INTRODUCTION
The osseointegration process is the basis for the treatment of edentulism with dental implants. Osseointegration consists of bone tissue formation in direct contact with implant surfaces 1 , which enables the implants to withstand occlusal chewing forces predictably for long-term periods 2, 3 . All types of edentulism have been treated successfully with implant-supported prostheses 3, 4 , however, the presence of bone tissue in good quantity and quality for implant placement is not always observed 5, 6 . Guided bone regeneration (GBR) techniques have therefore often been used to increase the availability of bone tissue for implant placement 6, 7 .
Although autogenous bone graft is considered the gold standard bone substitute biomaterial 8, 9 , autograft can cause side effects related to donor site morbidity, and has limitations due to the limited availability and high resorption rates of this kind of graft 10 . This has promoted the use of alternative bone substitutes, especially deproteinized bovine bone (DBB), in guided bone regeneration techniques 7, 11 .
DBB is an osteoconductive bone substitute that is efficient for treating bone defects with high success rates and predictability in humans 12, 13 . Preclinical studies have shown that DBB presents low rates of resorption, which benefits the maintenance of the volume ofthe grafted area 14-16 . However, this property is related to the reduction of bone formation observed in areas grafted with DBB 14, 17 . The impact of this reduced bone formation in the grafted area on the osseointegration process has been little explored. The objective of this preclinical study was to compare the osseointegration of implants placed in areas of native bone and DBB grafted area in rat tibias.
MATERIALS AND METHODS
This study was submitted and approved by the Animal Ethics Committee of our institution (CEUA: 26/2016). Twenty-eight rats (Rattus novergicus, Hotzman variation), 12 weeks old, weighing 250-300 g, were used. The animals were kept in an environment with controlled temperature (21 ± 1°C), humidity (65-70%), and light cycles (12 hours). They were offered water and food ad libitum. This study was conducted according to the ARRIVE protocol for preclinical studies.
Groups and study design
The animals were randomly assigned to 2 groups of 14 animals each, according to the type of substrate where the implants were to be placed: NB Group -implants were placed in native bone; DBB Group: implants were placed in areas previously grafted with Deproteinized Bovine Bone (Bio-Oss®, Geistlich AG, Wolhusen, Switzerland - Small granules 0.25-1mm). The bone defect was performed and grafted with the DBB 60 days before implant placement. At baseline, the implants were placed directly in the native bone (NB) or in the areas grafted with DBB. After 15 or 45 days, the animals were euthanized by anesthetic overdose (Fig. 1).
Surgical procedure - Bone defect and grafting procedures
The animals in the DBB group were anesthetized by a combination of Ketamine (Agener Uniao Ltda, Sao Paulo, SP, Brazil) at 0.08 ml / 100g body mass with Xylazine (Rompum, Bayer SA, Sao Paulo, SP, Brazil) at 0.04 ml / 100g body mass.
An incision was made in planes over the tibial tuberosity. The bone tissue was submitted to osteotomy by means of a counter-mounted spherical drill with the aid of a 1200 rpm electric motor (BLM 600 - Driller, Sao Paulo, SP, Brazil) under abundant irrigation with sterile saline solution. The final measurements of the defects formed were 4mm in length and width, and 1.5mm in depth, and they were subsequently filled with DBB. The tissue was sutured by planes internally with 5.0 resorbable thread (Vicryl Ethicon, Johnson & Johnson, Sao Jose dos
Campos, Brazil) and extemally with 4.0 silk thread (Ethicon, Johnson & Johnson, Sao Jose dos Campos, Brazil). The animals received a single intramuscular dose of streptomycin-associated penicillin at 0.1 ml / kg (Multibiotic Small, Vitalfarma, Sao Sebastiao do Paraíso, MG, Brazil) and 0.1 ml / kg ketoprofen (Ketoflex; Mundo Animal, Sao Paulo, Brazil).
