Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Cuadernos de herpetología
versión On-line ISSN 1852-5768
Cuad. herpetol. vol.29 no.1 San Salvador de Jujuy mayo 2015
TRABAJO
Discovery of two new species of Phymaturus (Iguania:Liolaemidae) from Patagonia, Argentina, and occurrence of melanism in the patagonicus group
Fernando Lobo1, Santiago Javier Nenda2
1 Instituto de Bio y Geociencias del NOA, Consejo Nacional de Investigaciones Cientificas y Tecnicas (CONICET)- Universidad Nacional de Salta, Avenida Bolivia 5150, 4400-Salta, Argentina.
2 Division Herpetologia, Museo Argentino de Ciencias Naturales ‘‘Bernardino Rivadavia''-CONICET, Avenida Angel Gallardo 470, C1405DJR Buenos Aires, Argentina
Recibido: 03/04/14
Revisado: 12/05/14
Aceptado: 25/06/14
ABSTRACT
Comprehensive studies recently published on the evolution and systematics of Phymaturus (morphological and molecular ones) revealed not only a historical pattern and subclades within the traditional P. palluma and P. patagonicus species groups but also a still not fully understood unsuspected diversity. Several populations in northern and southern Argentina may represent independent lineages that deserve formal description. Two of these populations were studied for the present contribution and are easily distinguished from all the other species in the genus. One of these populations is from Río Negro province and belongs to the P. patagonicus group; it exhibits a unique dorsal color pattern and several individuals are melanic, a characteristic never reported before for the genus, with the exception of P. tenebrosus. A careful examination of melanic individuals revealed the same dorsal pattern as that of non-melanic ones, although it is obscured. We also report the discovery of melanic individuals of two other species that are probably closely related: P. ceii and P. sitesi. The melanism found in the P. patagonicus group differs from the head melanism of males in certain species of the P. palluma group because in the former group melanism is not determined by sexual dimorphism and involves the whole animal. The other population studied in this contribution belongs to the P. palluma group and is described as a new species because its color pattern and scalation differ from those of all the other members of the P. palluma group. Finally, we discuss the position of these new lizard species in the context of the available phylogenetic hypothesis and the occurrence and evolution of melanism in the P. patagonicus group.
KEYWORDS: Lizard, Phymaturus cacivioi sp. nov., Phymaturus tromen sp. nov., Taxonomy, Phylogeny, Polymorphism.
RESUMEN
Estudios exhaustivos publicados recientemente sobre la evolución y la sistemática de Phymaturus (morfológicos y moleculares, respectivamente) revelaron no sólo un patrón histórico y subclados dentro de los grupos de P. palluma y de P. patagonicus tradicionales, sino también una diversidad insospechada todavía no entendida completamente. Varias poblaciones en el norte y el sur de la Argentina pueden representar linajes independientes que merecen descripción formal. Dos de estas poblaciones fueron estudiadas en el presente trabajo y se distinguen fácilmente de las otras especies del género. Una de estas poblaciones es de la provincia de Río Negro y pertenece al grupo de P. patagonicus, posee un patrón de coloración dorsal particular y varios individuos son melánicos, una característica nunca antes reportada para el género, con la excepción de P. tenebrosus. Un examen cuidadoso de los individuos melánicos revela el mismo patrón dorsal como el de los ejemplares no melánicos, aunque oculto. Se da a conocer también el descubrimiento de individuos melánicos de otras dos especies que probablemente están muy relacionados: P. ceii y P. sitesi. El melanismo que se encuentra en el grupo patagonicus difiere del melanismo de la cabeza de los machos de algunas especies del grupo de P. palluma porque en el primero, el melanismo, no se rige por el dimorfismo sexual e involucra a todo el animal. La otra población estudiada en este trabajo pertenece al grupo de P. palluma y se describe como una nueva especie ya que su patrón de coloración y escamación difieren del de todos los demás miembros del grupo de P. palluma. Finalmente, se discute la posición de estas nuevas especies de lagartos en el contexto de la hipótesis filogenética disponible y la aparición y evolución del melanismo en el grupo de P. patagonicus.
PALABRAS CLAVE: Lagarto, Phymaturus cacivioi sp. nov., Phymaturus tromen sp. nov., Taxonomía, Filogenia, Polimorfismo.
INTRODUCTION
Phymaturus is a distinctive clade of saxicolous lizards occurring in rocky environments across the arid west of Argentina, from Chubut province to the Puna steppe in Catamarca, and in central Chile. Its taxonomic composition after its first cladistic revision (Etheridge, 1995, 10 species) increased rapidly, becoming a genus composed of more than 40 species (Lobo et al., 2013). For a review of the whole sequence of descriptions and contributions on the taxonomy of Phymaturus, see Lobo et al. (2012a; 2013). The rate of increase of knowledge about this generic diversity is impressive, with 12 new taxa formally described for northern and western central Argentina, and central and southern Chile in the last two years (Avila et al., 2010; Lobo et al., 2010a; 2012b; 2012c; Núñez et al., 2010; Troncoso-Palacios and Lobo, 2012; Scolaro et al., 2012). Most species of Phymaturus show a restricted geographic distribution (Lobo et al., 2010a), and because of its habitat preference (saxicolous) and its exclusive herbivory, among other characteristics, these animals are the focus of attention for different research groups.
Regarding the evolution and phylogenetics of the genus Phymaturus, Etheridge (1995) divided it into two groups, the P. patagonicus and P. palluma groups, comprising four and six species, respectively. This division was based on synapomorphies and was confirmed by later studies. Espinoza et al. (2004) evaluated herbivory in liolaemid lizards and formulated a phylogenetic hypothesis. Morando (2004) analysed phylogenetic relationships within Liolaemidae, with emphasis on Liolaemus, using 12 species of Phymaturus. The analyses of Espinoza et al. (2004) and Morando (2004) were based on DNA sequences from different gene regions. Lobo and Quinteros (2005) analysed a morphological data set of 133 characters and 22 terminal taxa, including 10 taxa of the 12 available in the literature at that time. Recently, two more comprehensive studies have been published. One was based on 206 morphological characters that resulted in a hypothesis of relationships for 46 terminal taxa (Lobo et al., 2012a). The other analysed the information recovered from mitochondrial and nuclear genes for 49 terminal taxa (Morando et al., 2013). In both studies several unnamed populations of potential new species were included. Taking into account these cladistic analyses, plus the taxonomic contributions mentioned above, a considerable increase in our understanding of the composition and evolutionary relationships within the genus has been advanced in just a few years. The Phymaturus patagonicus group consists of 23 taxa including recent descriptions of Avila et al. (2010), Avila et al. (2012), Avila et al. (2014), Lobo et al. (2010a; 2012c; 2012d), Scolaro et al (2012) and Scolaro et al. (2013) (see Table 1 in Lobo et al., 2013). Within this group there are several unnamed populations, most of them requiring a detailed morphological study. The Phymaturus palluma group consists of 19 taxa (following Lobo et al., 2013), and several populations are the objects of taxonomic studies at this time. The populations that resemble "roigorum" lizards, which inhabit the Tromen volcano (Neuquén province) are among those of uncertain identity. The main goal of the present contribution is to describe formally two new taxa of the P. patagonicus and P. palluma groups, and to discuss the phenomenon of melanism that occurs in certain species of the genus.
MATERIALS AND METHODS
We examined the type series of most species of Phymaturus described to date (see Appendix). We were unable to examine directly described taxa of the patagonicus group (P. desuetus, P. sitesi, P. delheyi, and P. sinervoi), so we used information from their type descriptions for comparative purposes. For diagnoses of the two new taxa, we examined the external morphology (e.g., squamation and color patterns) of 583 specimens, representing 25 of the currently recognized species (see Appendix). Color in life of the new species (and most of the previously described species of the genus Phymaturus) was recorded in the field. All specimens from the type series of the new species are deposited at MCN-UNSa and MACN; they were fixed in 10% formaldehyde and preserved in 70% ethanol. Some character states were determined with the aid of a binocular dissection microscope, and measurements were taken with electronic calipers to the nearest 0.05 mm. Terminology for the description of squamation follows Smith (1946), and neck-fold terminology follows Frost (1992). Definitions and detailed descriptions for body patterns of Phymaturus are provided in Lobo and Quinteros (2005), Lobo et al. (2010a), Lobo et al. (2010b) and Lobo et al. (2012a). Optimization of melanism on a Phymaturus cladogram (Fig. 5) was performed using TNT (Goloboff et al., 2003). Institutional abbreviations follow Sabaj Pérez (2013), with the following additions: JAS-DC (José A. Scolaro Diagnostic Collection), MCN-UNSa (Museo de Ciencias Naturales, Universidad Nacional de Salta, Salta, Argentina), and UNCo-PH (Universidad Nacional del Comahue, Neuquén, Argentina, Programa de Herpetología).
RESULTS
Phymaturus cacivioi sp. nov.
Holotype.- MCN-UNSa 3895. Male. 12.6 km SW of Mencué on Provincial Route 67; 40°30'53.90" S, 69°42'25.00" W 1140 m above sea level. El Cuy Department. Río Negro Province. Argentina.
Paratypes.- 8 females, 10 males and 3 juveniles. MCN-UNSa 3888, MCN-UNSa 3890, MCN-UNSa 3894, MACN 44731 (ex MCN-UNSa 3896), MACN 44732 (ex MCN-UNSa 3897), MACN 44733 (ex MCN-UNSa 3898), MACN 44735 (ex MCN-UNSa 3935), MACN 44737 (ex MCN-UNSa 3938) females, MCN-UNSa 3936, MCN-UNSa 3889, MCN-UNSa 3891, MCN-UNSa 3892, MCN-UNSa 3899, MCN-UNSa 3901, MCN-UNSa 3902, MACN 44730 (ex MCN-UNSa 3893), MACN 44736 (ex MCN-UNSa 3937), MACN 44734 (ex MCN-UNSa 3900) males. MCN 3903-05 juveniles. Same data as holotype.
