INTRODUCTION
In Argentina, sunflower oil (SFO) is commonly used for frying and is consumed in large quantities, fried potatoes being the most consumed deep-fried food 1 . Fried food consumption contributes to the development of chronic diseases such as obesity 2 , atherosclerosis 3 , and liver damage 4 . It also contributes to deterioration of bone histomorphometric parameters 5 and altered mechanical strength of femoral diaphysis to external loading 6, 7 . The adverse effects of diets containing fried sunflower oil (SFOx) on total skeleton bone mineral content (BMC) are more evident on growing rats 8 .
Bone with a faster turnover rate (modeling) could reveal potential adverse effects of inappropriate types of fat intake on the skeletal and biomechanical bone properties of a growing animal. Nutritional intake is known to affect the mechanical properties of the appendicular and axial bones.
Our previous studies indicated that growing animals fed a diet rich in olive oil or high-oleic-sunflower oil (HOSO) exhibited negative mechanical strength of femoral diaphysis in response to external loading, compared to commercial rodent diet. Moreover, HOSO with phytosterols or fish oil did not reverse the detrimental effects on femur biomechanical competence 9 .
Mandible quality depends on diet intake and gender 10 . Our previous studies demonstrated impaired mechanical mandible competence in rats fed a suboptimal restricted diet 11, 12 and a diet containing inadequate quality or quantity of protein 13 . However, to our knowledge, there are no studies evaluating the effect of different types of dietary fat and the biomechanical competence of the mandible during growth.
The aim of the current study was to assess the effects of two oil diets on mandible morphometric properties and biomechanical competence. The study diets contained either SFO or SFOx were compared with a control diet (C). The three diets had similar viscosity and masticatory loading capacity since the mandible is a load-bearing bone that differs from other bones in the axial skeleton. It is influenced by mechanical masticatory loading, which rapidly impacts the mass density and microarchitecture of the mandibular alveolar bone 14, 15 . The importance of this study is the detrimental effect of consuming diets rich in fried oils on bone health during growth.
MATERIALS AND METHOD
Animals
The Animal Resources of the Department of Biochemistry, School of Dentistry of the University of Buenos Aires provided the male weaning Wistar rats for the study. The twenty-one rats, weighing 53.8 (42.9-64.7) g, were housed under standard conditions (light-dark 12:12 hours, 21±1oC and 50-60% humidity) in individual steel cages, following the Principles of Laboratory Animal Care established by the National Institute of Health (NIH), USA Ethics Committee.
The protocol for this animal study was approved by the Animal Research Committee of the School of Dentistry, University of Buenos Aires (Approval number 004/17).
The mothers’ nutritional status was adequate to factor directly associated with bones and body development.
Diets
The composition of the three diets consumed in the experiment is shown in Table 1. A modified commercial stock Purina chow (Gilardoni SA, Buenos Aires, Argentina) was used as the control diet (C). Chow was composed of vegetal-based ingredients such as soybean flour, corn gluten, wheat flour and wheat bran, and animal-based ingredients such as fish and meat flour. The main sources of fat in the C diet were corn oil and fish oil (Table 1).
