Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
BAG. Journal of basic and applied genetics
versión On-line ISSN 1852-6233
BAG, J. basic appl. genet. v.19 n.1 Ciudad Autónoma de Buenos Aires ene./jun. 2008
Aphidicolin induces break points in heterozygous Robertsonian translocation rob(1;29) from Uruguayan Creole cattle
Brief post
Rody Artigas, Andrés Iriarte, Beatriz Tellechea, Silvia Llambí, Miguel De Bethencourt, Alicia Postiglioni.
Área Genética. Depto. de Biología Molecular y Celular. Facultad de Veterinaria (UDELAR). Av. A. Lasplaces 1550. CP. 11600. Phone 598-02-68 58 02. FAX: 598-02-68-01-30. Montevideo, Uruguay.
Corresponding authors: Alicia Postiglioni. Área Genética. Depto. de Biología Molecular y Celular. Facultad de Veterinaria (UDELAR). Av. A. Lasplaces 1550. CP.11600. Phone 598-02-68 58 02. FAX: 598-02-68-01-30. Montevideo, Uruguay. E.mail:aliposvet@gmail.com
ABSTRACT
Bovine presents a complex genome due to high number of repetitive sequences involved in macro and micro-rearrangements that are essential in the bovids evolution. However, it has a morphologically simple karyotype, with acrocentric autosomes (2n=60,XX and 60,XY). The Robertsonian translocation rob(1;29), which produces embryo mortality, has been widely described in different breeds of Bos taurus but the complexity of its mechanism and its consequences in chromatin changes, are still not clear. Clastogenic agents are used as an approach to study this rearrangement in chromatin structure. Particularly, the aphidicolin (APC) inhibits the eukaryotic DNA polymerase a during replication. This fact permit to identify regions rich in dCTP due to a competition and spreading of the enzyme. Lymphocyte macro culture of 2 female Uruguayan Creole cattle carriers of rob(1;29) were APC-inducted (0,3uM) in one cell cycle. A new break point, rob(1;29)p13/21 (relative distance: p2c/p1=0.45), is located in a late replication region (RBG- band). Two fragile sites (c-fra) in the long arm of rob(1;29) (q13/ 21 and q43) and two other c-fra in BTAX (q12 and q31) were scored. C-fra: rob(1:29)q13/21 y Xq12 were located proximal to centromere in later replication region while rob(1;29)q43 and Xq31 were located proximal to telomere in early replication region. A high incidence in c-fra proximal to centromere in both biarmed chromosomes (BTAXc2=11.3 P<0.001; rob(1;29)c2=4.66P<0.05) was found. Comparing APC-effect: BTA1q13/21 and rob(1;29)q13/21, the high damage found in the second was equivalent to 75% of heterozygosis. This region (RBG- band) has a microsatellite associated to this rearrangement and tissue specific genes.
Key words: Aphidicolin; Break point; rob(1;29); Uruguayan Creole cattle.
RESUMEN
Los bovinos presentan un genoma complejo debido al número alto de secuencias repetidas involucradas en la formación de macro y micro-rearreglos, que fueron esenciales en la evolución de los bóvidos. Sin embargo presentan un cariotipo de morfología sencilla, con autosomas acrocéntricos (2n=60,XX y 60,XY). La translocación Robertsoniana rob(1;29) está ampliamente descripta en diferentes razas de Bos taurus, determinando mortalidad embrionaria temprana. La complejidad del reordenamiento y sus consecuencias en cambios de la cromatina, no son bien conocidas. Para estudiar este reordenamiento se utilizó afidicolina (APC), un agente clastogénico que inhibe a la ADN polimerasa a durante la replicación y permite identificar regiones ricas en dCTP debido a competencia y desplazamiento de la enzima. Se realizaron macro cultivos linfocitarios de 2 hembras de bovinos Criollos Uruguayos portadores de la rob(1;29). Se indujeron con APC (0,3uM) durante un ciclo celular. Se localizó un nuevo punto de ruptura, rob(1;29)p13/21 (distancia relativa: p2c/p1=0.45) en una región de replicación tardía (RBG-). Se analizaron dos sitios frágiles (c-fra) en el brazo largo de la rob(1;29) (q13/21 y q43) y otros dos c-fra en BTAX (q12 y q31). Los c-fra: rob(1:29)q13/21 y Xq12 se ubicaron próximos al centrómero en regiones de replicación tardía, mientras que rob(1;29)q43 y Xq31 se ubicaron próximos al telómero en regiones de replicación temprana. Se encontró una alta incidencia de c-fra proximal al centrómero en ambos cromosomas (BTAXc2=11.3 P<0.001; rob(1;29)c2=4.66P<0.05). Comparando los efectos de APC: BTA1q13/2 y rob1;29q13/21, se encontró mayor daño en el segundo, equivalente a un 75% de heterocigosis. Esta región (RBG-) contiene un microsatélite asociados al reordenamiento y genes tejido-específicos.
Palabras claves: Afidicolina; Punto de ruptura; rob(1;29); Ganado Criollo Uruguayo.