Diagnosis
Phymaturus cacivioi sp. nov. belongs to the Phymaturus patagonicus group because it exhibits apomorphies found for this group of species (Lobo et al., 2012a): bellies of females and males light orange or pink (not yellow, which is exclusive of males of the palluma group), a set of enlarged scales projected over the auditory meatus (not perpendicular), and external margins of postmental scales dark pigmented. Within the P. patagonicus group, P. cacivioi sp. nov. differs from P. payuniae, P. nevadoi, P. sitesi, P. delheyi, P. patagonicus, P. somuncurensis, P. sinervoi, P. calcogaster, P. camilae, P. yachanana and P. etheridgei in that it lacks a dorsal pattern formed by dispersed white spots. Phymaturus cacivioi sp. nov. does not exhibit a dorsal pattern of ocelli forming two paravertebral rows, unlike P. manuelae, P. spectabilis, P. excelsus, P. spurcus (juveniles), P. payuniae (females), P. nevadoi (females), P. castillensis, P. felixi (polymorphic) and P. camilae (females). Phymaturus cacivioi sp. nov. has a lateral dark band, which is absent in almost all species of Phymaturus with the exception of P. zapalensis, P. ceii, P. tenebrosus, P. sinervoi (females) and P. somuncurensis. Four species are phenetically similar to P. cacivioi sp. nov.: P. tenebrosus, P. manuelae, P. ceii and the recently described P. sinervoi, which can be easily distinguished by the following character combination: dorsal ocelli light brown, more marked than the rest of the background color, which is darker, conspicuous in all females of P. ceii and P. manuelae and in melanic males of P. ceii (but fading); dorsal ocelli always absent in P. cacivioi sp. nov., P. sinervoi and P. tenebrosus; presence of sexual dimorphism in color pattern in P. ceii and P. manuelae, but not in P. cacivioi sp. nov., P. sinervoi and P. tenebrosus. Transverse rows of white spots on the back are always present in Phymaturus cacivioi sp. nov.; this character is shared with P. calcogaster and an individual of P. manuelae but is absent in P. tenebrosus, P. sinervoi and P. ceii. Dorsum of trunk with red-brown background color only present in P. cacivioi sp. nov., and in a few individuals of P. manuelae and P. calcogaster (but combined with white dispersed spots). A similar color is observed in P. manuelae, but the dorsal pattern is quite different. The occurrence of complete melanic individuals (MCN collection) is different in the three species: Phymaturus cacivioi sp. nov. 36%, P. ceii 13.4%, and P. tenebrosus 76.9%. In P. sitesi, P. tenebrosus and P. ceii an individual with irregular melanic coloring on body or limbs was exceptionally observed. Melanic individuals of the three species are easily recognizable because they have the characters observed in the non-melanic individuals. Throats of P. cacivioi sp. nov. are variegated as in P. sinervoi, and P. tenebrosus; P. manuelae lacks any kind of pattern, and P. ceii exhibits similar variegation but fading, almost inconspicuous (see Fig. 2 in Scolaro et al., 2007) (P. sitesi idem P. tenebrosus, P. delheyi variegated). Tails of P. sinervoi are spotted in white as on the trunk; this character was not included in Lobo et al. (2012a) and probably is a synapomorphy of P. payuniae, P. nevadoi, P. sitesi and P. delheyi. In P. cacivioi sp. nov., P. ceii, and P. tenebrosus tails are slightly ringed or variegated or they lack pattern at all (P. tenebrosus). Color in life of the belly in both sexes is pink in P. cacivioi sp. nov. or pink-orange in males, pink/orange in P. sinervoi females and orange to yellow in males; yellow in males of P. manuelae and light pink to lack of color in females. In P. ceii it is yellow in males and white to light pink in females. Belly yellow/mustard in males of P. tenebrosus, and light orange in females. Midbody scale number in Phymaturus cacivioi sp. nov. (x = 203.2; SD = 8.2; range = 187-213), similarly to P. tenebrosus (x = 199.2; SD = 20.2; range = 171-236); but the number is lower than in P. manuelae (x = 218.3; SD = 9.7; range = 208-233), P. ceii (x = 223.8; SD = 10.7; range = 204-242), and P. sinervoi (x = 227.0; SD = 9.9; range = 213-248 after Scolaro et al., 2012).The number of scales in contact with nasal are fewer in P. cacivioi sp. nov. (x = 7.05; SD = 0.70; range = 6-8), in P. ceii (x = 8.18; SD = 0.87; range = 7-9) but similar to the number in P. manuelae (x = 7.43; SD = 0.8; range = 6-8) and P. tenebrosus (x = 7.56; SD = 1.09; range = 6-9); the number of scales in contact with mental in P. cacivioi sp. nov.( x = 4.26; SD = 0.65; range = 4-6) is also lower than in P. ceii (x = 5.18; SD = 0.98; range = 4-6) but similar to that in P. tenebrosus (x = 4.25; SD = 0.58; range = 4-6), P. manuelae (x = 4.28; SD = 0.5; range = 4-5) and P. sinervoi (x = 4.70; SD = 0.8; sensu Scolaro et al., 2012). Larger number of precloacal pores in males than in females in Phymaturus cacivioi sp. nov. (x = 9.36 SD = 1.91 range = 6-12); a similar range is reported by Scolaro et al. 2012 for P. sinervoi: (6-13); P. ceii (x = 7.71 SD = 1.6 range = 5-10); P. tenebrosus (x = 8.50 SD =1 range = 7-9); P. manuelae (x = 8.33 SD = 1.5 range = 7-10). Precloacal pores are present in 50% of females of P. cacivioi sp. nov. and absent in all females of P. ceii, P. sinervoi (according to Scolaro et al., 2012), P. manuelae and P. tenebrosus. Body size of P. cacivioi sp. nov. (SVL x = 91.93; SD = 5.02; range = 78.9-98,71) is similar to that of P. sinervoi (x = 91.7; SD = 5.5, after Scolaro et al., 2012) being a little smaller in P. ceii (x = 85.57; SD = 4.79; range = 74.35-92.03) and a little larger in P. manuelae (x = 93,1; SD = 6.6; range = 83.3-100,4) and in P. tenebrosus (x = 94.81; SD = 6.38; range = 85-107.52). Most informative characters for this diagnosis are presented in Table 1; character states exhibited by species closely related to P.cacivioi sp. nov. are shown.
Table 1. Morphological characters that exhibit variation (and are useful for its diagnosis) among those species more closely related and most phenetically similar to Phymaturus cacivioi sp. nov.

Description of holotype.- Male. SVL 89.09 mm. Head length 16.5 mm. Head width 15.4 mm. Head height (at parietal) 8.6 mm. Axilla-groin 42.2 mm (47.3% of SVL). Tail length (complete, not regenerated) 102.7 mm (1.15 times SVL). Body moderately wide, trunk width: 36.2 mm (40.7% of SVL). Nineteen smooth dorsal head scales. Seven, six, five organs in postrostrals. Nasal bordered by eight scales, not in contact with rostral. Canthal separated from nasal by two scales. Loreal region flat. Nine enlarged supralabial scales, none contacting subocular. Eight enlarged infralabials. Auditory meatus oval with five small, flat, projecting scales on the anterior margin. Auricular scale absent. Nine convex, juxtaposed temporals. Rostral undivided. Mental subpentagonal, in contact with four scales. Interparietal bordered by eight scales. Frontal region without an azygous scale. Supraorbital semicircles inconspicuous. No distinctly enlarged supraoculars. Eight imbricate flat superciliaries. Subocular not fragmented, separated from supralabials by one row of lorilabials. Nine lorilabials, the eighth and ninth contacting subocular. Preocular separated from lorilabial row by one scale. Scales of throat round, flat, and juxtaposed. Eighty-three gulars between auditory meata. Lateral nuchal folds well developed, with granular scales over longitudinal fold. Antehumeral pocket well developed. Sixty-nine scales between auditory meatus and shoulder. In ventral view, anterior gular fold absent and posterior gular folds present, their anterior margins with enlarged scales on their borders. Enlarged scales in the center of chest absent. Dorsal scales round, smooth, juxtaposed. Forty-one dorsal scales along midline of the trunk at a distance equivalent to head length. Scales around midbody: 204. Ventral scales larger than dorsals. Ventral scales between mental and precloacal pores: 177. Ten precloacal pores in a divided row without supernumerary pores. Brachial and antebrachial scales smooth, with round posterior margins. Supracarpals laminar, round, smooth. Subdigital lamellae of fingers with three keels. Number of subdigital lamellae of fingers I: 11; II: 15; III: 19; IV: 22; V: 15. Claws moderately long. Supradigital lamellae convex, imbricate. Infracarpals and infratarsals with round margins and 2-3 keels. Supracarpals and supratarsals smooth, with rounded posterior margins. Subdigital lamellae of toes I: 12; II: 17; III: 23; IV: 27; V: 19.
Holotype coloration: the holotype exhibit dorsal background brown color, with transversal rows of white small spots over dorsum, head reticulated dark brown and a conspicuous dark lateral band on flanks. Throat white with thin black variegation and chest entirely white, belly light orange. Limbs are irregularly spotted (light brown to dark brown markings). In ventral view, limbs are white and speckled with very few and dispersed small black spots. Ventral surfaces of thighs are yellow.
Variation: Based on 19 adult specimens (11 males and 8 females). SVL 78.9-98.7 mm (x = 91.9; SD = 5.0) (taken from four adult specimens to avoid ontogenetic variation). Head length 16.4-18.8% (x= 17.7%; SD = 0.6) of SVL. Tail length 1.05-1.26 (x = 1.17; SD = 0.06) times SVL. Scales around midbody 187-213 (x = 203.2; SD = 8.2). Dorsal head scales 17-22 (x = 19.9; SD = 1.2). Ventrals 152-196 (x = 178.2; SD = 9.5). Scales surrounding interparietal 6-9 (x = 7.6; SD = 0.9). Scales surrounding nasal 6-8 (x = 7.0; SD = 0.7). Number of scale organs on postrostrals 2-7 (x = 4.6; SD = 1.1). Superciliaries 7-10 (x = 7.9; SD = 1.0). Subocular fragmented in two scales only in one specimen. Mental scale in contact with 4-6 (x = 4.3; SD = 0.6). Number of chinshields 6-10 (x = 7.2; SD = 1.1). About two thirds of individuals (13 versus 6) show enlarged scales on the border of gular fold. Lorilabials 8-11 (x = 9.4; SD = 0.7). Enlarged scales on the anterior border of the auditory meatus 3-7 (x = 4.5; SD = 1.2). Scales of neck along longitudinal fold from posterior border of auditory meatus to shoulder 64-93 (x = 76.3; SD = 6.6). Gulars 67-93 (x = 81.4; SD = 6.1). Scales between rostral and frontal 7-10 (x = 8.8; SD = 1.0). Subdigital lamellae on fourth finger 19-26 (x = 22.6; SD = 1.7). Subdigital lamellae on fourth toe 24-32 (x = 27.5; SD = 1.9). Males with 6-12 precloacal pores (x = 9.4; SD = 2.0). Half of females show precloacal pores 1-9 (x = 4.7; SD = 3.3). Most individuals (except four) exhibit dorsal white thin spots arranged forming transverse rows on the back (Figs. 1 and 2). Throats show a thin dark reticulation, with the exception of three individuals.