Table 1 Diet composition
|
INGREDIENTS (g/100g) |
C |
SFO |
SFOx |
|---|---|---|---|
|
Starch |
48 |
30.7 |
30.7 |
|
Protein (mix of corn, wheat, soybean, fish, and meat flour) |
23 |
14.7 |
14.7 |
|
Fat (acid hydrolysis) (mix of corn oil and fish oil) |
7.0 |
4.5 |
4.5 |
|
SFO |
13 |
||
|
SFOx |
13 |
||
|
Other source of fats: Cholesterol (ppm) Linoleic acid Linolenic acid Arachidonic acid Omega-3 fatty acids Total saturated fatty acids Total monounsaturated fatty acids |
200 1.3 0.11 <0.01 1.90 1.89 1.98 |
128 8.79 0.08 0.03 1.98 2.53 4.88 |
128 6.91 0.07 0.04 1.97 3.57 5.58 |
|
Total polyunsaturated fatty acids |
3.32 |
10.9 |
8.99 |
|
Fiber Minerals |
6.0 6.0 |
3.8 3.8 |
3.8 3.8 |
|
Vitamins mixture Gelatin Water Total kcal |
1.5 3.0 5.5 359 |
0.96 3.0 23.5 351.1 |
0.96 3.0 23.5 351.1 |
The experimental diets were supplemented with either fresh sunflower oil (SFO) or fried sunflower oil (SFOx), thereby differing in fat concentration from the C diet. All diets were similar in particle size. The three diets were prepared by adding 3% gelatin to prepare cubes with similar viscosity and consistency 16 based on the rheological properties of several hydrogels. The ingredients of each diet were crushed and mixed, and the homogenized mixture was transferred to a press. The diets were cut manually into a similar size as the commercial chow. It was important to ensure similar consistency for all three diets because consistency affects the masticatory apparatus by producing high frequency loads of variable magnitude on the teeth and jaws. Jaws differ from the appendicular skeleton because mandibular loads cause complex patterns of bone deformation during normal function, as a result of the diverse force vectors acting on them. These forces produce bone modeling and remodeling, ultimately shaping the adult jaw and providing mechanically fit morphology. During the power stroke of mastication, maximal muscle activity and bone strain occur 17 , affecting mandibular bone mass, quantity and density, as well as mandibular length and width.
Lipids provided 17.5 % of the calories in the C diet, and 45% of the calories in the SFO and SFOx diets. SFO and SFOx were mixed with the rat chow at 13% (w/w) of diet. The oil compositions of the experimental diets were as follows: (1) SFO composition: n-6 Polyunsaturated Fatty
acids (n-6 PUFA): 61.25%; n-3 PUFA: 0.07%; Monounsaturated Fatty acids (MUFA): 27.8%; Saturated Fatty acids (SFA): 10.1%; Trans fatty acids (TFA): 0.7%; (2) SFOx composition: n-6 PUFA: 46.78%; n-3 PUFA: 0%; MUFA: 33.15%; SFA: 18.09%; TFA: 1.66%. The diets contained calcium carbonate anhydrous at 40.04%, potassium phosphate monobasic at 22.76% and Vitamin D-3 (400 000 IU/g) (AIN-93G); 0.6 mg alpha-tocopherol equivalents/g PUFA were added to the high fat diets, as recommended by Valk and Hornstra 18 .
Diets were prepared on alternate days and kept refrigerated. Food intake was measured (Mettler scale PC 4000; accuracy±1 mg) and the amount of diet consumed was expressed in grams per rat and per week (g/rat/wk) and as kcal per 100 g of body weight per day (kcal/100 g W/day).
Frying Procedure
The repeated frozen potato frying procedure was performed in standard commercially available 8L deep-fat fryer pots heated for 6 hours per day for a total 40 hours of frying. Sunflower oil oxidation was measured as previously described 8 . Polar compounds (triacylglycerol polymers, dimers, monomers, and oligomers, oxidized triacylglycerols, diacylglycerols and non-esterified fatty acids) were analyzed using a Testo device, Model 270, at 50o C.
Experimental design
The rats were randomly assigned to one of three different diets and had ad libitum access to food and water during the experiment.
Food was replaced on alternate days. The amount of food consumed and the total body weight were assessed weekly. Food efficiency for body weight gain (g of body weight gain/g of food intake in the same period) was calculated.
At the end of the experimental period (eight weeks), final body weights were determined and animals were euthanized by an intramuscular injection of anesthesia (0.1 ml of ketamine hydrochloride; 100 mg/ml, (Holliday Lab.) /100 g body weight mixed with 0.02 ml of xylazine; 100 mg/ml, (Konig Lab.) /100 g body weight. The right hemimandibles were dissected and cleaned of adhering soft tissue, weighed in a Mettler scale and stored at -20°C wrapped in gauze soaked with Ringer’s solution in sealed plastic bags, in accordance with Turner and Burr 6 . Each bone was thawed at room temperature and mandible weight was measured in g with a Mettler PE 600 scale (Zurich, Switzerland). Mandibular growth and mechanical properties were determined.
The same researcher measured all bone structures in order to reduce possible errors.
Zoometrics
Body weights (W) were measured in a Mettler PC 4000 scale; accuracy ± 0.001g and recorded weekly, after 2 to 4 hours fasting, throughout the experimental period. Body weight gain was expressed in grams per rat between weeks (g/rat/wk).
