H&E stain; original magnification x100) showing histologic features of the interdental septum bone located between the first and the second lower molars of rats subjected to different experimental conditions (control, LPS and LPS+HU 308). B) Whole perimeter of interdental septum bone expressed in pm. C) Bone formation surfaces expressed in % as osteoblast surface/total bone surface (Ob.S/BS). D) Bone resorption surfaces expressed in % as eroded surface/total bone surface (ES/BS). E) Number of osteoclasts expressed as N.Oc/mm. Results are presented as mean ± SEM. *p<0.05 and ***p<0.001 vs. control; #p<0.05 and ###p<0.001 vs. LPS.
• Radioimmunoassay of prostaglandin E2
Although early-stage experimental periodontitis caused a remarkable increase in prostaglandin E2 in comparison to controls, no significant difference was found between early-stage experimental periodontitis and HU 308-treated groups (Fig. 3). However, after the 3-week study period, a downward trend can be detected in the CB2r-agonist group with respect to early-stage experimental periodontitis.
DISCUSSION
Being one of the most prevalent disorders of the oral cavity, periodontal disease has been extensively studied in laboratory animals. To this end, endotoxin LPS-induced periodontitis has been a method which aimed to achieve a more physiological process of the spontaneous disease than traumatic methods24,25. The traumatic model consists of placing a ligature around molars and maintaining it, usually for two weeks, which produces a traumatic effect and facilitates dental plaque accumulation26. In the LPS-induced model, toxin reaches periodontal tissues and no traumatic effect is generated other than the needle injection. However, scientific literature has not reached a consensus with regard to the characteristics of this model or to the terms used for the induction. Indeed, different authors have run trials lasting 1 to 10 weeks24,25,27-30. Moreover, in previous studies we have demonstrated that LPS injections in first molar gums for six weeks produce a set of chemical and anatomical changes in periodontal tissues14,16,31. At this time, the increase in alveolar bone loss and inflammatory mediators such as TNFα, PGE2 and nitric oxide (NO) in gingival tissues indicate that EP has been established. Therefore, in the current study, we firstly focused on evaluating conditions of periodontal disease at several periods of time in order to clarify the emergence and severity of the damage at earlier terms than six weeks.
We found that no alveolar bone loss was induced by LPS injections after one week of the treatment, whereas after three weeks, the alveolar bone loss by the distance method was significantly higher in the LPS-injected group than in the control group. Furthermore, the difference between groups was even more pronounced after six weeks, when the alveolar bone loss was also evident from the diminished width. Regarding PGE2 gingival content, results showed variability in control values among different times assessed in separate experiments, which could be associated with the high volatility and complicated biology of PGE2, in part due to the high number of PGE2 synthesizer cells and their variable response to the presence of antigens32. Therefore, it is justified to analyse the results of each experiment separately. Despite these almost inevitable discrepancies, our results showed that gingival PGE2 content was significantly higher in LPS-induced periodontitis than in control rats at each assessed time.
Overall, these findings suggest that in early stages of LPS induction, even when alveolar bone damage is not clearly marked, gingival tissue expresses some sign of inflammation, a stage which could be related to gingivitis in human populations33. In addition, the first signs of alveolar bone loss at three weeks suggest that 3 weeks, or sometime between 1 and 3 weeks of LPS induction could be defined as the beginning of periodontitis in rats, though further studies are needed.
Since initial signs of alveolar bone loss were identified after three weeks, we subsequently assessed the effects of the HU 308 treatment at this time and considered it as an early stage of experimental periodontitis.
At 3-weeks, alveolar bone loss by the distance method increased on certain sides by LPS, was prevented in HU 308-treated rats. Some differences regarding control and LPS values between this study and the 3-week term assessed in the study time range might be caused by logical variability between experiments or due to random factors. In any case, distinct alveolar bone damage between control and LPS groups was also noticeable in this 3-week experiment, where even in group treated with HU 308, a mitigating effect emerged. In the histological analysis, the preventive effect of HU 308 on bone resorption was confirmed in the bone volume evaluation, even though a statistically nonsignificant tendency in the same direction could also be observed in the periodontal space evaluation. These results are consistent with those we found in the 6-week treatment, where these initial tendencies appeared to be strengthened. In fact, after six weeks, LPS-induced damage was clearer on both sides of the first upper and lower molars, while the HU 308 treatment efficiently attenuated it16. Furthermore, another study using in vitro human periodontal ligament cells demonstrated that LPS increases the concentration of IL-1β, IL-6, TNF-α, and RANKL, while HU 308 attenuates these parameters, normalizing bone metabolism34. On the other hand, histomorphometric measures on the interdental septum, such as perimeter of bone septum, bone formation areas, bone resorption areas and number of osteoclasts, lead to a scenario characterized by bone resorption in the LPS group and bone formation or resorption prevention in the HU 308-treated group. In this respect, in a recent study, Kamali et al. demonstrated the osteogenic effects of a scaffold based on cannabindiol, a natural cannabinoid, on bone defects in rats, employing histomorphometric measurements, among other techniques35.
PGE2 content evaluation in the gingival tissue showed a higher value in LPS-induced group with respect to controls, in agreement with the time range studies results. However, this increase was not significantly modified by the HU 308 treatment at three weeks, although a downward trend was evidenced. This result suggests that in a stage of emerging damage, HU 308 could start to show an incipient preventive effect, which is stronger and statistically significant at six weeks of induction20. Similarly, in our previous six-week study, other inflammatory mediators such as TNFα and NO, which were increased in gums by LPS-induced periodontitis, also showed the reducing effect of the HU 308 treatment. Thus, attenuation of PGE2 in gingival tissue reveals the therapeutic potential of the CB2r stimulation, providing a potential way to reduce inflammatory and immune responses as well as to prevent bone resorption, probably through the regulation of osteoblast and osteoclast activity36.
In conclusion, a 3-week term of LPS-induced periodontitis is a valid model of the early stage of the disease, since emerging damage is expressed in bone tissue, although it is not as clear as in a 6-week term. Additionally, we demonstrated herein that 3-week harmful effects could be prevented by local stimulation of CB2r with HU 308. Current outcomes shortly after the beginning of LPS-induced periodontitis also support the importance of initiating interventions in early stages of the