Figure 1. A and B Dorsal and ventral views of an adult male of Phymaturus cacivioi sp. nov. (regular pattern); C and D dorsal and ventral view of an adult male of the same species (melanic pattern). Recognizable diagnostic characters for the species, even in melanic specimens: transverse series of white spots over trunk, absence of ocelli and variegated throat.
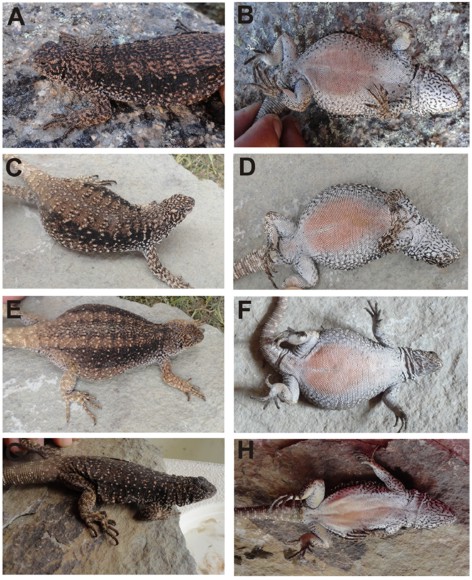
Figure 2. A, C and E dorsal views of Phymaturus cacivioi sp. nov. females; B, D and F ventral views of same individuals. G and H dorsal and ventral views of an adult male of Phymaturus cacivioi sp. nov.
Pattern of body and limbs, color in life (Figs.1, 2 and 3): almost all specimens studied exhibit dorsal back-ground brown color, with transversal rows of white small spots over dorsum. In several individuals a wide vertebral band of darker color is present. Heads are reticulated dark brown. Dark lateral bands on flanks are conspicuous. Throats and chest are white, the first showing a thin black variegation. Pink color is always present on bellies of females and young males; in adult males bellies become light orange. Limbs are irregularly spotted (light brown to dark brown markings). In ventral view, limbs are white and speckled with very few and dispersed small black spots. Ventral surfaces of male thighs are yellow, as in most males of Liolaemids. Juveniles show the same pattern and general color as adult specimens; here we report a single juvenile specimen with its body, limbs and head irregular and partially melanic (Fig. 3b).

Figure 3. Three color morphs of Phymaturus cacivioi sp. nov. juvenile found: A. melanic MCN 3903, B. an intermediate form (irregularly melanic, MCN 3904) and C. regular pattern (MCN 3905). This intermediate pattern is present at very low frequency, was unique among studied specimens (adult and juvenile combined).
Etimology: we name this new species in honour of our Argentine colleague and friend Pedro Matías Cacivio, in recognition of his enthusiasm and companionship during several field trips and laboratory work.
Distribution (Fig. 4): only known from its type locality.
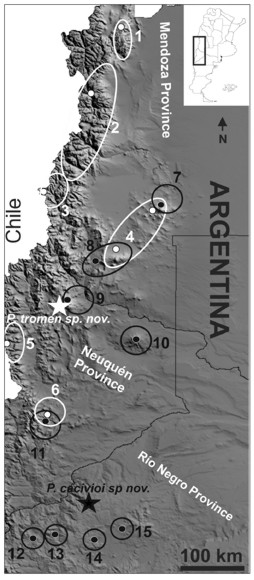
Figure 4. Geographical distribution of species of the palluma (white) and patagonicus (black) groups in argentine provinces of Mendoza, Neuquén and Río Negro. White star represents type locality of P. tromen sp. nov., black star type locality of P. cacivioi sp. nov. 1- P. sp. ("adrianae"), 2- P. palluma, 3- P. verdugo, 4- P. roigorum, 5- P. dorsimaculatus, 6- P. querque, 7- P. nevadoi, 8- P. payunie, 9- P. delheyi, 10- P. sitesi, 11- P. zapalensis, 12- P. tenebrosus, 13- P. manuelae, 14- P. sinervoi, 15- P. ceii. Dots indicate type localities.
Phylogenetic relationships (Fig. 5): We included P. cacivioi sp. nov. in the most recent data matrix of the morphological analysis (Lobo et al., 2012a) plus P. sitesi and P. delheyi (Avila et al. 2011) and we added additional morphological characters (29) (unpublished data). After running TNT we found a K3 hypothesis, P. tenebrosus is found as sister taxon of P. zapalensis and P.cacivioi sp. nov. sister taxon of P. ceii, all these species related to clade D (Lobo et al., 2012a; payuniae group of Morando et al., 2013) (Fig. 5). Optimization of melanism in the group suggests two alternative explanations: one of them is that melanism arose in the ancestral node of clade D (payuniae group) and was lost in P. zapalensis and in the node supporting P. delheyi, P. payuniae and P. nevadoi. The other possible explanation is that melanism occurred independently in P. tenebrosus, in the pair of sister taxa P. ceii-P. cacivioi sp. nov. and in P. sitesi.
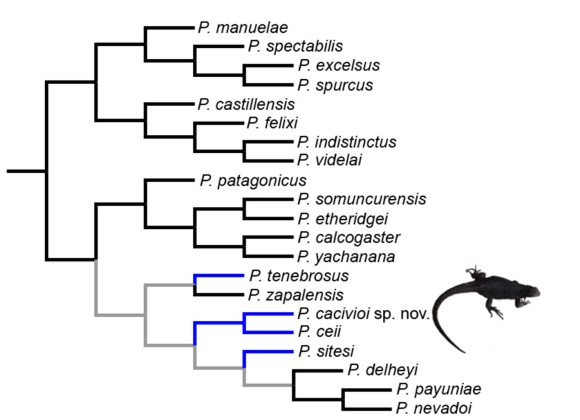
Figure 5. Cladogram showing phylogenetic relationships of species of the P. patagonicus group after a reanalysis performed over the original data matrix of Lobo et al. (2012a). The occurrence of complete melanic individuals or at least spotted ones is mapped in blue. Ancestral assignation of states for this subclade is ambiguous.
Comment: this new taxa could correspond to populations studied by Morando et al (2013) as sp. 18 and sp 19.
Phymaturus tromen sp. nov.
Holotype.- MCN-UNSa 3719 Male. Provincial Route 37, on the way from Chos Malal to Tromen, 37°10'38.5'' S, 70°10'16.2'' W. Chos Malal Department, Neuquén Province, Argentina.
Paratypes.- 5 females, one male, and one juvenile female. MCN-UNSa 3720 male, MACN 45430 (ex MCN-UNSa 3713), MCN-UNSa 3714, MCN-UNSa 3717-18 females, MACN 45431 (ex MCN-UNSa 3715), MCN-UNSa 3716 juvenile (female). Same data as holotype.
Diagnosis
Phymaturus tromen sp. nov. belongs to the palluma group of Phymaturus because it exhibits apomorphies found for this group of species (Lobo et al., 2012a): superciliary scales short and juxtaposed, loss of contact between lorilabial row and subocular scale, row of precloacal pores tending to be divided, spotted chests, and tail scales showing longitudinal thin grooves. Within the palluma group P. tromen sp. nov. differs from all members of the puna subclade (P. antofagastensis, P. punae, P. extrilidus, P. aguanegra, P. williamsi, P. mallimacci, P. laurenti, P. denotatus, P. paihuanense, P. alicahuense, P. darwini and P.bibroni) in that it lacks the homogeneous, thin, brown-spotted pattern ("spray pattern") typical of that group. Phymaturus tromen sp. nov. differs from P. roigorum, P. querque, P. sp1. (Lobo et al., 2012a), P. verdugo, and P. palluma in that it has the largest number of midbody scales of the group (Table 2). Dorsal head melanism is absent in P. tromen sp. nov. but conspicuous in P. verdugo, P. sp1., P. querque, and P. palluma. Dorsal reticulated pattern of males is thin, unlike in P. roigorum, P. sp1., and P. dorsimaculatus (thick) (see Fig. 1 in Lobo and Abdala, 2007). Female flank color is present in P. tromen sp. nov. but absent in P. querque, P. verdugo, and P. sp1. A scapular spot is conspicuous in P. tromen sp. nov. but absent in P. dorsimaculatus, P. roigorum and P. verdugo. Females of P. tromen sp. nov. exhibit an ocellate pattern (absent in P. maulense, P. damasense, P. dorsimaculatus, P. verdugo, P. palluma and P. sp1.), dorsal ocelli of each longitudinal row become fused without evident margins, unlike in P. roigorum and P. querque (Fig. 7). Phymaturus tromen sp. nov differs from P. maulense and P. damasense, in the dorsal pattern of females; females of both Chilean species lack dorsal ocelli, exhibiting transverse thin black stripes. Throat pattern of P. tromen sp.nov. is variegated, not black as in P. vociferator, P. damasense and P. maulense. Females of P. tromen sp.nov. lack a patch of enlarged scales in the center of gular fold, as in females of P. damasense.
The characters useful for species recognition within this group are listed in Table 2.
Table 2. Morphological characters that exhibit variation (and are useful for its diagnosis) among those species more closely related and most phenetically similar to Phymaturus tromen sp. nov.
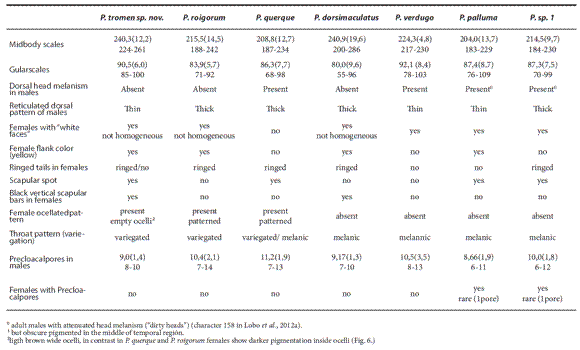

Figure 7. A and B dorsal and ventral views of an adult male of Phymaturus querque. C and D, same views for an adult female of this species. E-H dorsal pattern variation in females of P. querque. Recognizable diagnostic characters for the species: melanic color continued on the neck and shoulder of male, melanic and/or homogeneous dark gray/melanic throat, yellow color covering its entire ventral surface and projected also over flanks, and orange/yellow tail. Females with thick reticulated pattern over dorsum exhibiting individual small ocelli. No flank color in females.
