Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Cuadernos de herpetología
versión On-line ISSN 1852-5768
Cuad. herpetol. vol.30 no.2 San Salvador de Jujuy set. 2016
TRABAJO
Gonadal development in the Neotropical high Andean frog Dendropsophus labialis (Amphibia: Hylidae)
María Alejandra Pinto-Erazo1, Javier Goldberg2, Adriana Jerez1
1Laboratorio de Ecología Evolutiva, Departamento de Biología, Universidad Nacional de Colombia, Sede Bogotá. Calle 45 # 26 - 85. Bogotá, Colombia.
2Instituto de Bio y Geociencias del NOA (IBIGEO-CONICET), CCT-Salta. 9 de Julio 14. 4405. Rosario de Lerma. Salta, Argentina.
Recibido: 28/06/16
Revisado: 19/07/16
Aceptado: 19/08/16
ABSTRACT
Amphibians are one of the most threatened groups of vertebrates whose populations are declining due to many reasons including, among others, the presence of chemicals associated with human intervention and in particular, with agriculture. These chemicals are deposited where amphibian larvae develop provoking morphological abnormalities in different organs, such as the gonads, which have been used as biological indicators of these substances in the environment. Therefore, descriptions of normal gonadal development among anurans are important not only to increase our knowledge about the reproductive biology of species but also to identify the type of gonadal abnormalities. The main objective of this work was to describe the morphological changes that occur during gonadal differentiation in the Neotropical high Andean frog Dendropsophus labialis, an endemic species from the Eastern Andes of Colombia. We analyzed 211 individuals including larvae and adults. This species has a differentiated pattern of gonadal development and accelerated rates of ovary and testis differentiation since mature oocytes and seminiferous tubules are present during pre-metamorphic larval stages. In comparison with other phylogenetically related species, D. labialis has one of the most accelerated rates of gonadal differentiation described so far. We confirmed that the gonadal development is to some degree independent of somatic development and we described a case of a gonadal abnormality.
Key words: Tadpoles; Gonadal abnormalities; Ovarian differentiation; Testicular differentiation.
RESUMEN
Los anfibios constituyen uno de los grupos de vertebrados más amenazados, y sus poblaciones están declinando debido a diferentes factores que incluyen, entre otros, productos químicos provenientes de la actividad antrópica, en particular, la agricultura. Estos químicos se depositan en los cuerpos de agua donde se desarrollan las larvas de anfibios, generando malformaciones en diferentes órganos, como las gónadas, las cuales han sido usadas como indicadores biológicos del impacto de estas sustancias en el ambiente. Por lo tanto, las descripciones del desarrollo gonadal normal en anfibios son relevantes, ya que no solo nos permite conocer acerca de la biología reproductiva de las especies, sino que también es fundamental para identificar anormalidades gonadales. El objetivo de este trabajo es describir los cambios que ocurren durante la diferenciación gonadal en la rana neotropical altoandina Dendropsophus labialis, endémica de la cordillera oriental de Colombia. Analizamos 211 individuos incluyendo larvas y adultos. Esta especie presenta un patrón de desarrollo gonadal diferenciado; además presenta un desarrollo acelerado del ovario y el testículo, con ovocitos maduros y túbulos seminíferos presentes en estados pre metamórficos. Es así que respecto a otras especies relacionadas filogenéticamente, D. labialis exhibe una de las tasas más acelerada del desarrollo gonadal hasta ahora descritas. Finalmente, confirmamos que el desarrollo gonadal es en algún grado independiente del desarrollo somático, y describimos un caso de anormalidad gonadal.
Palabras clave: Renacuajos; Anormalidad gonadal; Diferenciación ovárica, Diferenciación testicular.
INTRODUCTION
Elucidating the normal pattern of gonadal differentiation and development in anurans has broad implications for many biological disciplines such as developmental biology, evolutionary biology, evolutionary developmental biology or conservation biology. On one hand, it has been stated to be conservative, but it has been studied in less than 1% of all recognized anuran species with practically no Neotropical species considered (Iwasawa et al., 1987; Lopez, 1989; Ogielska and Bartmanska, 1999; Chavadej et al., 2000; Gramapurohit et al., 2000; Falconi et al., 2001; Falconi et al., 2004; Ogielska and Kotusz, 2004; El Jamil et al., 2008; Downie et al., 2009; Fabrezi et al., 2010; Piprek et al., 2010; Sandoval and Gomez, 2010; Flament et al., 2011; Haczkiewicz and Ogielska, 2013; Phuge and Gramapurohit, 2013; Mali and Gramapurohit, 2015). On the other hand, it represents the best reference for an assessment of the effects of chemical exposure and their accumulated effects (Mann et al., 2009).
Descriptions of normal gonadogenesis (timing and sequence) represent a key factor for understanding the developmental basis of life cycle diversification. Descriptions also help to identify and discriminate those naturally occurring gonadal malformations from those provoked by external factors, such as the presence of chemicals in water bodies where the species live. Anurans, with their biphasic life cycle, are more exposed to toxins coming from agricultural and industrial runoff and, actually, several malformations have been described (Daughton and Ternes, 1999; Hayes et al., 2002; Hayes et al., 2003; McCoy et al., 2008; Rohr and McCoy, 2010; Papoulias et al., 2013), making them the most threatened group of vertebrates with the highest rates of population decline in the world (Stuart et al., 2004; Brühl et al., 2013; Costa and Nomura, 2016).
However, most of these studies have dealt with experimental procedures to evaluate the role of chemical exposure in gonadal development and descriptions of normal gonadogenesis from field samples are lacking. Such studies are needed as baselines as well as for the assessment of critical periods in which a species may be more vulnerable to the action of pesticides, endocrine disruptors, or pharmaceuticals.
Worldwide amphibian populations are experiencing massive declines (Muths and Fisher, 2015), and species inhabiting high altitude tropical habitats seem to be most at risk of declination because of direct and indirect anthropogenic factors (Lips, 1998; Stuart et al., 2004; Lips et al., 2005; Seimon et al., 2007), affecting the species along their altitudinal distributions. Considering that most declines have occurred above 1000 m in the Andes and anurans are the most diverse ectotherm group in the tropical Andes, with around 600 endemic species, there is a need for accurate descriptions of normal development in these species as plausible indicators of high altitude ecosystems and species health (Duellman, 1979; Navas, 2006).