Surgical procedure - Implant Placement
After 60 days, the animals in both groups were subjected to implant placement in the NB and DBB areas. An incisión similar to the first procedure was made over the tibial tuberosity in right and left tibias. The grafted region was prepared for implant placement by applying a progressive sequence of drills (spear drill; 2.0 mm spiral drill - Neodent®; Curitiba, PR, Brazil) to accommodate a machined surface implant 4 mm high and 2.2 in diameter (Neodent®; Curitiba, PR, Brazil). All drilling was performed with the aid of an electric motor, adjusted to 1200 rpm, under abundant irrigation with sterile saline solution. The implant was installed with the aid of a digital key (1.2mm hexagonal digital key - Neodent, Curitiba, PR, Brazil). The tissue suture and the postoperative drug protocol were similar to those used in the first surgery. The animals in the native bone group were only subjected to surgery for implant placement with the same surgical and post-surgical protocols. The right tibia was used for microtomographic and histomorphometric analysis, while the left tibia was used for biomechanical analysis.
Biomechanical Evaluation
After euthanasia, the left tibias were stabilized in a small vise. A hexagon wrench was attached to both the implant and torque wrench (Tohnichi, model ATG24CN-S, Tokyo, Japan) and a counterclockwise movement was performed to unscrew the implant. The maximum torque required to move the implant was noted as the removal torque value (Ncm).
Microtomographic evaluation
The right tibias were fixed in 4% paraformaldehyde for 48 hours and stored in 70° alcohol. These samples were scanned by micro CT scan (Skyscan, Aatselaar, Belgium) with the following parameters: Camera pixels: 12.45; x-ray tube power: 65 kVP, x-ray intensity: 385 pA, integration time: 300 ms, filter: Al-1 mm and voxel size: 18 pm3. The images were reconstructed, spatially repositioned and analyzed by specific software (NRecon, Data Viewer, CTAnalyser, Aatselaar, Belgium). The region of interest (ROI) was defined as a 0.5 mm circular region around the entire diameter of the implant. This ROI was defined as Total Volume (0.5mm margin around implants - 4.5mm x 3.2mm). The threshold used in the analysis was 25-90 shades of gray, and the volume values of mineralized tissue around the implants (BV/TV) were obtained as a percentage 18 . A trained examiner blinded to the experimental groups performed this analysis.
Histomorphometric evaluation
After scanning, the right tibias were dehydrated in a staggered ethanol solution (60 - 100%) and embedded in light-curable resin (Technovit 7200 VLC, Kultzer Heraus GmbH & CO, Wehrheim, Germany). The blocks containing the implant and bone tissue were cut at a central point using a disposable system (Exakt Apparatebeau, Hamburg, Germany). The final sections were approximately 45 pm thick. They were stained with Stevenel’s blue associated with acid fuchsin and analyzed under an optical microscope (DIASTAR - Leica Reichert & Jung products, Wetzlar, Germany) at 100X magnification. Histomorphometric evaluation was performed using image analysis software (Image J, San Rafael, CA, USA). The percentages of bone-implant contact (% BIC) and bone area between implant turns (% BBT) were evaluated separately in the first three threads. These analyses were performed by a blind, trained examiner.
Statistical analysis
GraphPad Prism 6 software (San Diego, CA, USA) was used for the statistical analysis. The data generated by the histometric, microtomographic and biomechanical analyses were numerical, so they were submitted to the Shapiro-Wilk Normality test to evaluate whether they were distributed according to the central distribution theorem. All data distributed according to the normallity. Then, the parametric unpaired t-test were used for the inferential analysis. All tests in this study were applied with a significance level of 95%. The sample size calculation was referenced to % BIC data from a previous study that evaluated the effect of an implant surface osseointegration in grafted areas in a similar experimental model and assessment as performed in this study 19 . Considering that the smallest difference between the means in the groups where there were statistically significant differences was 19.29% with standard deviation difference between these groups 6.59%, it was found that a sample of 7 animals per group / period was sufficient for application of statistical tests with type a error set at 0.05 and P power of 0.90.
RESULTS
All animals survived after the surgical procedures and were healthy throughout the experimental period.
Removal torque analysis
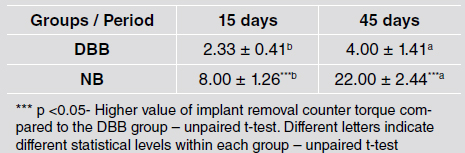
Removal torque increased in the longer evaluation times in both groups (p<0.05). The implants placed in DBB-grafted areas presented lower removal torque values than implants placed in native bone at both evaluation times (8.00 ± 1.26 Ncm vs. 2.33 ± 0.41 Ncm at 15 days and 22.00 ± 2.44 Ncm vs. 4.00 ± 1.41 Ncm at 45 days) (p <0.05) (Table 1).