Description of holotype (Fig. 6a and b) - Male. SVL 105.19 mm. Head length 20.1 mm. Head width 20.5 mm. Head height (at parietal) 9.6 mm. Axilla-groin 53.9 mm (51.3% of SVL). Tail length (complete, not regenerated) 104.9 mm. Body moderately wide, trunk width 47.7 mm (45.0% of SVL). Twenty-two rugose dorsal head scales. One to three scale organs per postrostral scale (x= 2.2). Nasal bordered by 11 scales, not in contact with rostral. Canthal separated from nasal by two scales. Flat loreal region. Thirteen enlarged supralabial scales. Twelve enlarged infralabials. Oval auditory meatus with five scales projecting on the anterior margin. Auricular scale absent. Ten convex, rugose and juxtaposed temporals. Rostral undivided. Mental subpentagonal, in contact with seven scales. Interparietal bordered by nine scales. Frontal region without an azygous scale. Supraorbital semicircles inconspicuous. Five scales between superciliaries and frontal region. Eleven juxtaposed flat superciliaries. Subocular fragmented in two scales and separated from supralabials by two rows of lorilabials. Preocular separated from lorilabial row by three scales. Scales of throat small, round, flat, and juxtaposed. Eighty-eight gulars between auditory meata. Well-developed lateral nuchal folds, with granular scales over longitudinal fold. Well-developed antehumeral pocket. One hundred and eighteen scales between auditory meatus and shoulder. In ventral view, gular fold present and posterior gular fold without enlarged scales on the borders of their anterior margins. Round, small, smooth, juxtaposed dorsal scales. Forty-three dorsal scales along midline of the trunk, covering a length equivalent to head length. Scales around midbody: 242. Mid-dorsal scales larger than those on flanks. Ventral scales larger than dorsals. Ventral scales between mental and precloacal pores: 213. Ten precloacal pores. Brachial and antebrachial scales smooth with round posterior margins. Flat, round, smooth supracarpals. Subdigital lamellae of fingers with 3‑5 keels. Number of subdigital lamellae of fingers I: 10; II: 14; III: 19; IV: 22; V: 15. Moderately long claws. Convex, imbricate supradigital lamellae. Infracarpals and infratarsals with round margins and 1‑3 obtuse mucrons. Smooth supratarsals, with round posterior margins. Subdigital lamellae of toes I: 10; II: 16; III: 21; IV: 24; V: 17.
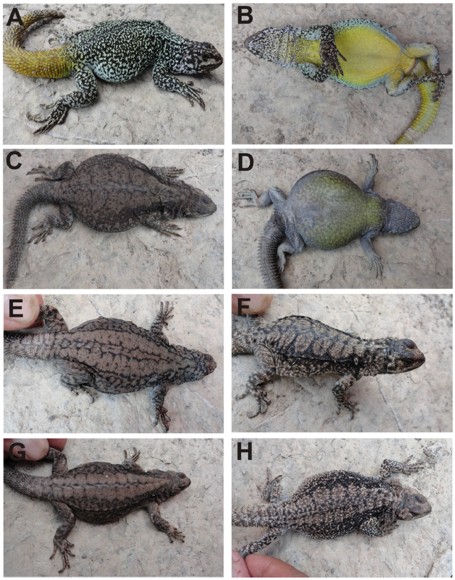
Figure 6. A and B dorsal and ventral views of the holotype (male) of Phymaturus tromen sp. nov. C and D, same views for an adult female of the species. E-H dorsal pattern variation in females of P. tromen sp. nov. Recognizable diagnostic characters for the species: homogeneous thin reticulated pattern over dorsum of trunk head and neck, variegated throat, yellow color more restricted to abdominal region and chest not extended over flanks, and yellow tail in males (Fig. 6A). Females with ocelli of the same side becoming confluent. Females with yellow flank color (Fig. 6D).
Holotype color (Fig. 6a and b): This specimen has a dorsal pattern of black reticulated pigmentation on a light gray background; a pale yellow color covers the dorsum of trunk. This pattern is extended over limbs. The holotype lack a melanic head, and the throat is partially variegated, as it occurs in P. roigorum and P. querque. Male chest has pale gray spots and the belly has yellow color.
Variation: Based on four females and two males (including the holotype). SVL 85.8-105.2 mm (x = 95.6; SD = 6.7). Head length 16.3‑20.1 mm (x = 17.6; SD = 1.3).With the exception of the holotype, all other individuals have regenerated tails. Scales around midbody 224‑261 (x = 240.3; SD = 12.2). Dorsal head scales 22‑25 (x = 22.7; SD = 1.2). Ventrals 192‑230 (x = 206.3; SD = 14.7). Scales surrounding interparietal 8‑9 (x = 8.3; SD = 0.5). Scales of neck along longitudinal fold from posterior border of auditory meatus to shoulder 94‑118 (x = 105.2; SD = 8.8). Gulars 85‑100 (x = 90.5; SD = 6.0). Scales between rostral and frontal 9‑11 (x = 10.2; SD = 0.7).
Pattern of body and limbs, color in life (Fig. 6): This new species shows a notable sexual dichromatism. Males have a dorsal pattern of black reticulated pigmentation on a light gray background; a pale yellow color covers the dorsum of trunk. This pattern is extended over limbs. Males lack a melanic head, and the throat is partially variegated, as in P. roigorum and P. querque. Male chest has pale gray spots and the belly has yellow spots. Females are brown (several of them "chocolate") and have a dorsal pattern formed by two longitudinal rows of ocelli that in most individuals are fused, losing their anterior and posterior margins. In a few individuals a scapular spot is conspicuous (Fig. 6f). A pale thin reticulation all over the belly and chest is evident, with a light yellow color on the chest and anterior sides of belly.
Etimology: The specific epithet ["Tromen"] refers to the name of the volcano at whose base this new species is found.
Distribution (Fig. 4): only known from its type locality. Other lizard species found in the same area are Liolaemus punmahuida (Avila et al., 2003) and Liolaemus tromen (Abdala et al., 2012a).
Phylogenetic relationships: When P. tromen sp. nov. is included in the morphological data set of Lobo et al. (2012a), it is always found related to P. roigorum and P. querque, but its position is uncertain because it changes according to different values of constant K (implied weights). Using a value of K = 3, the analysis was found congruent with other independent studies in Lobo et al. (2012a). Phymaturus tromen is the sister taxon of the pair of species formed by P. querque and P. roigorum. In Morando et al. (2013) this species is recorded as sp.1 and in one of their analyses it is related to P. querque and P. roigorum ("only nuclear BEST analysis") (Morando et al., 2013). The latter result is congruent with our morphological data.
Comment: this new taxa could correspond to populations studied by Morando et al. (2013) as sp. 1.
DISCUSSION
About melanism in Phymaturus
There are two kinds of melanic phenomena in Phymaturus. One of them is restricted to the neck and head and is ruled by the same processes as those that allow differentiation of all sexually dimorphic characters. This case is evident in species of the P. palluma group, whose males exhibit a melanic pigmentation that partially or totally covers the neck and head (P. palluma and P. sp1. of Uspallata, or in P. dorsimaculatus where it is restricted to the throat and sides of head, and the extreme case of P. verdugo). The second condition is an overall melanism that covers the head, body and limbs entirely (individuals of P. tenebrosus Lobo and Quinteros, 2005; P. cacivioi and P. ceii). The latter condition is not determined by sexual dimorphism because both sexes can exhibit this condition, and up to now we do not know any species whose entire population is melanic. Another phenomenon that also deserves attention is the condition exhibited by several species of the Puna clade of Phymaturus (i.e., P. extrilidus), which do not exhibit melanic heads as do southern species (P. palluma, P. verdugo). When individuals sunbathe in the field, their heads become dark, almost black, whereas the remaining pigmentation becomes brighter; in fixatives, these animals exhibit a patterned head with typical reticulation over head and neck. There is a kind of physiological condition that deserves further study. Under careful examination, melanic individuals of P. ceii exhibit (fading) dorsal ocelli, and a throat without variegation, characters that differentiates this species from P. cacivioi sp. nov. The pattern of non-melanic individuals of each species can be distinguished under the darkened surface of bodies, as in other melanic lizards (see Fig.1 in Lacerta vivipara, Jambrich and Jandzik, 2012). Within Liolaemus melanic heads or bodies have been reported for species of the L. fitzingerii group (Cei, 1998; Abdala, 2007; Abdala et al., 2012a; 2012b; Escudero et al., 2012; e.g., L. melanops, L. canqueli, L. tromen, L. purul), and in the "andinus" group (sensu Lobo et al., 2010b) (Koslowskyi, 1898, e.g., L. andinus, L. montanus, L. nigriceps). Within the chiliensis group partial or total melanism has been described in species belonging to different sub-groups in the nigromaculatus group (Müller and Hellmich, 1933, e.g., L. ater, L. sieversi), in the nigroviridis group (Núñez and Labra, 1985, e.g., L. curis) in the altissimus group (Núñez et al., 1991; Navarro and Núñez, 1993, e.g., L. cristiani, L. isabelae); in the petrophilus group (Avila et al., 2010; Abdala et al., 2010, e.g., L. punmahuida); and in the alticolor group (Shreve, 1938, e.g., L. tacnae). Hence, the occurrence of total or partial melanism is quite homoplastic and its expression is varied: head melanism (sexual dimorphic or not), flank melanism, ventral melanism, and complete melanism, among other forms. And because melanism is present in a wide range of latitudes and elevations, its potential adaptive significance can be addressed only by studying each case within those independent lineages.
The genetic basis of melanism has been studied in different vertebrates in recent years. The MC1R gene was found to be related to melanism in mammals (Anderson et al., 2009; McRobie et al., 2009). Buades et al. (2013) studied the MC1R gene in melanic and non-melanic populations of two species of Podarcis and found no statistical evidence of selection for MC1R, suggesting that here is no relationship between MC1R polymorphism and color variation in these lacertids lizards. However, other studies showed that different variants of the MC1R gene are directly related to phenotypes. These variants can result in similar phenotypes; species with darker morphs living in the desert become lighter when living on white sands (Sceloporus undulatus, Aspidoscelis inornata and Holbrookia maculata). Each one of these three species has a single coding mutation in MC1R that is statistically correlated with their phenotypes (Rosenblum et al., 2004; 2010).