The Neotropical high Andean frog Dendropsophus labialis is an endemic species from the Eastern Andes of Colombia that occurs in lentic and permanent water bodies in close association with highly disturbed, cultivated or urban, areas (Hunter and Valdivieso, 1962; Guarnizo et al., 2014). This species is distributed along a wide altitudinal gradient, from 1950 to 3600 masl, and represents one of the two species with the highest altitudinal distribution in the genus (Guarnizo et al., 2009). In this context, it exhibits a series of physiological, morphological and/or life history, specialized traits mainly related to the low and variable temperatures of the area (Lüddecke, 1997a; Navas, 2006). Tadpoles are present throughout the year and attain a large metamorphic size after a larval period of about two months (Ladino and Colmenares, 1987; Amézquita and Lüddecke, 1999). Since Dendropsophus labialis is a Neotropical frog that occurs in high altitude areas with low and fluctuating temperatures, it constitutes an interesting model to begin the study of gonadogenesis in Andean species. The complete histomorphological characterization of its gonadogenesis under the current conditions will allow us in the future to recognize possible changes related to land use and the accumulated effects of contaminants.
MATERIAL AND METHODS
We collected tadpoles (N=68) and adults (N=10) of Dendropsophus labialis in a permanent pond located at the Departamento de Biología of Universidad Nacional de Colombia, Bogotá, Colombia (4°38'28.09"N; 74°4'53.43"W) at 2558 m a.s.l., once a month during August 2014 to January 2015. This pond is characterized by being surrounded by open grassland and some dispersed trees. Physicochemical water conditions are within normal parameters for aquatic life and do not show presence of exogenous contamination (J.C. Donato, pers. comm.).
Additionally, we raised tadpoles (N= 133) in the laboratory at light conditions (12 light hours/12 hours darkness) and water temperature (19 °C) which is similar to the pond from stage 26 to metamorphosis with a low incidence of mortality (3, 79%). These tadpoles were raised and collected during February 2015 to October 2015 following the stages of Gosner (1960).
Overall, we analyzed 211 individuals including larvae and post metamorphic specimens. Individuals were collected at different stages of development, euthanized in an aqueous solution of chloretone, fixed in formalin (10%), adults were preserved in ethanol (70%) and larval stages were determined according to Gosner (1960). Specimens are housed as lots in the Colección de Anfibios of the Instituto de Ciencias Naturales (ICN) with the number ICN 055789.
To obtain data on external morphology, gonads along with the kidneys were dissected in tadpoles from the Gosner stage 25 to the end of metamorphosis and from adult specimens. For histological treatment, gonads along with the kidneys were dehydrated, cleared in xylene, embedded in paraffin, sectioned at 6 μm, and stained with haematoxylin and eosin following histological protocols by Luna (1968). In females adults, oocyte stages were determined according Dumont (1972) and ovarian developmental stages were determined following Ogielska and Kotusz (2004).
Photographs were taken with a Leica™ MC-170 HD digital camera attached to a Leica M205CA stereomicroscope, and then processed by the image stacking software Leica™ Application Suite version 4.6.0.
RESULTS
Undifferentiated gonads
In tadpoles at Gosner stages 25-27 (N= 22), the gonads were undifferentiated and resemble whitish, elongated, cylindrical, and paired cords attached to the top of the peritoneal cavity by means of the mesogonium (Fig. 1A). Histologically, the gonads contained large germ cells, devoid of yolk platelets, surrounded by darkly stained somatic cells, the nuclei of which appeared crescent-shaped (Fig. 1B; Stage II of Ogielska and Kotusz, 2004).
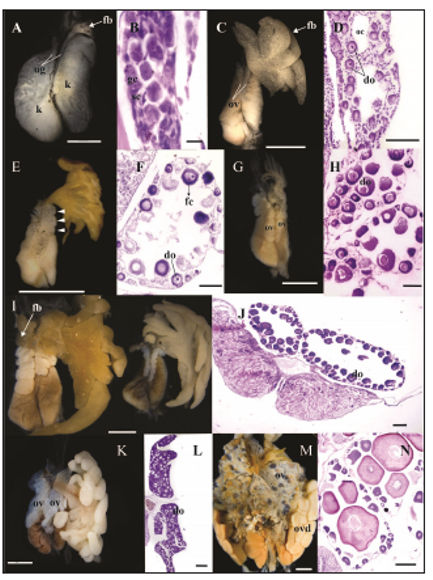
Figure 1. External morphology and histological sections of undifferentiated gonads and the events occurring during ovarian differentiation. (A-B) Gosner stage 27: (A) External morphology of the undifferentiated gonad. Fat bodies began the differentiation; (B) longitudinal section of the undifferentiated gonad with the presence of gonial and somatic cells. (C-D) Gosner stage 28: (C) Incipient lobulation of the ovary with developed fat bodies; (D) the ovarian cavities enlarged and previtellogenic diplotene oocytes appeared. (E-F) Gosner stage 33: (E) the degree of lobulation became more evident. Arrowheads indicate each lobule; (F) ovary with numerous diplotene oocytes surrounded by a single layer of prefollicular cells. (G-H) Gosner stage 35: (G) ovary increased in size; (H) diplotene oocytes are larger and surrounded by follicular cells. (I-J) Gosner stage 39: (I) ovary continued to grow and fat bodies of the right ovary began the differentiation. The gonads of the right corresponds to a specimen raised in laboratory while the gonads of the left corresponds to a tadpole collected in the field. Note that left gonads have smaller ovaries and lobules; (J) the ovary is fully developed with large diplotene oocytes and very few oogonia. (K-L): Gosner stages 42-46, (K) the lobules become wider; (L) the ovarian cavity is reduced due to the increase in number of diplotene oocytes. (M-N) Adults: (M) ovaries with numerous oocytes at different stages which is evident from its pigmentation; (N) each lobule contain oocytes at different stages. do= diplotene oocytes, fb= fat bodies, fc=follicular cells, gc= gonial cells, k= kidney, oc= ovarian cavity, ov=ovary, ovd=oviduct, sc= somatic cells, ug=undifferentiated gonads. Scale bars: 200 μm in A, D, F, H, N, J and L; 0.01 mm in B, 500 μm in C; 1 mm in E; 2 mm in G, I, K and M.
At stage 27 (Fig. 1A), fat bodies began to differentiate as fingerlike projections from the anterior end of the left gonad (= progonium) with a whitish coloring while there was no sign of differentiation in the right gonad.
