INTRODUCTION
Several strategies are currently available for the prevention and control of the tooth decay process. Dentists must base their decisions on the available evidence, as well as on the characteristics and needs of the injury and the patient himself/herself. The treatment of caries lesions has changed over the years, following an increasingly conservative and less invasive trend in order to avoid the restorative cycle and preserve dental tissue 1-2 .
Silver diamine fluoride (SDF) was originally formulated in Japan at 38% concentration (w/v) under the brand name Saforide®. This original formulation was presented in its composition AgF(NH3)2, with concentrations of 44,800 ppm F (μg/mL), 253,870 ppm Ag, and alkaline pH 3 .
The only FDA-approved SDF product in the United States (Advantage Arrest™ Silver Diamine Fluoride 38%) is a colorless topical agent composed of 24.4-28.8% (w/v) silver and 5.0-5.9% fluoride, with pH 10 4 . It is mainly used for lesión control, being an efficient, affordable, safe product, with good results reported by several laboratory and clinical studies 3,5, 6 . In addition to preventing caries, SDF stops the caries process efficiently 7 .
SDF was introduced for the treatment of cavities in both primary and permanent teeth, although its use was not frequent. However, it is currently being increasingly adopted as a strategy 8 . SDF inhibits demineralization, promoting the remineralization of enamel and dentin. The SDF concentrations available on the market range from 12% to 38% (80,170 to 254,000 ppm Ag ion concentration), with alkaline pH 5, 9-11 . The SDF mechanism is based on the combination of silver and fluoride in an alkaline solution, which results in a synergistic effect with the ability to arrest tooth decay, where in addition to inhibiting demineralization, it preserves the degradation of dentin collagen. SDF can react with calcium and phosphate ions to form fluorohydroxyapatite with reduced solubility, thus being one of the main factors in stopping carious lesions 12-14 . When SDF solution is applied to a dentin lesion, the fluoride ions interact with the free calcium ions in the hydroxyapatite, forming calcium fluoride (CaF2) and fluorapatite (CalO (P04) 6OH2-xFx). Then, the silver ions interact with the free phosphate and form a layer of silver phosphate deposits (Ag3PÜ4) on the treated surface and the dentinal tubules. In addition, SDF fluoride strengthens the dental structure of acidic by-products of bacterial metabolism, decreasing its solubility, and silver interacts with the cell membrane and bacterial enzymes, inhibiting microorganism growth 13, 15 .
Topical application of SDF is considered a non-invasive treatment for caries lesions, being an effective, easy, low-cost, painless option for the patient. SDF has been used successfully for controlling carious lesions in children is because even when access to dental services is available, traditional restorative treatment can be complicated 9,13, 15 . In addition, decayed dental tissues do not need to be removed before applying the SDF, which makes the process faster 14 . Another advantage of the SDF is that it can prevent and paralyze both coronary and root carious lesions, providing an effective option for socially vulnerable patients 9, 13 .
It is essential to assess the stability of the reagent in the SDF Solutions. In 2012, the first study was published evaluating two Brazilian brands and one Japanese brand of SDF solution 16 . Other studies have continued to assess the fluoride concentration in commercial SDF solutions3,11,17. Because there is a lack of information about Argentine brands, the aim of this study was to measure the pH, fluoride (F) and silver (Ag) ion concentrations, and short-term stability of 2 solutions of 38% silver diamine fluoride (SDF) manufactured in Argentina.
MATERIALS AND METHODS
Sampling
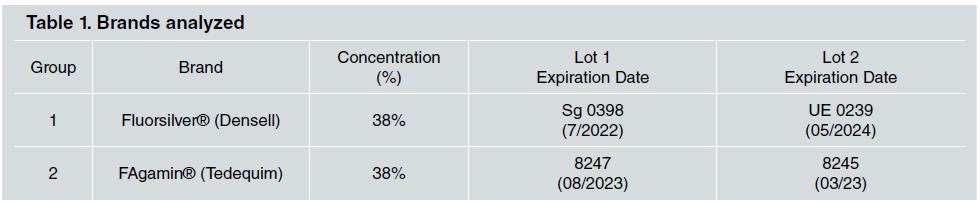
The two brands of SDF solutions manufactured in Argentina, Fluorsilver® (G1) and FAgamin® (G2), were used in this study. Two different lots of each brand were purchased and coded. In the sequence, the analyses were performed after randomization using Microsoft Office Excel to enable blind assessment ( Table 1 ).
Experimental design
The samples were analyzed by a prívate laboratory accredited by OAA (Argentine Accreditation Ageney as Le 209 test laboratory, complying with IRAM-ISO/IEC17025 Standards.)
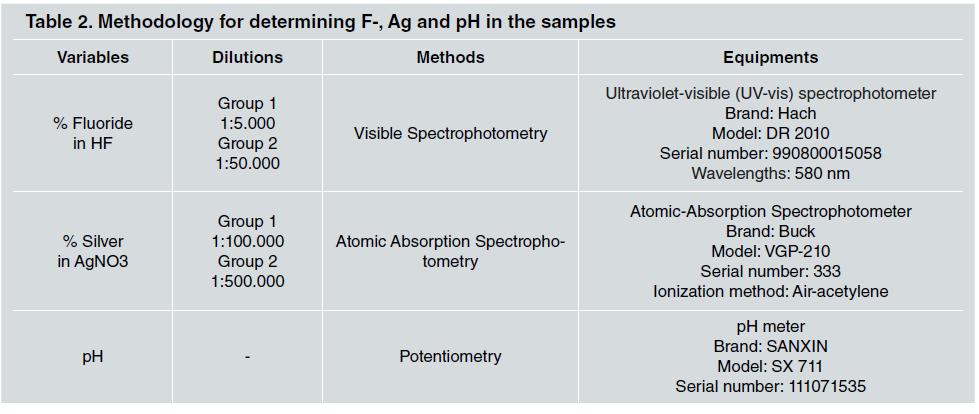
For the determinations, 10 ml of the solution were used for each bateh. To determine the dimensional stability of the groups, the following variables were evaluated: Ag concentration (% w/v) using Atomie Absorption Spectrophotometry; Fluoride concentration (% w/v) using Visible Spectrophotometry, and pH using potentiometry ( Table 2 ). All the equipment was calibrated with standard solutions before the analysis using a standard curve with a correlation coefficient of r > 0.99.