The occurrence of populations of lacertid lizards with some melanic individuals has been observed, but entire melanic populations are rare and have been seldom reported (Castilla, 1994; Jambrich and Jandzik, 2012; Trócsányi and Korsós, 2004). Common explanations supporting this phenomenon are related to conditions associated with insularity, either on oceanic islands, restricted alpine habitats or northern latitudes, which could favour the appearance of melanic forms. Reports on this lizard group included Podarcis hispanica (Pérez·Mellado, 1984), P. hispanica atrata, P. dugesii, P. lilfordi and P. muralis (Zuffi, 1986; Barbadillo and Sánchez Herráinz, 1992), Lacerta vivipara and L. agilis (Malkmus, 1976; Bruno, 1979; Bischoff et al., 1989; Kuranova, 1989; Pérez Mellado, 1989; Cirer and Martínez Rica, 1990). The melanism exhibited by the species studied in the present contribution cannot be explained by the fact that populations live at extreme latitudes because there are several species of Phymaturus living far to the south that do not exhibit melanic individuals. In addition, even when most species of Phymaturus show a kind of insular distribution (rocky isolated formations) non-melanic species are more numerous than the melanic ones reported in the present study. Melanism is believed to offer a thermoregulatory advantage (the "thermal melanism hypothesis" of Clusella-Trullas et al., 2008), but results reported in the literature about this hypothesis are contradictory. Tosini et al. (1991) and Gvozdík (1999) rejected or did not confirm this assumption, whereas Pearse and Pogson (2000) suggested that the two populations of the subspecies Anniella pulchra nigra may have arisen from different ancestral populations in response to selection in cool, coastal habitats. Studies that compare heating rates of individuals differing in skin reflectance under the same environmental conditions generally support the hypothesis that melanism plays an adaptive role in thermoregulation (Clusella-Trullas et al., 2008). Different authors have suggested that low skin reflectance would be important for animals inhabiting cold regions (e.g., Watt, 1968); however, among the species sampled they did not include representatives from South America (see Fig. 3, Clusella-Trullas et al., 2008). Our knowledge about thermal biology in Phymaturus does not provide a reasonable answer to this phenomenon; in fact, Cruz et al. (2009) found that thermal biology for Phymaturus is conservative, and detected low levels of variation in the thermal parameters studied, with no clear relationships between climatic and thermal variables. Specific studies to propose an explanation of melanism in Phymaturus are needed. To date, no studies have been conducted on Phymaturus about skin reflectance and its relation to thermoregulation.
A lower melanism rate in females for Lacerta vivipara was reported and was explained by their higher vulnerability to visual predators, mainly during pregnancy, when females rely more heavily on crypsis (Bauwens and Thoen, 1981; Gvozdík, 1999; Jambrich and Jandzik, 2012). Our samples and observations of Phymaturus are limited, so any conclusion should be considered with caution. Phymaturus cacivioi sp. nov. exhibits a sex-biased proportion in melanic individuals (five males versus two females), whereas the only two melanic P. ceii individuals collected are both males, and in P. tenebrosus (those deposited at MCN) four are males and one is a female. We consider that increased predation pressure on melanic females is an interesting hypothesis to be tested in this group.
Phymaturus phylogenetics
There is a significant degree of congruence between morphological and molecular analyses of relationships within Phymaturus (Lobo et al., 2012a; Morando et al., 2013), although different criteria for building trees were implemented. The K3 topology is the morphological hypothesis most congruent with the all-genes molecular analysis: both P. patagonicus and P. palluma groups are recovered; clades A, B, C, and D within the patagonicus group in Lobo et al. (2012a) are indistinctus, spurcus, somuncurensis, and payuniae groups, respectively, in Morando et al. (2013). The only important incongruence is the split of the C clade of Lobo et al. (2012a) into two clades, calcogaster and somuncurensis, in Morando et al. (2013) and the relationships among subclades. Within the palluma group clades F, G, H, and I of Lobo et al. (2012a) are verdugo and mallimacci groups (mallimacci group formed by two subclades with the same internal relationships as those of the morphological analysis). Although basal taxa of the palluma group (vociferator group of Morando et al., 2013) were poorly sampled in the morphological analysis, it is congruent with P. dorsimaculatus, being basal to the remaining species of the group. The use of implied weight in the morphological analysis resulted in better supported clades than in the current un-weighted analyses (Goloboff et al., 2008), but there are no criteria about the magnitude of the weight that should be used in any analysis. Because of this, Lobo et al. (2012a) used several values of K. Comparisons with independent studies, like the one of Morando et al. (2013) based on DNA sequences, allowed us to test the value of morphological information in recovering phylogenetic relationships in this lizard clade. The analysis performed, which yielded a value of 3 to the constant K, provides the most congruent hypothesis with other independent (DNA-based) analysis, the probability that these two independent studies arrive at the same arrangements by chance is almost impossible (see Omland, 1994). Both analyses are congruent between them because they recover only one history of the group.
Phymaturus cacivioi sp. nov. relationships
We were not able to examine samples of sp.18 and sp. 19 of Morando et al. (2013), which could be conspecific with P. cacivioi. In their Bayesian analysis using all-genes information (Fig. 5, Morando et al., 2013) sp. 18 and sp. 19 are found as sister taxa of the payuniae group, P. tenebrosus related to the clade formed by Chubut species, and P. ceii nested within that last group and sister taxon of P. somuncurensis. Morando et al. (2013) for all genes (BEST) sampled found a polytomy of P. sp.18, P. sp.19, P. tenebrosus, and the payuniae and somuncurensis groups (Morando et al., 2013, Fig. 6B). It seems that the Bayesian hypothesis is more congruent with our morphological tree (Lobo et al., 2012a) than the BEST analysis because, both hypotheses have almost the same hierarchical structure: (P. zapalensis (P. sitesi (P. delheyi (P. payuniae-P. nevadoi)))). Therefore, because incongruence between morphological and molecular hypotheses for the patagonicus group remains unexplained, we cannot arrive at definite conclusions about melanism evolution and further studies are necessary. Hypotheses of melanism evolution within the patagonicus group may change with future analyses.
Acknowledgements
J. Faivovich (MACN), L. Avila and C. Pérez (CENPAT) and J. Williams (MLP), allowed us to study specimens under their care. We thank two anonymous referees for their useful comments and suggestions on this contribution. We thank A. Laspiur, S. Valdecantos, J. Grosso, M. Paz, M. Pereyra, B. Blotto and L. Díaz Fernández for helping us in the field or lab. This study was supported by grants (FL) from CONICET Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina (PIP 2841) and CIUNSA Consejo de Investigaciones de la Universidad Nacional de Salta, Argentina (CIUNSA 1663).
Specimens of Phymaturus examined (583 individuals) representing 25 recognized species. Phymaturus calcogaster (n=16): MACN 39990-91 (paratypes), JAS-DC 799, 803, 1096-97: Argentina, Chubut Province, Telsen Dept., Laguna de las Vacas; JAS-DC 1154-55,
Argentina, Chubut Province, Telsen Dept., Bajo Amarillo. MCN-UNSa 4295-98, 4301-04. Laguna de las Vacas, southwestern end of the lake, 42º29'54.60'' S, 67º21'07.03'' O. 651 m above sea level .Telsen Dept., Chubut Province. Argentina.
Phymaturus castillensis (n=15): IBA 869-1, 869-2, 869-3, Argentina, Chubut Province, Sarmiento Department, NW Lago Colhué Huapi, Sierra Castillo, 1000 m. MCN-UNSa 3960-64, 3967-69 3975-78. Sierra del Castillo, Estancia La Juanita, near Provincial Route 24, 58 km NW of Sarmiento, Sarmiento Dept., Chubut Province 45°08'11.30" S, 69°10'10.40"W. 405 m.
Phymaturus ceii (n=21): MCN-UNSa 910-18, Argentina, Río Negro Province, 25 de Mayo Dept., Provincial Road 8, 17 km S of San Antonio del Cuy. MACN 44738 (ex MCN-UNSa 3914), MACN 44739 (ex MCN-UNSa 3918), MACN 44740 (ex MCN-UNSa 3921), MACN 44741 (ex MCN-UNSa 3923), MACN 44742 (ex MCN-UNSa 3928), MACN 44743 (ex MCN-UNSa 3941), On Provintial Route 6, El Cuy Dept., Río Negro Province, Argentina; 40º20'47,1"S; 68º58'50,3"W (1194 m) MCN-UNSa 3913, 3916, 3920, 3939-40, 3942. On Provintial Route 6, El Cuy Dept., Río Negro Province, Argentina; 40º20'47,1"S; 68º58'50,3"W. 1194 m.
Phymaturus damasense (n=6): MNHN 4782 (Holotype). "Las Damas" river, approximately 1.5 Km to east from Termas del Flaco (34º57´56´´S - 70º24´45´´W), 66 km SE from San Fernando, Región del Libertador Bernardo O´Higgins, Chile. SSUC; Re 0413-17 (Paratypes). Same data as the holotype . Between 1765 and 2032 m MNHN; 4745-48. Termas del Flaco (Río Las Damas).
Phymaturus dorsimaculatus (n=35): MCN 1573 (Holotype). Copahue, Dept. Ñorquin.
37°49'S; 71°06'W. Neuquén, Argentina. MCN-UNSa 1571-1572, 1574-1575 (Paratypes). Same data as holotype. MCN 1568-1570. Termas de Copahue, Dept. Ñorquin, Neuquén, Argentina. 37°49'14"S; 71°05'12"W; 2050 m. MCN-UNSa 921.Termas de Copahue, 2050 m. MVZ 232503. Depto. Ñorquin, Barda W Termas de Copahue; elevation 2050 m. Prov. Neuquén, Argentina. MCN-UNSa 1565-1567, 1573 and MCN-UNSa 1484-1487 Copahue, Dept. Ñorquin, Neuquén, Argentina. MCN-UNSa 1579-1581. R Provincial Route 4, 14.8 km W Colipilli. 37º 44' 06''S; 70º 28' 53''W. 1590 m. Ñorquin Dept. Neuquén Province, Argentina. MCN-UNSa 3727-32. Between El Huecu and Colipilli 37°44'06.6S; 70°28'52.1W. 1545 m. MCN-UNSa 3733-40. North of El Huecu, 37° 36´31.1´´S; 70°37´53.5´´W; 1638 m.
Phymaturus etheridgei (n=17): FML 23495 (Holotype) FML 23496-501 (paratypes) Argentina, Río Negro Province, 25 de Mayo Dept., between Ingeniero Jacobacci and Moligüe, on Provincial Route 76, 41°34'47.2"S, 69°23'33.0"W,818 m; FML 8435, MCN-UNSa 3109-13, Argentina, Río Negro Province, 25 de Mayo Dept., 43 km N of Moligüe, 41°35.880'S, 69°22.628'W. MCN-UNSa 4305, 07-08, 10. Between Ingeniero Jacobacci and Molihue (Provincial Route 76), 41°34'47.2"S, 69°23'33.0"W. 818 m.