Ovarian differentiation
The first sign of ovarian differentiation was discernible at Gosner stage 28 (N= 22 female frogs) with an incipient lobulation of the cords (Fig. 1C). Scattered pigmented cells appeared distributed on the anterior surface of both ovaries (Fig. 1C). Fat bodies continued to be more developed in the left ovary and became separated from the gonad proper with a uniformly granular aspect. It is important to note that the degree of lobulation is not associated with the degree of development of fat bodies because there are cases in which lobulation is more conspicuous and fat bodies are small. The gradually disappearance of the somatic cells in the sterile medulla formed small lumina that later fused to form a larger cavity, the ovarian cavity, which appeared surrounded by a well-defined epithelium (Fig. 1D). The cortex was composed of primary and secondary oogonia, the latter configured as small nests lined by prefollicular cells. Several oogonia have entered into meiosis and appeared as previtellogenic primary oocytes in groups surrounded by somatic cells. These diplotene oocytes had a highly basophilic cytoplasm and a discernible (acidophilic), spherical, central nucleus (Fig. 1D).
At stage 33 (N= 9), the ovaries increased in size which was attributable to the growth of the cortex, the ovarian cavity and the increased number of diplotene oocytes. The degree of lobulation, even when more defined than in previous stages, was variable among specimens. Fat bodies became larger and displayed a yellowish color indicating the accumulation of lipids (Fig. 1E). In light microscopy, the epithelium surrounding the ovarian cavity appeared well defined. Each oocyte appeared individualized and surrounded by proliferating prefollicular cells. Primary oogonia were less numerous than in previous stages (Fig. 1F).
At stage 35 (N= 15) ovaries reached bigger lobules than in previous stages. On average the left ovary had seven lobules whereas the right one had five lobules. Fat bodies showed variable sizes among specimens (Fig. 1G). Histologically, diplotene oocytes appeared encircled by follicular cells and became larger with larger nuclei with numerous nucleoli (Fig. 1H). It is important to highlight that those specimens at this stage raised under laboratory conditions exhibit underdeveloped ovaries with no lobulation and big fat bodies (Fig. 2).
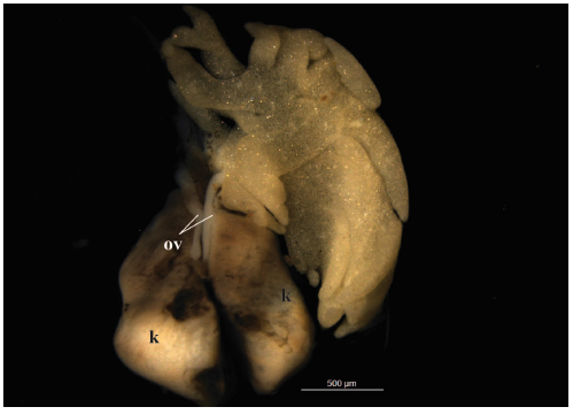
Figure 2. External morphology of the ovary of an individual at Gosner stage 36 raised in the laboratory. Note the lack of lobulation of the ovaries and the presence of big fat bodies in comparison with Fig. 1G. k=kidney, ov=ovary.
At stage 39 (N= 8), the ovaries continued to grow in size with bigger lobules than in previous stages. The right ovary always had fewer lobules than the left one, eight versus eleven, respectively. Fat bodies of the right ovary began to differentiate (Fig. 1I). Most peripheral oogonia entered meiotic activity and differentiated into oocytes. Therefore, the number and size of diplotene oocytes progressively increased and fulfilled the ovarian cavity (stage X of Ogielska and Kotusz, 2004) (Fig. 1J). As in previous stages, those specimens from laboratory conditions had less developed and smaller ovaries than those specimens from the wild (Fig. 1I).
In metamorphic tadpoles (N= 4), from stage 42-46, lobules appeared much wider than in previous stages, with a lower number in the right ovary in respect of the left one. Pigmentation was evident as randomly distributed black points throughout the ovaries (Fig. 1K). The ovarian cavity shrank in size due to the ovary was almost fully occupied by diplotene oocytes, with the largest ones adjoining the ovarian cavity (Fig. 1L).
In adults (N= 5), ovaries are wider with almost no pigmentation. Fat bodies were smaller with thinner projections (Fig. 1M). Individual lobules contained developing oocytes of variable size in stages I to IV (sensu Dumont, 1972), encircled by follicular cells and a theca (internal and external) (Fig. 1N).
Testicular differentiation
By Gosner stage 28 (N= 9), testes appeared as short, cylindrical cords with no external sign of differentiation beyond the absence of lobulation that characterize ovarian differentiation. Pigmentation appeared only in the left testis which was larger than the right one. This asymmetry in testis lengths, together with a slightly more advanced stage of differentiation of the left testis, remained during the whole development. At this stage a (thicker) proximal part and a (thinner, spindle-shaped) distal part of the developing testes was already evident. Fat bodies associated to the left testis were well developed and had a whitish coloring (Fig. 3A). Histologically, each testis appears as a massive structure without the characteristic lumen of the ovarian cavity. In the medulla, big primary spermatogonia are evenly distributed and among crescent-shaped, darkly stained somatic cells (Fig. 3B).
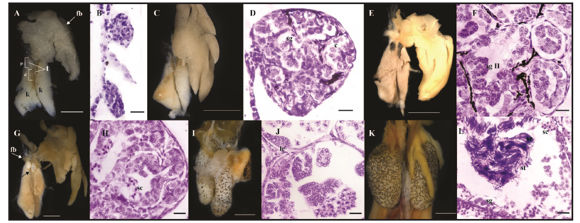
Figure 3. External morphology and histological sections of the testes during their differentiation. (A-B) Gosner stage 28: (A) testes showed a proximal and a distal part and fat bodies were fully developed; (B) The testes showed no sign of a lumen and presence of spermatogonia. (C-D) Gosner stage 30: (C) Testes were thicker and more pigmented; (D) first signs of seminiferous tubules differentiation with presence of spermatogonia. (E-F) Gosner stage 36: (E) Testes became thicker; (F) Presence of several seminiferous tubules with cysts of secondary spermatogonia. (G-H) Gosner stage 40: (G) the distal part of the testis degenerated (black arrow) and fat bodies in the right testis appeared (white arrow); (H) seminiferous tubules present spermatogonia, spermatocytes and Leydig cells. (I-J) Gosner stages 42 - 46: (I) testis became bigger and slightly pigmented; (J) detail of a seminiferous tubule with the cystic configuration. (K-L) Adults: (K) testis become bigger and thicker ovoid structures with variable pigmentation; (L) seminiferous tubules contains spermatogonia, spermatocytes, spermatids and spermatozoa. c=cysts, d=distal part, fb=fat bodies, k=kidneys, lc=Leydig cell, p=proximal part, sc=spermatocyst, sg=spermatogonia, sg II= secondary spermatogonia, st=spermatid, sz=spermatozoa, t=testis. Scale bars: 1 mm in A, E, I and K; 2 mm in C, G; 200 μm in B and D; 0.02 mm in F, H, J and L.