Micro Ct analysis
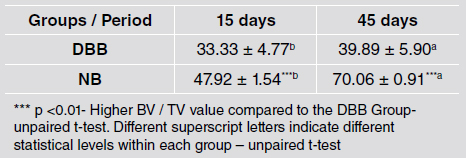
The BV/TV around the implants was higher at 45 days than at 15 days for both groups. Implants placed in NB areas presented higher BV/TV values than implants placed in DBB grafted areas at both times (47.92 ± 1.54% vs. 33.33 ± 4.77% at 15 days and 70.06 ± 0.91% vs. 39.89 ± 5.90% at 45 days) (p <0.01) (Table 2).
%BIC and %BBT analysis
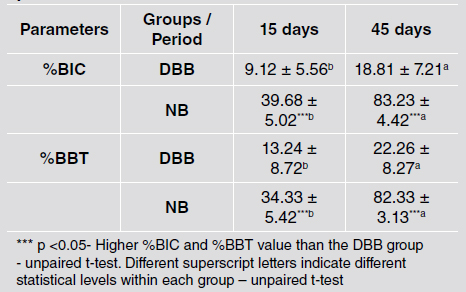
Histometric analysis showed that the degree of osseointegration improved at 45 days compared to 15 days for both groups. However, the implants placed in DBB grafted areas presented lower %BIC (39.68 ± 5.02% vs. 9.12 ± 5.56% at 15 days and 83.23 ± 4.42% vs. 18.81 ± 7.21% at 45 days), and %BBT (34.33 ± 5.42% vs. 13.24 ± 8.72% at 15 days and 82.33 ± 3.13% vs. 22.26 ± 8.27% at 45 days) values than implants installed in NB at both evaluation times (p <0.001) (Table 3). Fig. 2 shows representative histomorphology images.
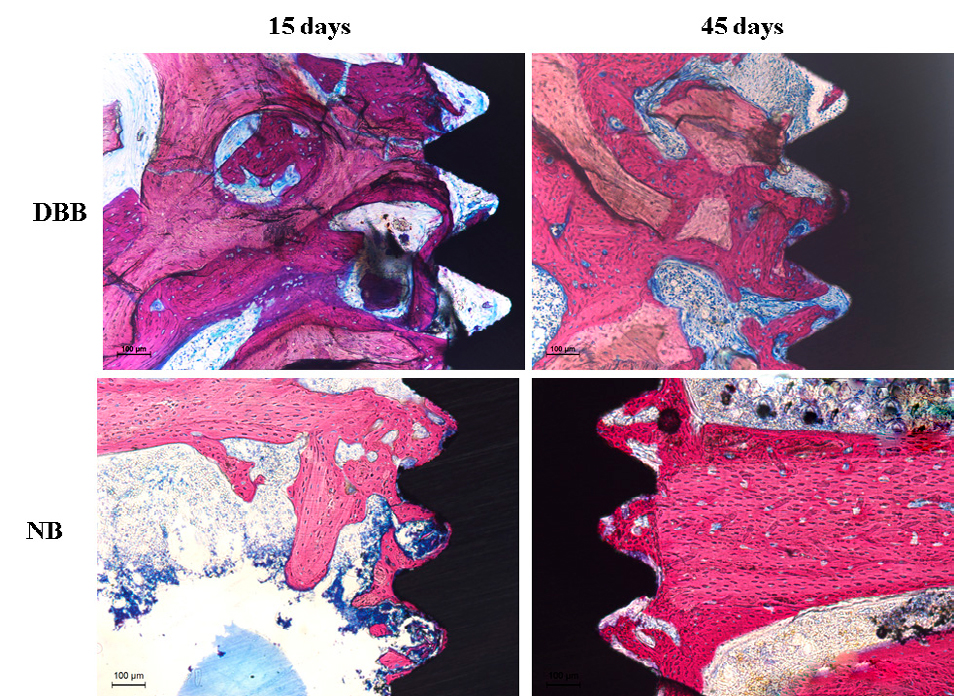
Fig. 2 Representative images of the non-decalcified sections showing a better pattern of the osseointegration of implants placed in NB than of implants placed in DBB.