Mandibular morphometric properties
Mandibular growth was assessed directly on the right hemimandible by obtaining measurements (to the nearest 0.05 mm) with digital calipers, as per Eratalay et al. 19 with modifications by Alippi et al. 20 . The dimensions, including mandibular area, length of the base, length of the mandible and mandibular height are shown in Fig. 1.
The mandibular length measurement was divided into anterior and posterior sections by a vertical line drawn perpendicular to the occlusal plane of the molars immediately posterior to the surface of the third molar.
These specific measurements were selected to provide data on the growth of the bone as a whole without taking into consideration its morphological units.
Biomechanical testing of the mandible
Mechanical properties of the rat right hemimandible were determined using a three-point bending mechanical test. Before testing, each bone was thawed at room temperature and biomechanical competence was determined as described in our previous publication 12 .
Mechanical properties of the rat hemimandible were determined using an Instron model 4442 (Instron Corp., Canton, MA, USA), as described previously 21 . The plots of load v. deformation (W/d) were analyzed to determine the structural mechanical properties of the hemimandible; which measure the resistance to deformation (stiffness) and fracture (strength). The following measurements were recorded: Load at fracture (Wf, N), which directly expresses the resistance of the whole bone to fracture, incorporating both the elastic and the plastic behaviors; Load at yielding (Wy, N), which defines a threshold above which unrecoverable permanent deformation occurs, and Yielding deformation (dy, mm) at the yielding point, and structural stiffness or bone rigidity that represents the rigidity of the bone or the resistance to deformation (Wy/dy, N/mm) 21 .
Statistical Analysis
Results were presented as mean values ± SD and/ or SE. Statistical analyses were performed using SPSS (v. 20.0 IBM Corp., Chicago, IL. USA) and GraphPad Prism (version 6.0). Comparisons between groups were analyzed by one-way analysis of variance. When a statistically significant difference was encountered, a Student-Newman-Keul’s test or Dunn (non-parametric test) was performed. In all analyses, Bartlett’s test for homogeneous variances was applied. Statistical significance was set at p = 0.05. The Kolmogorov-Smirnov test was used to determine whether data had normal distribution.
RESULTS
The incorporation of fatty acids in gelatin hydrogels caused no difference in diet viscosity; all three diets had a similar viscosity with 1.6 cPa at 20°C. These data were similar to those reported by Lorenzo, Checmarev, Zaritzky and Califano 22 .
Animal body weight patterns and food intake of all three groups are presented in Table 2 and Fig. 2A and 2B. At the beginning of the study, animal body weights did not differ significantly (p = 0.6705). However, total body weight was significantly altered by the type of dietary fat consumed during the experimental period. Total body weights were lower in the SFOx rats than in the other groups (p = 0.0074). From wk=2 to wk=6, SFOx rats ceased gaining weight (p > 0.05), while SFO rats gained weight (p < 0.01). By the 6th week of the experiment, the SFOx group had the lowest body weight gain (Fig. 2B), while the body weight of SFO rats were similar to the C group (p > 0.05).
Table 2 Body weight, diet and fat intake
|
CONTROL |
SFO |
SFOx |
p† |
|
|---|---|---|---|---|
|
Initial Body Weight (g) |
53.8±10.9a |
53.9±8.8a |
57.7±7.6a |
0.644ns |
|
Final Body Weight (g) |
344.3±20.4b |
328.4±22.3b |
307.4±13.8a |
0.007** |
|
Energy Intake (kcal/100gW/day) |
24.8±1.4a |
30.9 ± 2.7b |
30.6 ± 2.9b |
0.007** |
|
Fat Intake (g/100gW/day) |
0.49±0.1a |
1.51±0.3b |
1.52±0.3b |
0.001*** |
|
SFA (g/100gW/day) |
0.13±0.01a |
0.22±0.02b |
0.28±0.02c |
0.001*** |
|
MUFA (g/100gW/day) |
0.14±0.01a |
0.44±0.07b |
0.45±0.06b |
0.001*** |
|
PUFA (g/100gW/day) |
0.23±0.01a |
0.98±0.02c |
0.72±0.01b |
0.001*** |
|
Linoleic acid (g/100gW/day) |
0.07±0.01a |
0.79±0.01c |
0.55±0.02b |
0.001*** |

Fig. 2 Diet intake per week throughout the study (A) and Food efficiency for body weight gain per week throughout the study (B).