At stage 30 (N= 8), both testis became shorter and thicker and more pigmented. Fat bodies acquired a yellowish coloration (Fig. 3C). Primary spermatogonia divided mitotically and produced clusters of smaller cells, the secondary spermatogonia. By this stage testes showed the first signs of seminiferous cord organization through the formation of small lumina within each cord. Pigmentation, distributed along the interstitial space, individualizes each presumptive seminiferous tubule (Fig. 3D).
At stage 36 (N= 6), the shortening and thickening of the testes was evident; the development and differentiation of the proximal part proceeded while the distal part degenerated, and there were only vestiges of it. Differences in size between the left and the right testis became conspicuous. The pattern of pigmentation among specimens was variable because some individuals presented darkly stained testes while in others they were slightly pigmented (Fig. 3E). The medulla appeared organized in abundant seminiferous tubules with several cysts of secondary spermatogonia which can be recognized by their round shape and big size (although smaller than primary spermatogonia) with prominent nucleoli (Fig. 3F). At stage 37, fat bodies related to the right testis began to differentiate.
By stage 40, the distal part of the testes was almost lost. As a rule, the left testis was always longer than the right one. Fat bodies related to the left testis were well developed while those of the right testis began to differentiate and developed as fingerlike projections (Fig. 3G). The testes appeared organized in seminiferous tubules with several cysts of germ cells at different stages of differentiation. Within each cyst, germ cells were at approximately the same stage. Some cysts contained spermatocytes in different phases of the first meiotic division with different degrees of chromatin condensation (Fig. 3H).
During metamorphic stages (N= 10 frogs), testis increased in size and pigmentation was variable, from reticulated to uniformly dark patterns (Fig. 3I). Histologically, there was no change in morphological organization, but an increased number of secondary spermatogonia and spermatocytes were present. Leydig cells differentiated next to the seminiferous tubules and were triangular in shape with a highly basophilic cytoplasm and large nuclei (Fig. 3J). In a specimen at stage 46, testis developed several testicular oocytes which appeared located at the center of the seminiferous tubules and separated from the surrounding testicular tissue. These oocytes had the morphology of a previtellogenic oocyte (Fig. 4).
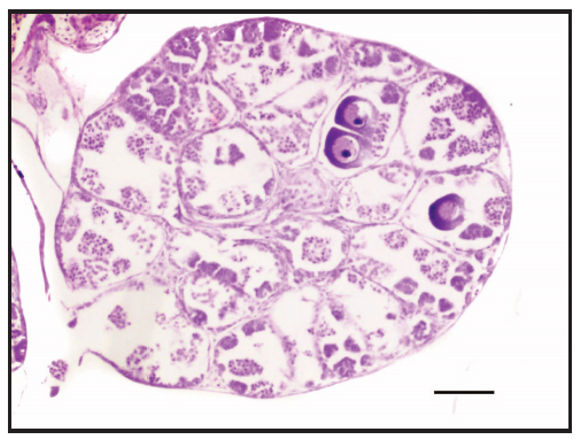
Figure 4. Gonadal abnormality of an individual at Gosner stage 46 in which some diplotene oocytes are located within seminiferous tubules. Scale bar: 200 μm.
Adult testes (N= 6) were paired, ovoid structures, with a larger size than that of the testes in metamorphic specimens. The size of fat bodies was variable as well as the pattern of pigmentation which could vary between both testes of a single specimen or between specimens (Fig. 3K). Each seminiferous tubule was composed of several cysts of germ cells at different stages of differentiation but within each cyst, germ cells appeared at approximately the similar stage: spermatogonia, spermatocytes, spermatids and spermatozoa; the latter indicating gonadal maturity (Fig. 3L).
DISCUSSION
Gonadal differentiation in Dendropsophus labialis follows the sequence of morphological events described for most amphibians with a differentiated pattern (Gramapurohit et al., 2000) in which undifferentiated gonads differentiate, by stage 28 in the case of D. labialis, directly into an ovary or a testis. This pattern was observed in Bombina orientalis, Lithobates catesbeianus, Microhyla ornata, Pelophylax nigromaculatus and Xenopus laevis (Gramapurohit et al., 2000; Mali and Gramapurohit, 2015). Two other patterns of gonadal sex differentiation have been described: "undifferentiated" where the gonad differentiates into an ovary or remains in an undifferentiated condition for a while until it becomes a testis; and "semidifferentiated" in which the gonads regardless of the genetic sex differentiate into ovaries but in males there occurs a degeneration of oocytes to generate testis (Witschi, 1929; Dumont, 1972; Gramapurohit et al., 2000, Saidapur et al., 2001; Ogielska and Kotusz, 2004; Goldberg, 2015).
Regarding the rate of differentiation, Ogielska and Kotusz (2004) identified three types of ovarian differentiation rates with respect to somatic development: 1) basic rate, where the ovarian cavity appears for a time at the end of metamorphosis; 2) delayed rate, where the first diplotene oocytes are evident after metamorphosis, and 3) accelerated rate where previtellogenic oocytes appear before metamorphosis. In Dendropsophus labialis, the ovarian cavity and the first diplotene oocytes are evident from stage 28 and previtellogenic oocytes appear in early premetamorphic stages; therefore the rate of ovarian differentiation of this species is accelerated. The same rate was described in Euphlyctis cyanophlyctis (Phuge and Gramapurohit, 2013), Pseudis paradoxa (Downie et al., 2009), Scinax fuscovarius (Goldberg, 2015), Microhyla ornata (Mali and Gramapurohit, 2015), and in several ranid species (Ogielska and Kotusz, 2004; Gramapurohit et al., 2000).
Oogenesis (i.e., the process of producing the female gametes) and folliculogenesis (i.e., the maturation of the ovarian follicle) are two processes that occur in conjunction with each other, interacting via reciprocal induction, but they do not necessarily proceed simultaneously (Tanimura and Iwasawa, 1988). However, in D. labialis, oogenesis is initiated almost simultaneously with the formation of the ovarian cavity and folliculogenesis proceeds immediately after as in Scinax fuscovarius (Goldberg, 2015).