DISCUSSION
In some clinical conditions, limited bone availability for direct implants warrants grafting procedures 7 . Due to the limitations of autogenous bone grafts 10 , the use of osteoconductive bone substitutes has become more commonplace 11 . Placing implants in areas grafted with osteoconductive biomaterials can improve implant success rates 20 . Although previous systematic reviews have shown that DBB induces sufficient bone formation for implant installation with high success rates 21, 22 , some clinical studies have shown that implants placed in areas grafted with DBB presented relatively lower success rates 13, 20 than implants placed in native bone 23 . In fact, it is likely that the lower formation of bone tissue associated with the presence of biomaterial particles that are included in the matrix, but remain non-vital, may influence the resistance of these grafted areas to microbial challenges, as well as reducing the osseointegration process. In general, this study demonstrated that implants placed in areas grafted with DBB presented worse parameters than implants placed in the native bone area in all the analyses performed to evaluate the osseointegration process.
The implants placed in areas grafted with DBB had lower removal torque values than implants placed in native bone area. When dental implants are placed in grafted areas, it is recommended clinically to lock the apical portion of the implants in native bone in order to ensure good primary stability and avoid having a large portion of the implant remaining within the grafted area 24 . In the experimental model used in this study, two thirds of the implant were within the grafted area 25 . Thus, the experimental model used may explain the poor result of secondary stability achieved by the implants placed in the DBB group.
Moreover, the smaller amount of mineralized tissues around the implants placed in the grafted areas may also have had a negative influence on the biomechanical parameters of the DBB group in this study. The BV/TV data recorded in this study contradict other studies that have reported that areas grafted with DBB have good properties for maintaining volume and filling bone defects 15, 26 . However, it should be noted that in this study, the comparison was performed with the native bone of the tibia, which has cortical morphology, certainly influencing the BV/TB results for the NB group. It is also likely that a good part of the tissue repaired in the grafted areas with DBB in its coronal portion is soft tissue. Since a membrane was not used to cover the defects, the more coronal DBB particles may have been involved by fibrous connective tissue, a finding that has been described previously in a preclinical study evaluating the healing of post-extraction sockets filled with DBB in dogs 15 . Another interesting finding in this study was that the data from the histometric analysis (%BIC and %BBT) were also lower in implants placed in areas grafted with DBB than in implants placed in a native bone area. These results agree with histological findings from another preclinical study that demonstrated that implants placed in edentulous canine jaws previously grafted with DBB presented higher osseointegration with the native lingual bone crest than with the buccal bone crest contained the grafted area 26 . In addition, implants placed in mini-pig maxillary sinuses that were grafted with autogenous bone also showed a higher degree of osseointegration than implants placed in maxillary sinuses grafted with DBB (42.9% vs. 13.9%) 27 . The data from the current and the abovementioned studies showed that the reduced bone formation in areas grafted with DBB has a negative influence on the osseointegration process in these areas.
Despite the findings of the current study, the limitations regarding the use of autogenous bone graft and in healing critical defects commonly present in the oral cavity do not contraindicate the use of DBB as a bone substitute material. It is worth mentioning that DBB has been applied with great success in different clinical situations, such as in the maintenance of post-extraction dental sockets 28 , maxillary sinus lifting 29 , and in augmentation of vertical and horizontal bone tissue in edentulous edges 30 . However, the waiting time for implants placement in the grafted areas and the application of prosthetic loads should be performed later in grafted areas when the implants could not be placed immediately in a good amount of native bone 20 . In addition, supportive therapy in these regions should be performed more frequently than with implants that have been installed in areas of native bone 31 . Finally, the search for associations of growth factors that can improve the pattern of bone tissue formation in areas grafted with DBB should be investigated. The current study has some drawbacks that should be considered for the interpretation of our findings. It used implants with untreated surfaces, which are rarely used in daily clinical practice. The pattern of osseointegration in grafted areas is better when treated surfaces are used 19 . Another important limitation is that these results are more applicable in situations of previously grafted and healed alveolar ridges, and these findings are not applicable to clinical situations where the implants are placed immediately. On the other hand, it is possible to infer that the prosthetic loading protocols for implants placed in grafted areas with DBB may be