There was a substantial difference in food intake among the three rat groups during the first two weeks of the experiment. The SFOx rats ate less (73.4 (8.2) g/rat/wk) than the SFO (95.6 (10.7) g/ rat/wk) (p < 0.05) and C groups (84.2 (5.3) g/rat/ wk) (p < 0.01). However, after the third week, food intake was similar among all groups, both between weeks 2 and 5, and between weeks 6 and 8 of the experiment (Fig. 2A). The efficiency of body weight gain per g of diet intake was assessed in the three groups when the amount of food consumed was stable (Fig. 2A, 2B). The SFOx group showed the lowest efficiency for gaining weight (C: 1.585 (0.138) = SFO: 1.516(0.136) > SFOx: 1.388(0.090) g/g; p = 0.021).
Animals that consumed SFO diets containing either fresh oil (SFO) or fried oil (SFOx) had higher energy consumption than the C rats (p = 0.0002), but there was no difference in energy intake between the two SFO groups (Table 2). SFOx consumption caused a highly significant (p = 0.0074) negative consequence on body weight gain and final body weight, in comparison with the other two groups. SFO and SFOx rats ate more total fat, monounsaturated fatty acids (MUFA) and SFA than C rats. SFO and SFOx groups did not differ significantly from each other. However, SFOx rats consumed less polyunsaturated fatty acids (PUFA), counting linoleic, arachidonic and linolenic acids, than SFO (Table 2).
The mandibular weight and length as an index of mandibular size were significantly lower in SFOx than in SFO and C rats (p=0.0001 and p=0.0002, respectively). The posterior section of the mandible of the SFOx rats was the most significantly affected area compared to the anterior section of the mandible of the rats fed on the other two diets (p = 0.0005); the anterior/posterior ratio indicated that SFOx (p=0.0001) induced mandibular deformation (Table 3 and Fig. 3).
Table 3 Morphometry of bone mandible
|
CONTROL |
SFO |
SFOX |
p† |
|
|---|---|---|---|---|
|
Hemimandible weight (g) |
0.43±0.02c |
0.39±0.02b |
0.36±0.02a |
0.001*** |
|
Mandible height (mm) |
12.6±0.24b |
11.9±0.73a |
11.8±0.24a |
0.019* |
|
Mandible length (mm) |
25.6±0.24c |
25.1±0.49b |
24.6±0.24a |
0.001*** |
|
Mandible base (mm) |
26.03±0.65b |
25.00±0.50a |
24.80±0.26a |
0.001*** |
|
Mandible area (mm2) |
140.2±5.5b |
129.5±6.9a |
128.3±5.3a |
0.006** |
|
Mandible anterior part (mm) |
14.24±0.45a |
14.17±0.23a |
14.09±0.43a |
0.797ns |
|
Mandible posterior part (mm) |
11.4±0.3b |
11.1±0.2b |
10.7±0.2a |
0.001*** |
|
Mandible anterior/posterior ratio |
1.25±0.02a |
1.27±0.02a |
1.32±0.03b |
0.004** |

Fig. 3 Photograph of rat mandible, lateral side, showing dfferential mandibular growth due to a diet containing fried sunflower oil.
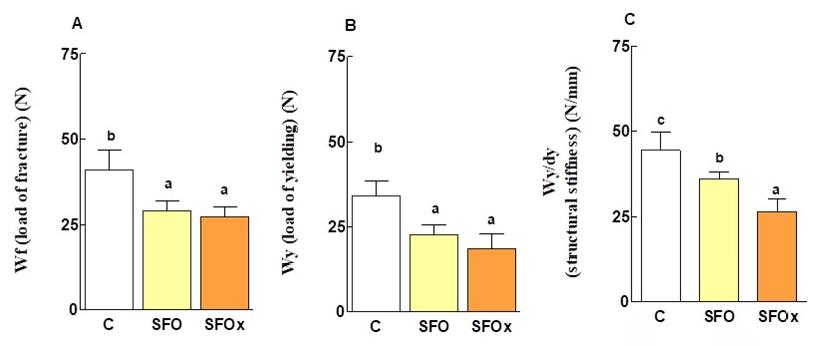
Structural properties resulting from the slope of the load/deformation curve in the linear region of the elastic behavior are presented in Figure 4A, 4B, 4C. Load-bearing capacity (Wf) (A), load of yielding (Wy) (B) and stiffness (Wy/ dy) (C) of the mandible were adversely affected in rats that consumed either fresh SFO oil or fried SFOx, in comparison to the C group (P = 0.001, P = 0.002 and P = 0.003, respectively). Even though Wf and Wy did not differ significantly between SFO and SFOx groups, fried oil consumption caused a major reduction in Wy/dy.