Phymaturus excelsus (n=9): MCN-UNSa 1582 (Holotype) Provincial Route 6, 1 km NW of Ojo de Agua, 41°32'30"S, 69°51'33"W, 1141 m, Ñorquinco Dept., Río Negro Province, Argentina. MCN-UNSa 1386, 1388 (paratypes) Argentina, Río Negro Province, Ñorquinco Dept., Provincial Route 6, Ojo de Agua; MCN-UNSa 1587-88, no data. MCN-UNSa 1385, 1387. Provincial Route 6, Ojo de Agua; Ñorquinco Dept., Río Negro Province, Argentina. MCN-UNSa 1590. Provincial Route 6, 1 km NW Ojo de Agua, 41°32'30"S, 69°51'33"W, 1141 m. Ñorquinco Dept., Río Negro Province, Argentina.
Phymaturus felixi (n=18): MCN-UNSa 1280 (Holotype) MCN-UNSa 1279, 1281-83 (paratypes) Argentina, Chubut Province, Paso de Indios Dept., 108 km S Paso de Indios on Provincial Route 24. MCN-UNSa 3979-91.84.5 km S to Paso de Indios on Provincial Route 24. Paso de Indios Dept., Chubut Province, Argentina.44º 27' 10.5'' S 69º 17' 48.3'' W. 734 m.
Phymaturus indistinctus (n=24): IBA 666-1, (Holotype) IBA 666-2-3, Lago Munsters, 2 km W. Las Pulgas, 700-800 m, Sarmiento Dept., Chubut Province, Argentina. MCN-UNSa1274-77, Las Pulgas, hill opposite the Virgin Grotto, Sarmiento Dept., Chubut Province, Argentina. MCN-UNSa 3943-55. 19 km W to Los Manatiales, Provincial Route 20, 45º27'S; 69º42'W, 669 m.
Phymaturus manuelae (n=7): UNCo-PH 201-02 (paratypes) JAS-DC 1251, 26 km W Comallo, adjacent to National Route 23. Pilcaniyeu Dept., Río Negro Province, Argentina. MCN-UNSa 3929-30, 3932-33. Between Pilcaniyeu and Las Bayas on National Road 40, 41°12´11.1´´S; 70°41´30.9´´W; 1014 m. Pilcaniyeu Dept., Río Negro Province, Argentina.
Phymaturus maulense (n=17): MNHN 3938-42, 3945, 4038-39. Vilches Alto, El Enladrillado, Reserva Nacional Altos de Lircay, (35º35'S; 70º58'W, 2189 m). MZUC 35959-60. Lircay, Provincia de Talca, Región del Maule. MVZ 232506-07. On the road to Laguna del Maule (Los Cóndores Pass), Talca Prov.; elevation 1800 m. Región VII (= Región del Maule), Chile. SSUC Re 0410-11. Laguna del Maule, Talca Prov. Región VII; F. MNHN 2353, 2460-61. Baños del Campanario (1500 m), Talca, San Clemente.
Phymaturus nevadoi (n=17): IBA 999, 3 individuals, type series, Argentina, Mendoza Province, Malargüe Dept., Macizo Nevado, Agua de la India Muerta, 1750 m. MCN-UNSa 3647, 3652-64. On Provincial Road 186 35°55'44.8''S; 68°32'36.7''W. 1711 m. Malargüe Dept., Mendoza Province, Argentina.
Phymaturus palluma (n=40): MCN-UNSa 3130-3131. On the road to Portillo Argentino (Cordón del Portillo) S33°36'53.8''; W69°29'16.7'', Mendoza Province, Argentina. MCN-UNSa 3612-13, 3619-22. On the road to Portillo Argentino, Arroyo Guardia Vieja S33°36'53.8''; W69°29'16.7'', Mendoza Province, Argentina. MCN-UNSa 2897, 2899-2900. Valle Hermoso, Malargüe Dept., Mendoza Province. MVZ 126991. Valle Hermoso, Malargüe Dept., Mendoza Province, Argentina. 35°20'S; 70°15'W. MVZ 126992-126894. Laguna de la Niña Encantada. 6 km E de los Molles, 33°18´S; 69°83´W. 2000 m. Mendoza Province, Argentina. MVZ 126995. North of Valle Hermoso, 35°11'S; 70°10'W. Malargüe Dept., Mendoza Province, Argentina.MVZ 126996-126999. 4 km NW Cerro Chupasangral; 2800 m. 33°21'S; 69°51'W. Tupungato Dept., Quebrada de Chupasangral, Mendoza Province, Argentina. MVZ 127025-127027. 2 km E Agua Botada, 35°62' S; 69°95' W, Malargüe Dept., Mendoza Province, Argentina. MVZ 180771-180774. Quebrada Cruz de Piedra, 34°26'S; 68°90'W. San Carlos Dept., Mendoza Province, Argentina. MCN-UNSa 3627-30, 3635-43, 3645.On the road to Laguna Diamante. 34°14'33.6''S; 69°24'00.0''W. San Carlos Dept., Mendoza Province, Argentina.
Phymaturus sp. (n=55): MCN-UNSa 2104-12. Near Paramillos. S 32º 28' 38.6''; W 69º 09' 06.4''.2746 m. Las Heras Dept., Mendoza Province, Argentina. SDSU 1969-1970. 20 km NE Uspallata, 2500 m. Mendoza Province, Las Heras Dept., Argentina: SDSU 3387. 27 km NE Uspallata. 32°28'52.2"S-69°09'59.2"W. 2768 m. Mendoza Province, La Heras Dept., Argentina: SDSU 3388. 27 km NE Uspallata. 32°28'52.2"S 69°09'59.2"W. 2768 m. La Heras Dept., Mendoza Province, Argentina MVZ 145146. Pampa de Canota, 20 km E, 8 km S Estancia Uspallata. 32°65'S; 69°27'W.; 3000 m. Las Heras Dept., Mendoza Province, Argentina. MVZ 92902, 92904, Las Heras Dept., Mendoza Province, Argentina. REE-SDSU 2306-2307, 2312-2313, 20 km NE Uspallata, 2500 m. IADIZA-CH. S/N (2 individuals) Paramillos, Mendoza Province, Argentina. IBA 760 (4 individuals).Paramillos, Mendoza Province. 2000 m. Argentina. MCN-UNSa 2650-2653, 2659-2662,2696-2708. El Portezuelo, San Juan Province, Argentina. MCN-UNSa 3614-17, 3624-26. Paramillos, Mendoza Province, Argentina. 32°28'59.3''S; 69°07'36.5''W. MVZ 127023. 2 km E of Los Hornillos, 32°51'S; 68°99'W. Las Heras Dept., Mendoza Province, Argentina.
Phymaturus patagonicus (n=35): MLP 778 (lectotype), MLP 777 (paralectotype) Argentina, Territorio del Chubut, Patagonia. FML 10077-85. 1 km W intersection of Provincial Routes 53 and 90, 2.2 km SW Meseta El Sombrero Paso de Los Indios Dept., Chubut Province, Argentina. IADIZA 80. 40 km W Dolavon, 350 m, Gaiman Dept., Chubut Province, Argentina. IBA 783, 5, 20 km W Sombrero; Paso de Los Indios Dept., Chubut Province, Argentina. IBA 789, 7. MCN-UNSa 1284-86. 40 km W Dolavon, Gaiman Dept., Chubut Province, Argentina. MCN-UNSa 1250-58,1261, Paso de Los Indios Dept., hills in front of El Sombrero, Chubut Province, Argentina. SDSU 1980, 40 km WSW Dolavon, Gaiman Dept., Chubut Province, Argentina.
Phymaturus payuniae (n=45): IBA 769-2, 769-4-8, 769-10, 769-12, 769-17, 769-20, 769-24, 769-26, type series, Argentina, Mendoza Province, Malargüe, Dept., Payún Plateau, 5 km from Volcán Payún, 2000 m; IADIZA 87-8-9, Argentina, Mendoza Province, Malargüe Dept., 20 km SE Volcán Payún, 1800 m. MCZ 152079-81. Basaltic rocks of the Payún Plateau Argentina, Mendoza Province, Malargüe Dept. REE-SDSU 2330-32, 2339, SDSU 1981-84, Argentina, Mendoza Province, Malargüe Dept., 10 km SW base of Volcán Payún. MCN-UNSa 3648-51, 3665-79. On Provincial Road 183 16 km S to Payún vulcano 36°40'20.8''S; 69°16'10.9''W. 1737 m.
Phymaturus querque (n=26): FML 21556 (holotype). Laguna Blanca, Laguna Blanca National Park, Zapala Dept., Neuquén province, Argentina. Paratypes: FM L 21211. One female.Same data as holotype. IBA 793 (4 individuals). Laguna Blanca. Neuquén province, Argentina. MACN 34514 (5 individuals). Laguna Blanca. Neuquén. MVZ 232504-05. Puesto Control, 3.5 km N Co. de 1 Laguna PN Laguna Blanca. 23°80'S, 56°83'W. 1800 m. Zapala Dept., Neuquén Province, Argentina. SDSU 1971. South shore of Laguna Blanca, Zapala Dept., Neuquén Province, Argentina.MCN-UNSa 3854-66. 9,5 km S Laguna Blanca, on road 46, 39°08'02.40"S; 70°25'45.80"W. 1387 m. Catán Lil Dept., Neuquén Province, Argentina.Phymaturus roigorum (n=37): MCN 1963 (holotype) Puesto Rojas, 16 km. de Ruta Provincial 180. El Nevado. Departamento de San Rafael, Mendoza Province. MCN 1962, same data holotype. FML 17705-708 (paratypes) same data as holotype. MCN 2096-2103 (paratypes). 6 km S Real del Molle, base of Payún Liso vulcano, 2128 m. 36°28'51,1"S; 69°22'27,9"W. Malargüe Dept., Mendoza Province, Argentina. SDSU 1948-51, 56, 62, 64-65. 3 km NW of base of Volcán Payún. Malargüe Dept. Mendoza Province, Argentina. SDSU 1972, 1974-75. 10 km S of base of Volcán Payún, Malargüe Dept., Mendoza Province, Argentina. IADIZA-CH 00091. Base of Volcán Payún. 1800-2000 m., Mendoza Province, Argentina. IBA 733 (5 individuals). Base Campamento. SW side of Payún. Mendoza Province. Argentina. MCN-UNSa 2113. Real del Molle. S 36º 28' 23.6''; W 69º 22' 46.8''. Malargüe Dept., Mendoza Province, Argentina.