With respect to testicular differentiation, three types of rates were recognized considering the differentiation of the seminiferous tubules regarding metamorphosis (Goldberg, 2015): 1) basic rate, in which the seminiferous tubules differentiate during metamorphosis, 2) delayed rate, in which the differentiation occurs after metamorphosis, and 3) accelerated rate in which the tubules differentiate before metamorphosis. In D. labialis, the first signs of seminiferous tubules differentiation were observed at stage 30, therefore this species has an accelerated rate of testicular differentiation. The same type has been described in Clinotarsus curtipes, Lythobates sylvaticus, P. minuta and P. paradoxa (Gramapurohit et al., 2000; Fabrezi et al., 2010; Goldberg et al., 2016). However, even when it exhibits an accelerated pattern as do other species, the very early differentiation of seminiferous tubules has only been described, among anurans, in Pseudis paradoxa and P.minuta in which it has been found that seminiferous tubules are fully developed by stage 31 and males already produce sperm at the end of metamorphosis (Downie et al., 2009; Goldberg et al., 2016). Interestingly, despite the development of seminiferous tubules occurring in an accelerated manner, spermatogenesis does not proceed rapidly since the differentiation of primary spermatocytes begins during metamorphosis.
An interspecific comparative approach between rates of ovarian and testicular development among some phylogenetically related species (Phyllomedusa azurea, P. boliviana, P. sauvagii, Pseudis minuta, P. platensis and Scinax fuscovarius) revealed that rates vary considerable among species and gonadal differentiation and development progress in a general pattern but with heterochronic changes that characterize each species. In this context and compared to the scarce available literature, the only evidence to suggest that Dendropsophus labialis presents a specialization in gonadal differentiation in relation to the low and variable temperatures of the area it inhabits comes from the early differentiation of both testis and ovaries.
Visceral pigmentation of the urogenital system has been extensible studied in adult anurans (Oliveira et al., 2002, 2003; Oliveira and Zieri, 2005; Zieri et al., 2007; Franco-Belussi et al., 2009), and although morphofunctional aspects of this visceral pigmentation are still unknown, a large survey has allowed to establish a species-specific pattern with three different categories (Franco-Belussi et al., 2009). In this context, Dendropsophus labialis displayed Category 1 which consists of few pigmented cells, constituting a discrete pigmentation. Interestingly, there is no literature data about the ontogeny of testis pigmentation and its variation, even less its function; although a thermoregulation role has been suggested (Zieri et al., 2007).
Additionally, it must be take into account that this species has a prolonged breeding activity with peaks that correspond to local monthly rainfall patterns. Females that can potentially reproduce can be found in any time of the year, however, they only spawn once during the breeding season laying 300-1600 eggs per clutch in permanent ponds (Lüddecke, 1997b; Amézquita and Lüddecke, 1999; Lüddecke, 2002). Futhermore, Amézquita and Lüddecke (1999) found that males attained sexual maturity in laboratory 80 days after metamorphosis, hence an accelerated sexual maturity and therefore a short juvenile period could be related with the early differentiation of gonads as suggested for Pseudis minuta (Goldberg et al., 2016) and Microhyla ornata (Mali and Gramapurohit, 2015). Some of the phylogenetically related species who have also available data of gonadal development and reproductive patterns, such as Scinax fuscovarius, Pseudis paradoxa, Phyllomedusa azurea and P. sauvagii, are from lowlands and experienced a prolonged reproductive activity (Prado and Haddad, 2005; Rodrigues et al., 2005; 2007) but dissimilar gonadal differentiation rates and age at sexual matury (Goldberg, 2015). Hence, although the variation observed in gonadal development could be related with the reproductive phenology of species, a relation that must be further explored, it is evident that each species has its own developmental pattern.
In the analysis of morphological variation, both growth heterochrony and sequence heterochrony offer numerous tools for the analysis of that variation as a result of development (Smith, 2002; Fabrezi, 2012). With this analysis we can identify intraspecific (between sexes) and interspecific variation but we cannot reliably polarize the direction of the event due to: 1) the limited information currently available on gonadal development in the Hylidae family and 2) testes and ovaries have independent developmental paths and are different from each other in sequence and timing of differentiation. On the other hand, the comparison of the timing and sequence of events of somatic and gonadal development corroborate that gonadal differentiation is independent of somatic development as gonadal maturation can occur before, during or after metamorphosis. This dissociation in the fundamental processes of ontogeny (sensu Needham, 1933): maturity and development (and growth, although this factor has not been tested in this study) provides evidence of heterochrony in life cycles involving how related species show divergent developmental programs that define specific characteristics for each species.
Differences in gonadal developmental rates have been linked to the susceptibility of some species for developing abnormal morphologies during development; the faster gonads mature they will have less exposure time. However an accelerated gonadal development, which involves gonads differentiating during early (aquatic) tadpole stages could represent a disadvantage when the differentiating gonad is in contact with chemicals because the exposure time is longer. In this study, a specimen at stage 46 (metamorphic) collected in a remnant wetland was found to have oocytes in diplotene within seminiferous tubules. This situation represents a case of gonadal abnormality, wherein the condition in which the oocytes are not present as a whole tissue but dispersed in testicular tissue is termed "Testicular Oocytes" (sensu Hecker et al., 2006) (Fig. 4). In particular, for this species it has been found that nitrate concentrations in water of 0.38 and 1.53 ppm can provoke morphological abnormalities in the mouth, tail, skin, among others, in tadpoles (Cortes and de Dulce, 1996). Tadpoles with testicular oocytes were found in a pond with nitrate concentration between 0.2 and 0.99 ppm (J.C. Donato, pers. comm.), a range that could influence the observed abnormality based on the previously mentioned study. As our sampled specimens were collected in a seemingly undisturbed wetland, this case represents an interesting report (although with a very low incidence) of malformations in tadpoles in natural environments which are frequently reported in terms of experimental treatments (Hayes et al., 2002). However, the dynamics, origin and induction of such malformations are unknown even when it has been found that it is derived from primary and secondary spermatogonia and occurs mainly in young individuals that are sexually maturing (Kobayashi et al., 2015).
An interesting issue comes from the variation in size and time of gonadal differentiation observed in laboratory reared individuals vs. individuals collected in the wild. Variations in size and timing of gonadal differentiation were evident as development progressed. The most conspicuous differences were observed in relation to the size of the gonad, the size of fat bodies, the degree of lobulation of the ovaries and the duration of gonadal development (Fig. 2). The observed variations in fat body size of D. labialis may be related to the availability of food or predator free environment, as suggested by Downie et al., (2009), because those individuals with larger fat bodies correspond to those raised in the laboratory. Differences in the degree of lobulation of ovaries or differences in gonadal size in which those individuals with less lobulation and smaller gonadal sizes are those maintained in captivity have not been previously reported in the literature. This is also true regarding the duration of gonadal development, which is slower in laboratory reared individuals. However, several studies reported differences in sex determination (Hsu and Liang, 1970), synthesis of yolk oocytes (Kemp, 1953), occurrence of sex reversal in populations reared in laboratory but collected in the field (Eggert, 2004) or different growth rates and age at sexual maturity between laboratory and wild amphibians (Houck, 1982).