DISCUSSION
To our knowledge, this is the first demonstration that a diet rich in fried sunflower oil has an impact on mandible morphometric properties and biomechanical competence, highlighting the importance of the research. This study demonstrated
that in healthy, growing male rats, a diet containing fried sunflower oil was detrimental to body and mandible growth, and mandible biomechanics. It demonstrated that SFOx consumption caused a highly significant negative effect on body growth, compared to the other two rat groups. Additionally, the mandible of SFOx rats had significantly lower bone weight and length, as indices of mandibular size.
The study also showed that the differences in mandible stiffness and strength induced by SFOx consumption appeared to be the result of decreased gain of bone structural properties. Bone mechanical quality (structural properties) depends on the combination of the mechanical quality of the mineralized tissue (material stiffness mostly related to collagen mineralization) and the architectural quality of the structural bone design, size, shape and architectural distribution of mineralized tissue 23 .
The observed mandibular changes in bone mineral content and spatial distribution of mineralized tissue did not reflect altered bone material properties of other bones; a previous study showed that the mechanical competence of the femur of SFOx rats was not affected 8 . In contrast, thermoxidazed oil consumption has been found to alter cardiovascular status, femur mass and biomechanical competence 6, 7 . Rat mandible and femur have also been demonstrated to have different performance in growing rats subjected to chronic suboptimal nutrition 12 or in those fed imbalanced diets 24 .
The rat mandible may be divided into an anterior section comprising the alveolar and symphysial regions and a posterior section comprising the condyloid, the coronoid and the angular process. In the weaning rat, the length of the posterior section of the mandible is about one-half of the anterior section 15 . There is a relative enlargement of the posterior section of the mandible to more than twice as much as that of the anterior section due to the rapid growth of the condyle and the cartilage of the mandible, situated in the posterior section. The growth rates are different between the two sections of the bone mandible, which attain equal lengths in adulthood.
The condyle located in the posterior area operates as the main center of regional adaptive growth. The regulation of its development is defined by genetic and epigenetic factors that modify the expression of transcription and growth factors 25 . The mandibular condyle is a secondary cartilage that it is not surrounded by a cartilaginous matrix, and is therefore not isolated from the influences of local factors. However, the condyle responds to functional and mechanical loads; its frequent stimulation triggers a series of events that increase the number of replicating mesenchymal cells 25 .
In the current study, the masticatory load did not differ among the three diets fed to the rats. All three diets were prepared to attain similar consistency, since studies in growing rodents have demonstrated that masticatory function induces morphological modifications in the mandible 26 . Moreover, the masticatory system is a complex musculoskeletal system where activation of the masticatory muscles, movements of the jaw, loads and deformations, in both the jaw and the temporomandibular joint, are directly interconnected. The potential harmful effect of fried oil on mandibular growth becomes evident on the posterior section of the bone (“anterior/ posterior ratio” altered in SFOx versus SFO and C rats [p = 0.0001]). In contrast, the anterior section, where the teeth are located and which determines the mandibular movements, was unaffected.
In rats, as in humans, most muscles are inserted in the posterior section of the mandible. In rodents, mandibular movements slide from front to back, whereas in humans, the movements open, close, and move laterally. Most of these movements depend on the incorporated muscles and are facilitated by the temporo-mandibular joint 17 . The consumption of a SFOx diet during growth appears to affect mandibular dynamics. Further studies may elucidate which sector of the posterior section was altered or whether the reduction was harmonious.