Phymaturus somuncurensis (n=29): IBA 470, 2, type series, MACN 37436-40, MCZ 156909, 170443-44, Laguna Raimunda, Meseta de Somuncurá, 9 de Julio Dept., Río Negro Province, Argentina. FML 1038, Laguna Raimunda, Meseta de Somuncurá, 1400 m; Valcheta Dept., Río Negro Province Argentina. IADIZA 212, Meseta de Somuncurá, Cerro Corona; 9 de Julio Dept., Río Negro Province, Argentina. IBA 507, 4, Argentina, Río Negro Province, 9 de Julio Dept., ca. Laguna Raimunda, Meseta de Somuncurá. MACN 37431-35, 2 km N Casco Cecchi, Meseta de Somuncurá, 9 de Julio Dept., Río Negro Province Argentina. REE-SDSU 2433-35. N Laguna Raimunda, Meseta de Somuncurá. Valcheta Dept., Río Negro Province, Argentina. SDSU 1780-83, 2 km N Laguna Raimunda, Meseta Somuncurá. 9 de Julio Dept., Río Negro Province, Argentina. MCN-UNSa 4550 (SJ 25). 41°12'13.95" S; 66°53'31.94" W, 1060 m. Meseta Somuncurá. 9 de Julio Dept., Río Negro Province, Argentina.
Phymaturus spectabilis (n=27): MCN-UNSa 1203 (holotype) MCN-UNSa 1204-15 (paratypes), 28 km S Ingeniero Jacobacci on Ruta Provincial 6; 25 de Mayo Dept., Río Negro Province, Argentina. FML 23502-15. Provincial oute 6, 27 km S of intersection with Provincial Route 23, 41°25'943.250 S, 69°45'924.000 W, 924 m. 25 de Mayo Dept., Río Negro Province, Argentina.
Phymaturus spurcus (n=16): MCZ 14791 (Holotype), MCZ 14914-15 (paratypes), Argentina, Río Negro Province, Pilcaniyeu Dept., Huanuluan; MCN-UNSa 1238-40, 1244-
49, 22 km W Ingeniero Jacobacci, National Route 23, Hills opposite Estancia Huanuluan; 25 de Mayo Dept., Río Negro Province, Argentina. MVZ 188904-07. Along rimrock 4 km S and 1 km E Alto del Escorial, 1100 m. Ñorquinco Dept., Río Negro Province, Argentina.
Phymaturus tenebrosus (n=18): MCN-UNSa 1271 (Holotype), MCN-UNSa 1264-70, 1272-73 (paratypes), National Road 40, 20 km S Cerro Alto; Pilcaniyeu Dept., Río Negro Province, Argentina. MCN-UNSa 1591-95, 1597-99, between Bariloche and Pilcaniyeu, Pilcaniyeu Dept., Río Negro Province, Argentina.
Phymaturus verdugo (n=8): MCN-UNSa 1958, 1960-1961. Río El Gancho 4 km. from Las Loicas. Mendoza Province, Argentina. MCN 1973-1977. 12.5 km from Las Loicas to Bardas Blancas, road to El Pehuenche. Mendoza Province, Argentina.
Phymaturus videlai (n=8): FML 21240-43, 126 km N Alto Río Senguer, 7 km N intersection of National Routes 40 and 26. Río Senguer Dept., Chubut Province, Argentina.
MCN-UNSa 4203-04, 07. Near Buen Pasto 85 km NW of Sarmiento 45°04'11"S, 69°25'25"W. 700 m, Sarmiento Dept., Chubut Province, Argentina.
Phymaturus zapalensis (n=37): IBA 792, 4, type series, Argentina, Neuquén Province, Zapala Dept., Laguna Teru, Laguna Blanca. IBA 866-1, 998-3, 2, 55 km S Piedra del Aguila. Collón Curá Dept., Neuquén Province Argentina. MCN-UNSa 1600-02, National Route 40, 1 km S Salitral, 39°40.600'S, 70°36.925'W, 994 m. Catán Lil Dept., Neuquén Province, Argentina. MVZ 188908-10, 8 km N and 4 km E Junín de los Andes on rocks along Río Malleo, 800 m; Huiliches Dept., Neuquén Province, Argentina. MVZ 232508-12. Provincial Route 46, 9.5 km S, 5 km Cerro Chachil, 1580 m; Catán Lil Dept., Neuquén Province, Argentina. MVZ 232513, 0.5 km W Primeros Pinos, 1600 m; Pirunches Dept., Neuquén Province, Argentina. MVZ 232514, Puesto de Control, 3.5 km N of Laguna, Parque Nacional Laguna Blanca, 39°02'32"S, 70°21'52"W, 1300 m, Zapala Dept., Neuquén Province, Argentina, Laguna Blanca. MVZ 232515-16, Provincial Route 46, Zapala Dept., Neuquén Province, Argentina. SDSU 1985-88, S shore Laguna Blanca; Zapala Dept., Neuquén Province, Argentina, SDSU 1989-90, S shore Laguna Blanca, 1275 m. Zapala Dept., Neuquén Province, Argentina. MCN-UNSa 3844-53. 9,5 km S to Laguna Blanca on Provincial Route 46, 39°08'02.40"S; 70°25'45.80"W 1387 m. Catán Lil Dept., Neuquén Province, Argentina.
LITERATURE CITED
1. Abdala, C. S. 2007. Phylogeny of the boulengeri group (Iguania: Liolaemidae, Liolaemus) based on morphological and molecular characters. Zootaxa, 1538, 1-84. [ Links ]
2. Abdala, C. S.; Quinteros, A. S.; Scrocchi, G. J. & Stazzonelli, J. C. 2010. Three new species of the Liolaemus petrophilus group (Iguania: Liolaemidae) from Argentina. Cuadernos de Herpetología 24: 25-40. [ Links ]
3. Abdala, C. S.; Semhan, R. V.; Moreno Azócar, D. L.; Bonino, M.; Paz, M. M. & Cruz, F. 2012a. Taxonomic study and morphology based phylogeny of the patagonic clade Liolaemus melanops group (Iguania: Liolaemidae), with the description of three new taxa. Zootaxa 3163: 1-32. [ Links ]
4. Abdala, C. S.; Díaz Gómez, J. M. & Juárez Heredia, V. I. 2012b. From the far reaches of Patagonia: new phylogenetic analyses and description of two new species of the Liolaemus fitzingerii clade (Iguania: Liolaemidae). Zootaxa 3301: 34-60. [ Links ]
5. Anderson, T. M.; von Holdt, B. M.; Candille, S. I.; Musiani, M.; Greco, C.; Stahler, D. R.; Smith, D. W.; Padhukasahasram, B.; Randi, E.; Leonard, J. A.; Bustamante, C. D.; Ostrander, E. A.; Tang, H.; Wayne, R. K. & Barsh G. S. 2009. Molecular and evolutionary history of melanism in North American gray wolves. Science 323: 1339-1343. [ Links ]
6. Avila, L. J.; Pérez, C. H. F. & Morando, M. 2003. A new species of Liolaemus (Squamata: Iguania: Liolaemidae) from Northwestern Patagonia (Neuquén, Argentina). Herpetologica 59: 534-545. [ Links ]
7. Avila, L. J.; Morando, M.; Pérez, D. R. & Sites Jr. J. W. 2010. A new species of the Liolaemus elongatus clade (Reptilia: Iguania: Liolaemini) from Cordillera del Viento, northwestern Patagonia, Neuquén, Argentina. Zootaxa 2667: 28-42. [ Links ]
8. Avila, L. J.; Pérez, C. H. F; Pérez, D. R. & Morando, M. 2011. Two new mountain lizard species of the Phymaturus genus (Squamata: Iguania) from northwestern Patagonia, Argentina. Zootaxa 2924: 1-21. [ Links ]
9. Avila, L. J.; Pérez, C. H. F; Minoli, I. & Morando, M. 2014. A new lizard of the Phymaturus genus (Squamata: Liolaemidae) from Sierra Grande, northeastern Patagonia, Argentina. Zootaxa 3793: 099-118. [ Links ]
10. Barbadillo, L. J. & Sánchez-Herráiz M. J. 1992. Melanismo en una población de Podarcis muralis (Reptilia, Lacertidae) de Cantabria (N. de España). Boletín de la Asociación Herpetológica Española 3: 15-17. [ Links ]
11. Buades, J. M.; Rodríguez, V.; Terrasa, B.; Pérez Mellado, V.; Castro, J. A.; Picornell, A. & Ramon, M. 2013. Variability of the mc1r Gene in Melanic and Non-Melanic Podarcis lilfordi and Podarcis pityusensis from the Balearic Archipelago. Plos One 8: 1-9. [ Links ]
12. Bauwens, D. & Thoen, C. 1981. Escape tactics and vulnerability to predation associated with reproduction in the lizard Lacerta vivipara. Journal of Animal Ecology 50: 733-743. [ Links ]
13. Bischoff, W.; Osenegg, K. & Mayer, W. 1989: Untersuchungen zur subspezifischen Gliederung der Madeira Mauereidechse, Podarcis dugesii (Milne-Edwards 1829). Salamandra 25: 237-259. [ Links ]
14. Bruno, S. 1979. Rettili d'ltalia. Tartarughe-Sauri-Serpenti. Giunti Martello, Firenze. 363 pp [ Links ]
15. Castilla, A. M. 1994. A case of melanism in a population of the insular lizard Podarcis hispanica atrata. Bolletí de la Societat d'Història Natural de les Balears 37: 175-180. [ Links ]
16. Cei, J. M. 1998. La mélanocéphalie chez les Lézards liolamines et redécouverte de l'holotype de Liolaemus melanops Burmeister, 1888 longtemps considéré comme perdu (Reptilian: Squamata: Iguania: Tropiduridae). Revue Français de Aquariologie 25: 59-62. [ Links ]
17. Cirer, A. M. & Martínez-Rica, J. P. 1990. The polymorphism of Podarcis pityusensis and its adaptive evolution in the Mediterranean isles. Herpetological Journal 1: 465-473. [ Links ]
18. Clusella-Trullas, S.; Terblanche, J. S.; Blackburn, T. M. & Chown, S. L. 2008. Testing the thermal melanism hypothesis: a macrophysiological approach. Functional Ecology 22: 232-238. [ Links ]
19. Cruz, F. B.; Belver, L.; Acosta, J. C.; Villavicencio, H. J.; Blanco, G. & Cánovas, M. G. 2009. Thermal biology of Phymaturus lizards: evolutionary constraints or lack of environmental variation? Zoology 112: 425-432. [ Links ]
20. Escudero, P. C.; Minoli, I.; Frutos, N.; Avila, L. J. & Morando, M. 2012. Estudio comparativo del melanismo en lagartijas del grupo Liolaemus fitzingerii (Liolaemini: Liolaemus). Cuadernos de Herpetología 26: 79-89. [ Links ]
21. Espinoza, R. E.; Wiens, J. J. & Tracy, C. R. 2004. Recurrent evolution of herbivory in small, cold-climate lizards: breaking the ecophysiological rules of reptilian herbivory. Proceedings of the National Academy of Sciences 101: 16819-16824. [ Links ]