On the other hand, it should be taken into account that there are several abiotic factors affecting the larval stage of amphibians, including temperature, density of conspecifics, presence of predators, UV radiation and the availability of foods that lead to changes in size, age and individual development (Amézquita and Lüddecke, 1999; Broomhall et al., 2000). In this case, the average temperature of the natural pond is the same as that of the laboratory which is 18, 50 °C (J.C. Donato pers. comm.) Therefore, laboratory data should be used with caution as laboratory conditions may not produce the same developmental patterns observed in the field. If we are looking to have a characterization of normal development in order to baseline for future comparison, then strong field surveys are needed.
The Neotropical high Andean frog, Dendropsophus labialis, belongs to the clade Dendropsophini which includes the genera Sphaenorhynchus, Pseudis, Scinax, among others (Faivovich et al., 2005). Given the remarkable temporal variation of gonadal development rates among the studied species of these genera and the sexual maturity reached with somatic larval morphology in Sphaenorhynchus bromelicola (Bokerman, 1974) and Pseudis species (Downie et al., 2009, Goldberg et al., 2016), it is suggested that this clade might represent an interesting model for the study of heterochrony (Fabrezi, 2012), a phenomenon that has shaped the life cycles of anurans (Fabrezi et al., 2009, Alberch, 1989).
Describing the ontogenetic variation in timing and sequences of those events related to the development and maturity of ovaries and testes between larval and post metamorphic stages of a greater number of species that share historical patterns and / or similar environmental conditions can lead to interesting interpretations of how the expression of developmental programs determine the influence and response to similar/dissimilar extrinsic factors. Additionally, this study could contribute to the discussion of evolutionary patterns in sexual differentiation and the evolution of reproductive cycles among anurans, since information from Neotropical high Andean species is scarce.
Acknowledgments
We thank the Laboratorio de Equipos Ópticos Compartidos (L.E.O.C), and the Laboratorio de Histología Vegetal y Animal of Departmento de Biología, Universidad Nacional de Colombia, Sede Bogotá (Bogotá, Colombia) for allowing us the use of equipment and space to develop the present research. Specimen collection permit were provided by means of Permiso Marco de Recolección de Especímenes, number 0255. We thank Camilo Yara, Miguel Mendez and Miller Castañeda for collaboration in the fieldwork, Sebastian Vega for technical assistance and Thomas Defler for improving the grammar of the manuscript. We also thanks the editor and two anonymous reviewers for their valuable comments that greatly improve the manuscript. This research was partially supported by Agencia Nacional de Promoción Científica y Tecnológica, PICT 2014-510.
LITERATURE CITED
1. Alberch, P. 1989. Development and the evolution of amphibian metamorphosis: 163-173. In: Splechtna, H. & Hilgers, H. (eds.), Trends in Vertebrate Morphology. Gustav Fischer Verlag, Stuttgart. [ Links ]
2. Amézquita, A. & Lüddecke, H. 1999. Correlates of intrapopulational variation in size at metamorphosis of the High-Andean frog Hyla labialis. Herpetologica 55: 295-303. [ Links ]
3. Bokermann, W. 1974. Observações sobre desenvolvimento precoce em Sphaenorhynchus bromelicola Bok.1966 (Anura, Hylidae). Revista Brasileira de Biologia 34: 35-41. [ Links ]
4. Broomhall, S.D.; Osborne, W.S. & Cunningham, R.B. 2000. Comparative effects of ambient Ultraviolet-B radiation on two sympatric species of australian frogs. Conservation Biology 14: 420-427. [ Links ]
5. Brühl, C.A.; Schmidt, T.; Pieper, S. & Alscher, A. 2013. Terrestrial pesticide exposure of amphibians: An underestimated cause of global decline?. Scientific Reports 3: 1-4. [ Links ]
6. Chavadej, J.; Jerareungrattana, A.; Sretarugsa, P. & Sobhon, P. 2000. Structure and development of the testis of bullfrog Rana catesbeiana, and their changes during seasonal variation. Science Asia 26: 69-80. [ Links ]
7. Cortes, F. & de Dulce, B. 1996. Descripción de las alteraciones de embriones de Hyla labialis expuestos a Hg², Cl¯ y NO3¯. Universitas Scientiarum 3: 41-52. [ Links ]
8. Costa, R.N. & Nomura, F. 2016. Measuring the impacts of Roundup Original on fluctuating asymmetry and mortality in a Neotropical tadpole. Hydrobiologia 765: 1-85. [ Links ]
9. Daughton, C.G. & Ternes, T.A. 1999. Special report: pharmaceuticals and personal care products in the environment: agents of subtle change?. Environmental Health Perspectives 107: 907-938. [ Links ]
10. Downie, J. R.; Sams, K. & Walsh, P.T. 2009. The paradoxical frog Pseudis paradoxa: larval anatomical characteristics, including gonadal maturation. Herpetological Journal 19: 1-10. [ Links ]
11. Duellman, W. 1979. The herpetofauna of the Andes: patterns of distribution, origin, differentiation, and present communities: 371-459. In: Duellman, W. (ed), The South American Herpetofauna: Its Origin, Evolution and Dispersal. Monographs of the Museum of Natural History, Kansas. [ Links ]
12. Dumont, J. 1972. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. Journal of morphology 136: 153-179. [ Links ]
13. Eggert, C. 2004. Sex determination: the amphibian models. Reproduction Nutrition Development 44: 539-549. [ Links ]
14. El jamil, A.; Magre, S.; Mazabraud, A. & Penard-Mobayed, M. 2008. Early aspects of gonadal sex differentiation in Xenopus tropicalis with reference to an antero-posterior gradient. Journal of Experimental Zoology A 309: 127-137. [ Links ]