The ingredients in the diets employed for testing could also play a role in the decreased mandibular mass. The findings of the rats fed SFOx could be related to the decreased PUFA levels and/or the increase in total saturated fat rather than the source of the dietary oil. In previous studies, we demonstrated that rats fed a high-saturated fatty acid diet for eight weeks had lower bone mineral density (spine BMD) and total skeleton bone mineral content (BMC) than rats that consumed other types of vegetable oils 27 . The rats that consumed high-fat vegetable oil diets, regardless of the diverse omega-6 (n-6) polyunsaturated fatty acid (n-6 PUFA)/omega-3(n-3) PUFA ratio, gained total skeleton BMD, BMC, and BMC per total body weight (BMC/W) similar to the animals fed the control diet proposed by the American Institute of Nutrition (AIN) committee in 1993 to provide the increased nutritional requirements for rat or mouse growth (AIN 93G) formulation 28 . It is recognized that a diet rich in saturated fatty acids could increase inflammatory cytokine expression and NF-kB ligand receptor activator, which has been found to stimulate bone resorption and disturb osteoblast genesis, leading to negative effects on bone 29, 30 .
The presence of dietary n-6 and n-3 PUFA are important because prostaglandins (PGs) are a group of lipid mediators formed from arachidonic acid in various tissues under several physiological and pathophysiological circumstances, and serve to sustain local homeostasis 31 . Among them, PGE works in a bimodal manner in bone metabolism; PGE2 at high concentrations powerfully induces bone resorption 32 . Further, in vitro studies have demonstrated that cyclooxygenase 2 (COX-2) and PGE2 stimulate receptor activator of nuclear factor kappa-B ligand (RANKL) expression and downregulate osteoprotegerin (OPG) expression, and that disruption of the balance of RANKL/ OPG stimulates osteoclastogenesis 33 . Other studies demonstrated that the type of dietary fat conditioned age-related alveolar bone loss, and that the presence de n-6PUFA from sunflower oil was responsible for higher age-related alveolar bone loss. The mechanisms involved in this phenomenon were associated with an ablation of the cell ability to adapt to aging.
In this study, the lack of decrease in mandibular mass in the SFO group compared to SFOx rats suggests that the high n-6 PUFA content exceeded the rat requirements. Fried oils, particularly sunflower oil, induced more deleterious effects on mandible than did the excess of n-6 PUFA. The adverse consequence of consuming SFOx diet on bone mass was weakened bone strength and structural stiffness of the mandible and the mandibular area. However, mandibular mass and structural mandible strength increased proportionally with body mass. Our previous studies demonstrated the prominent role of thermoxidazed oil consumption in determining the risk for growth and cardiovascular and bone effects. The consumption of a diet rich in sunflower oil did not affect final body weight and length; in contrast, the intake of SFOx induced a lower final body growth 8 .
The alterations in bone stiffness and strength induced by the consumption of SFOx diet by growing healthy male rats appeared to decrease bone structural properties with a loss in bone. In fact, the structural properties of the entire bone are established by the physicochemical nature of its calcified matrix (material properties focused on the rigidity of the tissue) and by the architectural distribution. Changes in bone structural properties could be due to modifications in mass and its spatial distribution (geometry) 7 . Mechanical loading of the mandible during mastication affects the mass, density and microarchitecture of the mandibular alveolar bone 14, 34 . Finally, our results raise an important consideration for future studies on rat mandible using micro-computed tomography (pCT) system for the evaluation of bone micro-architecture. This study was designed to feed young male rats a diet rich in fried oil that resembles the diet patterns of Argentinian children. These experimental results in growing rats may enable some responses in children fed similar diets to be foreseen. However, according to the Argentinian Second National Nutrition and
Health Survey (2019), children and adolescents have eating patterns that differ from those of adults: children consume twice as many pastry products or salted snacks, and 2- to 12-year-olds eat almost three times more confectionery products than adults (26.5% vs. 10.5% respectively) 35 .
CONCLUSIONS
Traditionally, the consumption of fried foods has been associated to cardiovascular diseases and obesity. We demonstrated that SFOx diets are inversely related to bone health. These findings evidenced the negative effects of fried sunflower oil consumption on total body growth and mandible alterations. These adaptations induced alterations in mandible dynamics and bone biomechanical competence, in terms of bone stiffness.