The SDF solutions were evaluated at baseline (day 0) and 30 days after they were opened (day 30).
The results were expressed in % w/v (1% w/v = 10,000ppm).
Determination of acidity, and fluoride and silver ion concentration
For the fluoride and silver concentration analyses, the samples were diluted (see Column 2 in Table 2) so that their concentrations corresponded to the ranges covered by their respective calibration curves (Lambert and Beer law compliance).
As a quality control of the results, the recovery of a solution of known silver concentration, added to the samples, was measured, complying with the recovery values found with the quality standards.
The pH was measured directly, without diluting. Sample storage: After opening, the samples were stored for 30 days in their original packaging in the dark at room temperature.
DISCUSSION
There is undoubtedly renewed interest in the use of SDF Solutions, especially since the COVID-19 pandemic triggered a search for procedures that generate the least amount of aerosols possible 18, 19 . The advantages of using SDF solutions are that application time is fast, without the need for cavity preparation, and the product is effective in primary and permanent dentition, making it an important resource, especially for public dental healthcare 19-21 .
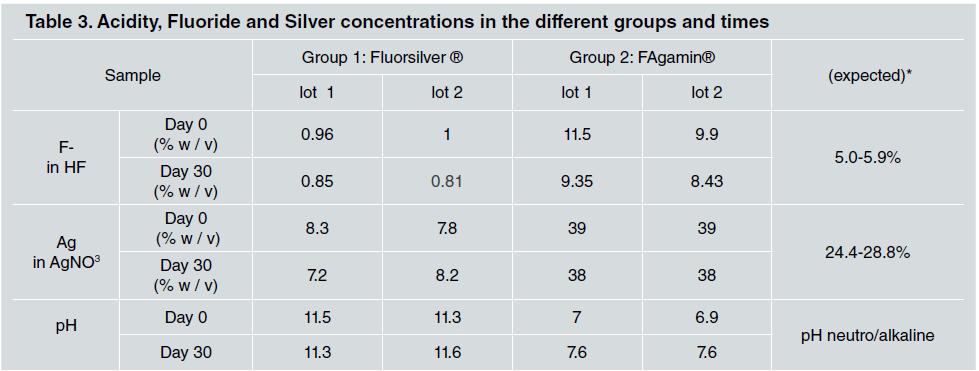
For SDF solutions to be stable, their pH must be alkaline. However, fluoride bioavailability is higher at low pH valúes, so to compénsate for this, fluoride concentrations are increased 3 . In this study, the pH remained basic in G1 and neutral in G2, in both periods evaluated. The values obtained in this study for both brands may compromise their therapeutic activity. Several studies have evaluated fluoride concentration in products for personal and professional use, but only four studies in the literature deal with SDF3,16-18. Soares-Yoshikawa et al. 3 evaluated the concentration and pH of six 12% to 30% SDF formulations marketed in Brazil and their bioavailability with demineralized dentin, using ion-selective electrode (ISE) and pH strips. All the formulations had concentrations different from those reported by the manufacturers. In the analysis, the concentrations of fluoride and silver ions detected were different from the concentration values declared by the original *Saforide solution and those published by Advantage Arrest™. Several studies have shown that the concentrations of free F and Ag ions in SDF solutions change over time 16, 17 .
Patel et al. 18 analyzed one of the SDF brands manufactured and marketed in Argentina, FAgamin finding the valúes F- 120,760ppm/Ag 258,000, which are similar to the findings in the current study. Crystal et al. 17 studied the stability of F and Ag in SDF solutions >and found that F concentrations increase slightly over time due to water evaporation. At 28 days, the pH of the product is stable, while the fluoride content tends to increase and the silver content tends to decrease. Another important point is that manufacturers do not disclose all the ingredients in their SDF products, so the ingredients of different SDF brands may vary and influence the results, as reported by Mei et al. 13 .
In the present study, measured and expected fluoride concentrations differed from the expected values in all samples, with smaller variations in Group 2. Fluoride concentrations were 20% of the expected value in Group 1, and double the expected value in Group 2. Fluoride concentrations decreased over time in both groups.
A concentration of 38% is sufficient to prevent caries progression. However, this requires guaranteed product quality 3 . The current study is the first to evaluate SDF formulations made in Argentina, which comprise only two brands.
Limitations to the study include the small number of samples and the short evaluation period. The results reinforce the knowledge that there is a need for greater rigor by regulatory institutions in order to prevent the marketing of products that are ineffective for managing dental caries. Future studies on a larger number of samples and tests that assess the anti-caries action, comparing the different brands, are suggested.
CONCLUSION
Over 30 days, the pH remained stable, while the fluoride and silver content tended to decrease. The F and Ag values found differed from those expected, and fluoride ion concentration changed over the 30-day period in both brands.
These findings point to a failure in quality control in these products, which could compromise their anticaries effect.