15. Fabrezi, M.; Quinzio, S.I. & Goldberg, J. 2009. Giant tadpole and delayed metamorphosis of Pseudis platensis Gallardo, 1961 (Anura, Hylidae). Journal of Herpetology 43: 228-243. [ Links ]
16. Fabrezi, M.; Quinzio, S.I. & Goldberg, J. 2010. The ontogeny of Pseudis platensis (Anura, Hylidae): heterochrony and the effects of larval development on postmetamorphic life. Journal of Morphology 271: 496-510. [ Links ]
17. Fabrezi, M. 2012. Heterocronía y variación morfológica en anuros. Cuadernos de Herpetología 26: 29-47. [ Links ]
18. Faivovich, J.; Haddad, C.F.B.; Garcia, P.C.A.; Frost, D.R.; Campbell, J.A. & Wheeler, W.C. 2005. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bulletin of the American Museum of Natural History 294: 1-240. [ Links ]
19. Falconi, R.; Petrini, S.; Quaglia, A. & Zaccanti, F. 2001. Fine structure of undifferentiated gonads in Rana dalmatina tadpoles. Italian Journal of Zoology 68: 15-21. [ Links ]
20. Falconi, R.; Dalpiaz, D. & Zaccanti, F. 2004. Ultrastructural aspects of gonadal morphogenesis in Bufo bufo (Amphibia, Anura) 1: sex differentiation. Journal of Experimental Zoology 301A: 378-388. [ Links ]
21. Flament, S.; Chardard, D.; Chesnel, A. & Dumond, H. 2011. Sex determination and sexual differentiation in Amphibians: 1-19. In: Norris, D.O. & Lopez, K.H (eds), Hormones and Reproduction of Vertebrates. Amphibians. Academic Press, United States of America. [ Links ]
22. Franco-Belussi, L.; Zieri, R.; Santos, L. R. S.; Moresco, R. M. & Oliveira, C. 2009. Pigmentation in anuran testes: anatomical pattern and variation. The Anatomical Record 292: 178-182. [ Links ]
23. Goldberg, J. 2015. Gonadal differentiation and development in the snouted treefrog, Scinax fuscovarius (Amphibia, Anura, Hylidae). Journal of Herpetology 49: 468-478. [ Links ]
24. Goldberg, J.; Barrasso, D.A.; Agostini, M.G. & Quinzio, S. 2016. Vocal sac development and accelerated sexual maturity in the lesser swimming frog, Pseudis minuta (Anura, Hylidae). Zoology doi: 10.1016/j.zool.2016.07.001 [ Links ]
25. Gosner, K.L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183-190. [ Links ]
26. Gramapurohit, N.P.; Shanbhag, B.A. & Saidapur, S.K. 2000. Pattern of gonadal sex differentiation, development and onset of in the frog Rana curtipes. General and comparative endocrinology 119: 256-264. [ Links ]
27. Guarnizo, C.E.; Amézquita, A. & Bermingham, E. 2009. The relative roles of vicariance versus elevational gradients in the genetic differentiation of the high Andean tree frog Dendropsophus labialis. Molecular Phylogenetics and Evolution 50: 84-92. [ Links ]
28. Guarnizo, C.E.; Armesto, O. & Acevedo, A. 2014. Dendropsophus labialis Peters, 1863. Catálogo de Anfibios y Reptiles de Colombia 2: 56-61. [ Links ]
29. Haczkiewicz, K. & Ogielska, M. 2013. Gonadal sex differentiation in frogs: how testes become shorter than ovaries. Zoological society of Japan 30: 125-134. [ Links ]
30. Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N. & Stuart, A.A. 2002. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proceedings of the National Academy of Science USA 99: 5476-5480. [ Links ]
31. Hayes, T.B.; Haston, K.; Tsui, M.; Hoang, A.; Haeffele, C. & Vonk, A. 2003. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environmental Health Perspectives 111: 568-575. [ Links ]
32. Hecker, M.; Murphy, M.B.; Coady, K.K.; Villeneuve, D.L.; Jones, P.D.; Carr, J.A.; Solomon, K. R.; Smith, E.E.; Van Der Kraak, G.J.; Gross, T.; Preez, L.; Kendall, R.J. & Giesy J. 2006. Terminology of gonadal anomalies in fish and amphibians resulting from chemical exposures. Reviews of Environmental Contamination and Toxicology 187: 103-131. [ Links ]
33. Hsu, C. & Liang, H. 1970. Sex races of Rana catesbeiana in Taiwan. Herpetologica 26: 214-221. [ Links ]
34. Houck, L. D. 1982. Growth rates and age at maturity for the plethodontid salamander Bolitoglossa subpalmata. Copeia 2: 474-478. [ Links ]
35. Hunter, A. S. & Valdivieso, B. 1962. La reproducción de la rana Hyla labialis. Caldasia 8: 573-583. [ Links ]
36. Iwasawa, H.; Nakazawa, T. & Kobayashi, T. 1987. Histological observations on the reproductive organs of growing Rana nigromaculata frogs. Science Reports of Niigata University-Series D (Biology) 24: 1-13. [ Links ]
37. Kemp, N. 1953. Synthesis of yolk in oocytes of Rana pipiens after induced ovulation. Journal of Morphology 92: 487-511. [ Links ]
38. Kobayashi, T.; Kumakura, M.; Yoshie, S.; Sugishima, T. & Horie, Y. 2015. Dynamics of testis-ova in a wild population of Japanese pond frogs, Rana nigromaculata. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 323: 74-79. [ Links ]
39. Ladino, E. & Colmenares, I. 1987. Tabla del desarrollo del estado metamórfico en Hyla labialis. Revista de la Facultad de Ciencias: Universidad Javeriana 1: 85-100. [ Links ]
40. Lips, K.R. 1998. Decline of a tropical montane amphibian fauna. Conservation Biology 12: 106-117. [ Links ]
41. Lips, K.R.; Burrowes, P.A; Mendelson, J.R. & Parra-Olea, G. 2005. Amphibian population declines in Latin America: A synthesis. Biotropica 37: 222-226. [ Links ]
42. Lopez, K. 1989. Sex differentiation and early gonadal development in Bombina orientalis (Anura: Discoglossidae). Journal of Morphology 199: 299-311. [ Links ]
43. Luna, L.G. 1968. Manual of histologic staining methods of the armed forces institute of pathology. McGraw-Hill, New York. [ Links ]
44. Lüddecke, H. 1997a. Colonization of the eastern Andes of Colombia by anurans: Evidence from natural history data of Hyla labialis. Salamandra 33: 111-132. [ Links ]