22. Etheridge, R. E. 1995. Redescription of Ctenoblepharys adspersa Tschudi, 1845, and the taxonomy of Liolaeminae (Reptilia: Squamata: Tropiduridae). American Museum of Natural History Novitates 3142: 1-34. [ Links ]
23. Frost, D. R. 1992. Phylogenetic analysis and taxonomy of the Tropidurus group of lizards (Iguania: Tropiduridae). American Museum of Natural History Novitates 3033: 1-68. [ Links ]
24. Goloboff, P. A.; Carpenter, J. M.; Arias, J. S. & Miranda Esquivel, D. R. 2008. Weighting against homoplasy improve phylogenetic analysis of morphological data sets. Cladistics 24: 758-773. [ Links ]
25. Gvozdík, L. 1999. Colour polymorphism in a population of the common lizard, Zootoca vivipara (Squamata: Lacertidae). Folia Zoologica 48: 131-136. [ Links ]
26. Jambrich, A. & Jandzik, D. 2012. Melanism in the topotypic population of the Pannonian subspecies of the common lizard, Zootoca vivipara pannonica (Reptilia: Lacertidae). Herpetology Notes 5: 219-221. [ Links ]
27. Koslowsky, J. A. 1898. Enumeración sistemática y distribución geográfica denlos reptiles argentinos. Revista del Museo de La Plata 8: 13-200. [ Links ]
28. Kuranova, V. N. 1989. On melanism in the viviparous lizard and common adder. Vestnik Zoologii 2: 59-61. [ Links ]
29. Lobo, F. & Abdala, C.S. 2007. Descripción de una nueva especie de Phymaturus del grupo de P. palluma de la provincia de Mendoza, Argentina. Cuadernos de Herpetología 21: 103-113. [ Links ]
30. Lobo, F. & Quinteros, S. 2005. A morphology-based phylogeny of Phymaturus (Iguania: Liolaemidae) with the description of four new species from Argentina. Papéis Avulsos de Zoologia 45: 143-177. [ Links ]
31. Lobo, F.; Abdala, C. & Valdecantos, S. 2010a. Taxonomic studies of the genus Phymaturus (Iguania: Liolaemidae): description of four new species. South American Journal of Herpetology 5: 102-126. [ Links ]
32. Lobo, F.; Slodki, D. & Valdecantos, S. 2010b. Two new species of lizards of the Liolaemus montanus group (Iguania: Liolaemidae) from the northwestern Uplands of Argentina. Journal of Herpetology 44: 279-293. [ Links ]
33. Lobo, F.; Abdala, C. & Valdecantos, S. 2012a. Morphological diversity and phylogenetic relationships within a South-American clade of iguanian lizards (Liolaemidae: Phymaturus). Zootaxa 3315: 1-41. [ Links ]
34. Lobo, F.; Cruz, F. B. & Abdala, C. S. 2012b. Multiple lines of evidence show that Phymaturus agilis Scolaro, Ibargüengoytía, and Pincheira-Donoso, 2008 is a junior synonym of Phymaturus spectabilis Lobo & Quinteros, 2005. Cuadernos de Herpetologia 26: 21-27. [ Links ]
35. Lobo, F.; Espinoza, R.; Sanabria, E. & Quiroga, L. 2012c. A new Phymaturus (Iguania: Liolaemidae) from the southern extreme of the Argentine Puna. Copeia 1: 12-22. [ Links ]
36. Lobo, F.; Nenda, S. J. & Slodki, D. 2012d. A new lizard of Phymaturus (Iguania: Liolaemidae) from Argentina. Herpetologica 68: 121-133. [ Links ]
37. Lobo, F.; Laspiur, A. & Acosta, J. C. 2013. Description of new Andean species of the genus Phymaturus (Iguania: Liolaemidae) from northwestern Argentina. Zootaxa 3683: 117-132. [ Links ]
38. Malkmus, K. 1976. Ein negrino der Bergeidechse (Lacerta vivipara) aus den schladminger Tauern. Naehr Naturwissenschaftliche Museum Aschaffenburg 84: 11-16. [ Links ]
39. McRobie, H.; Thomas, A. & Kelly, J. 2009. The genetic basis of melanism in the gray squirrel (Sciurus carolinensis). Journal of Heredity 100: 709-714. [ Links ]
40. Morando, M. 2004. Sistemática y filogenia de grupos de especies de los géneros Phymaturus y Liolaemus (Squamata: Tropiduridae: Liolaeminae). Unpublished Ph.D. Dissertation, Universidad Nacional de Tucumán, Argentina. [ Links ]
41. Morando, M.; Avila, L.; Pérez, C. H. F.; Hawkins, M. & Sites Jr., J. W. 2013. A molecular phylogeny of the lizard genus Phymaturus (Squamata, Liolaemini): Implications for species diversity and historical biogeography of southern South America. Molecular Phylogenetics and Evolution 66: 694-714. [ Links ]
42. Müller, L. & Hellmich, W. 1933. Beiträge zur Kenntnis der Herpetofauna Chiles. VII. Der Rassenkreis der Liolaemus nigromaculatus. Zoologischer Anzeiger 103: 128-142. [ Links ]
43. Navarro, J. & Núñez, H. 1993. Liolaemus patriciaiturrae and Liolaemus isabelae, two new species of lizards for northern Chile: Biogeographic and cytotaxonomic aspects (Squamata, Tropiduridae). Boletín del Museo Nacional de Historia Natural, Chile 44: 99-113. [ Links ]
44. Núñez, H.; Navarro, J. & Loyola, J. 1991. Liolaemus maldonadae y Liolaemus cristiani, dos especies nuevas de lagartijas para Chile (Reptilia, Squamata). Boletín del Museo Nacional de Historia Natural, Chile 42: 79-88 [ Links ]
45. Núñez, H. & Labra, M. A. 1985. Liolaemus curis, a new lizard from the Los Andes range, central Chile. Copeia 1985: 556-559. [ Links ]
46. Núñez, H.; Veloso, A.; Espejo, P.; Veloso, C.; Cortés, A. & Araya, S. 2010. Nuevas especies de Phymaturus (grupo "palluma") para la zona cordillerana central de Chile (Reptilia, Sauria, Liolaemidae). Boletín del Museo Nacional de Historia Natural, Chile 59: 41-74. [ Links ]
47. Omland, K. E. 1994. Character congruence between a molecular and a morphological phylogeny of Dabbling ducks (Anas). Systematic Biology 43: 369-386. [ Links ]
48. Pearse, D. E. & Pogson, G. H. 2000. Parallel evolution of the melanic form of the California legless lizard, Anniella pulchra, inferred from mitochondrial DNA sequence variation. Evolution 54: 1041-1046. [ Links ]
49. Pérez-Mellado, V. 1984. Sobre un ejemplar melánico de Podarcis hispanica (Steindacher, 1870). Doñana, Acta Vertebrata 11: 320-321. [ Links ]
50. Pérez-Mellado, V. 1989. Estudio ecológico de la lagartija balear Podarcis lilfordi (Günther, 1874) en Menorca. Revista de Menorca 80: 455-511. [ Links ]
51. Rosenblum, E. B.; Hoekstra, H. E. & Nachman, M.W. 2004. Adaptive reptile color variation and the evolution of the Mc1r gene. Evolution 58: 1794-1808. [ Links ]
52. Rosenblum E. B.; Rompler, H.; Schoneberg, T. & Hoekstra, H. E. 2010. Molecular and functional basis of phenotypic convergence in white lizards at White Sands. Proceedings of the National Academy of Sciences USA 107: 2113-2117. [ Links ]
53. Sabaj Pérez, M. H. (editor). 2013. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an Online Reference. Version 4.0 (28 June 2013). Available at <http://www.asih.org/, American Society of Ichthyologists and Herpetologists, Washington, DC> [ Links ].
54. Scolaro, J. A. & Ibargüengoytía, N. R. 2007. A new species of Phymaturus from rocky outcrops in the central steppe of Rio Negro province, Patagonia Argentina (Reptilia: Iguania: Liolaemidae). Zootaxa 1524: 47-55. [ Links ]
55. Scolaro, J. A.; M. Jara & D. Pincheira Donoso 2013. The sexual signals of speciation? A new sexually dimorphic Phymaturus species of the patagonicus clade from Patagonia Argentina. Zootaxa 3722: 317-332. [ Links ]
56. Scolaro, J. A; Méndez de la Cruz, F. & Ibarguengoytia, N. R. 2012. A new species of Phymaturus of the patagonicus clade (Squamata, Liolaemidae) from isolated plateau of southwestern Rio Negro Province, Argentina. Zootaxa 3451: 17-30. [ Links ]
57. Shreve, B. 1938. A new Liolaemus and two new Syrhopus from Perú. Journal of the Washington Academy of Sciences 28: 404-407. [ Links ]
58. Smith, H. M. 1946. Handbook of Lizards: Lizards of the United States and of Canada. Comstock Publishing Company, Ithaca, New York. [ Links ]
59. Tosini, G.; Lanza, B. & Bacci, M. 1991. Skin reflectance and energy input of melanic and non-melanic populations of wall lizard (Podarcis muralis). Pp. 443-448 En: Korsós, Z. & Kiss, I. (eds.), Proceedings of the Sixth Ordinary General Meeting. Budapest (SEH-HNHM). [ Links ]
60. Trócsányi, B. & Korsós, Z. 2004. Recurring melanism in a population of the common wall lizard: numbers and phenotypes. Salamandra 40: 81-90. [ Links ]
61. Troncoso-Palacios, J. & Lobo, F. 2012. A new species of Phymaturus (Iguania: Liolaemidae) of the palluma group from Central Chile. Cuadernos de Herpetología 26: 69-78. [ Links ]
62. Watt, W. B. 1968. Adaptive significance of pigment polymorphisms in Colias butterflies. I. Variation of melanin pigment in relation to thermoregulation. Evolution 22: 437-458. [ Links ]
63. Zuffi, M. 1986. Su Podarcis muralis maculiventris (Werner, 1981) melanica in Risaia a Bereguardo (Pavia). Atti Societa italiana Di Scienze Naturale E Museo Civico Di Storia Naturale Milano 127: 293-296. [ Links ]