45. Lüddecke, H. 1997b. Besiedlungsgeschichte der kolumbianischen Ostanden durch Anuren: Hinweise aus naturgeschichtlichen Daten von Hyla labialis. Salamandra 33: 111-132. [ Links ]
46. Lüddecke, H. 2002. Association between breeding cycle and male body condition in Hyla labialis. Journal of herpetology 36: 607-614. [ Links ]
47. Mali, P.V. & Gramapurohit, N.P. 2015. Pattern of gonadal differentiation and development up to sexual maturity in the frogs, Microhyla ornata and Hylarana malabarica: A comparative study. Journal of Experimental Zoology Part A 323: 666-678. [ Links ]
48. Mann, R.M.; Hyne, R.V; Choung, C.B. & Wilson, S.P. 2009. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environmental Pollution 157: 2903-2927. [ Links ]
49. McCoy, K.A.; Bortnick, L.J.; Campbell, C.M.; Hamlin, H.J.; Guillette, L.J. & St. Mary, C.M. 2008. Agriculture alters gonadal form and function in the toad Bufo marinus. Environmental Health Perspectives 116: 1526-1532. [ Links ]
50. Muths, E. & Fisher, R.N. 2015. An alternative framework for responding to the amphibian crisis. Oryx 1-4. [ Links ]
51. Navas, C.A. 2006. Patterns of distribution of anurans in high Andean tropical elevations: Insights from integrating biogeography and evolutionary physiology. Integrative and Comparative Biology 46: 82-91. [ Links ]
52. Needham, J. 1933. On the dissociability of the fundamental processes in ontogenesis. Biological Reviews 8: 180-223. [ Links ]
53. Ogielska, M. & Bartmanska, J. 1999. Development of testes and differentiation of germ cells in water frogs of the Rana esculenta complex (Amphibia, Anura). Amphibia-Reptilia 20: 251-263. [ Links ]
54. Ogielska, M. & Kotusz, A. 2004. Pattern and rate of ovary differentiation with reference to somatic development in anuran amphibians. Journal of morphology 259: 41-54. [ Links ]
55. Oliveira, C.; Zanetoni, C. & Zieri, R. 2002. Morphological observations on the testes of Physalaemus cuvieri (Amphibia, Anura). Revista Chilena de Anatomia 20: 263-268. [ Links ]
56. Oliveira, C.; Sant'Anna, A.C.; Omena, P.M., Santos, L.R.S. & Zieri, R. 2003. Morphological considerations on the seminiferous structures and testes of anuran amphibians: Bufo crucifer, Physalaemus cuvieri, and Scinax fuscovarius. Biociências 11: 39-46. [ Links ]
57. Oliveira, C. & Zieri, R. 2005. Testicular pigmentation in Physalaemus nattereri (Steindachner) (Amphibia, Anura) with anatomical observations on the extracutaneous pigmentary system. Revista Brasileira de Zoologia 22: 454-460. [ Links ]
58. Papoulias, D.M.; Schwarz, M.S. & Mena, L. 2013. Gonadal abnormalities in frogs (Lithobates spp.) collected from managed wetlands in an agricultural region of Nebraska, USA. Environmental Pollution 172: 1-8. [ Links ]
59. Phuge, S.K. & Gramapurohit, N.P. 2013. Gonadal sex differentiation, development up to sexual maturity and steroidogenesis in the skipper frog, Euphlyctis cyanophlyctis. General and Comparative Endocrinology 181: 65-71. [ Links ]
60. Piprek, R.P.; Pecio, A. & Szymura, J.M. 2010. Differentiation and development of gonads in the yellow-bellied toad, Bombina variegata L., 1758 (Amphibia: Anura: Bombinatoridae). Zoological Science 27: 47-55. [ Links ]
61. Prado, C.P.A. & Haddad, C.F.B. 2005. Size fecundity relationships and reproductive investment in female frogs in the Pantanal, south-western Brazil. Herpetological Journal 15: 181-189. [ Links ]
62. Rengel, D., Pisanó, A. & Lavilla, E. 1995. Diferenciación sexual de Phyllomedusa boliviana. Cuadernos de Herpetología 9: 15-20. [ Links ]
63. Rodrigues, D.J.; Uetanabaro, M. & Lopes, F.S. 2005. Reproductive patterns of Trachycephalus venulosus (Laurenti, 1768) and Scinax fuscovarius (Lutz, 1925) from the Cerrado, Central Brazil. Journal of Natural History 39: 3217-3226. [ Links ]
64. Rodrigues, D.J.; Uetanabaro, M. & Lopes, F.S. 2007. Breeding biology of Phyllomedusa azurea Cope, 1862 and P. sauvagii Boulenger, 1882 (Anura) from the Cerrado, Central Brazil. Journal of Natural History 41: 1841-1851. [ Links ]
65. Rohr, J.R. & McCoy, K.A. 2010. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environmental Health Perspectives 118: 20-32. [ Links ]
66. Saidapur, S.K.; Gramapurohit, N.P. & Shanbhag, B.A. 2001. Effect of Sex Steroids on Gonadal Differentiation and Sex Reversal in the Frog, Rana curtipes. General and Comparative Endocrinology 124: 115-123. [ Links ]
67. Sandoval, M.T. & Gomez, M.L. 2010. Desarrollo y morfología del sistema urogenital de Physalaemus santafecinus Barrio 1965 (Anura: Leiuperidae). Facena 26: 29-41. [ Links ]
68. Seimon, T.A.; Seimon, A.; Daszak, P.; Halloy, S.R.P.; Schloegel, L.M.; Aguilar, C.A.; Sowell, P.; Hyatt, A.D.; Konecky, B. & Simmons, J.E. 2007. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Global Change Biology 13: 288-299. [ Links ]
69. Smith, K.K. 2002. Sequence heterochrony and the evolution of development. Journal of Morphology 252: 82-97. [ Links ]
70. Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L. & Waller, W.R. 2004. Status and Trends of Amphibians Declines and Exctintions Worldwide. Science 306: 1783-1786. [ Links ]
71. Tanimura, A. & Iwasawa, H. 1988. Ultrastructural observations on the origin and differentiation of somatic cells during gonadal development in the frog Rana nigromaculata. Development, Growth and Differentiation 30: 681-691. [ Links ]
72. Witschi, E. 1929. Studies on sex differentiation and determination in amphibians. I. Development and sexual differentiation of the gonads of Rana sylvatica. Journal of Experiment 52: 235-265. [ Links ]
73. Zieri, R.; Taboga, S. R. & Oliveira, C. 2007. Melanocytes in the testes of Eupemphix nattereri (Anura, Leiuperidae): histological, stereological and ultrastructural aspects. The Anatomical Record 290: 795-800. [ Links ]