INTRODUCTION
Bos taurus genome is characterized by a high number of repetitive sequences (Vaiman, 1999). Renaturation in liquid phase revealed that at least 50% of this genome is composed of repetitive and highly repetitive sequences (Britten and Kohne, 1968). Schibler et al. (1998) presented a highresolution integrated bovine comparative map where the analysis of break point regions revealed specific repeated density patterns, suggesting that transposons (TEs) may have played a significant role on chromosome evolution and genome plasticity. Macro-rearrangements like the Robertsonian translocation rob(1;29) are considered a particular problem of sub-fertility due to embryo mortality (Gustavsson, 1969). This aneuploid alteration causes a reduction in reproductive efficiency that leads to economic loss. Most chromosome change constitution cause a delay on embryo development (Kawarsky et al.,1996). Furthermore, King (1991) suggested an important role of gene mutation over the early embryonic development of rob(1;29) carriers, probably involving collagen genes.
Molecular genetic characterization of rob(1;29), using 4 microsatellites (MS) of BTA1 (AGLA17, BM6438, TGLA49, BMS4015) and 3MS of BTA29 (BM4602, BMC2228, BMS1857) revealed heterozygosity in BMS4015 of a rob(1;29) bull carrier, hypothesizing its use as a marker of Mendelian segregation in families with this aneuploid anomaly (Joerg et al., 1998). This molecular marker, which is proximal to collagen typeVI-á1 gene (Col6A1), is located at BTA1q21/31 region, at 31cM of the bovine physical map (Shibler et al., 1998; Taylor et al., 1998).
Characterization of a genetic reserve of Uruguayan Creole cattle showed the presence of heterozygous Robertsonian translocation rob(1;29) carrier, with a frequency of 4% (Postiglioni et al., 1996). Clastogenic agents are currently used for analyzing rearrangements in chromatin structure (Sutherland and Richard, 1995).
Particularly, the aphidicolin (APC) inhibits the eukaryotic DNA polymerase a during replication, allowing to identify rich regions in dCTP due to a competition and spreading of the enzyme (Sutherland and Hecht, 1985). The DNA metabolism is affected (replication, transcription, recombination) which is evidenced in condensed metaphase chromosomes as a discontinuity of the chromatin (Sutherland and Richard, 1995; Carme et al., 1999; Wang, 2006). In one cell cycle of cultured human lymphocytes, the APC produces common fragile sites (c-fra) permitting to localize regions of chromosomal instability where rearrangements may occurred (Carme et al., 1999). Recently, an aphidicolin-induction map in cattle using RBG-banding was carried out by Rodriguez et al. (2002), showing dynamic chromosome regions where a minority of fragile sites are located on R+ bands. In this paper, we hypothesize that scoring these c-fra over homologous region proximal to centromere of rob1;29 will show heterogeneity in APC-effects, as was postulated by Sutherland and Hecht (1985). This particular APC-effects could be related to epigenetic mechanisms (Wang, 2006).
In the present study we analyzed the effect of aphidicolin on the chromatin structure in the trivalent: rob(1;29), BTA1 and BTA29, and the biarmed BTAX, in metaphases of female Uruguayan Creole cattle carrying the rob(1;29). We focused on the role of fragile regions as destabilizing agents of cattle genome and their implications in microrearrangements.
MATERIAL AND METHODS
Two female Uruguayan Creole cattle heterozygous for rob 1;29 from the genetic reserve located in San Miguel National Park (33º40´S y 53º38´W) were cytogenetically analyzed to identify and evaluate aphidicolin-induced chromosome break point regions in the trivalent (rob1;29, BTA1, BTA29) and the biarmed X chromosome.
Peripheral whole blood (0.2mL) from each animal was cultured in RPMI-1640 medium (5mL), supplemented with 15% foetal bovine serum, penicillin (100 IU. mL-1), streptomycin (100 μg. mL-1) and phytohemaglutinin (0,2μg. mL-1) for 72 hours at 38.5ºC. Four culture tubes were prepared for each animal, with different treatments: A) control without APC induction (0μM); B) APC induction (0,3μM, added 24 hours before harvesting); C) 5- bromo-2'-deoxyuridine induction (BrdU, 20μg.mL-1, added 6 hours before harvesting) to produce RBG-banding; D) APC induction (0,3μM) and RBG-banding (B+C). Colcemid (0.06 μg.mL-1) was incorporated to the cultures 30 minutes before harvesting. All culture tubes were set up simultaneously in Memmert batches at 38.5ºC. Airdried chromosome slides of tubes A and B were stained with Giemsa (pH 6,8) to detect spontaneous and induced break points. To confirm fragile regions, metaphases (tube B) were destained and reexamined after exposing them to GTG-banding (0,025% trypsin in phosphate-buffered saline, pH7.2, for 40 seconds at room temperature, following Verma and Babu (1995). Replication bands were detached in preparations of tubes C and D following Rodríguez et al. (2002). An Olympus microscope (BX60) with an image capture software (Kodak Digital Science 1D) was used with a 100X magnification. The analysis of chromosome structure was done according to the latest cattle standard chromosome nomenclature (Di Berardino et al., 2001).
To measure the different fragment lengths generated by the treatments, preparations were examined with a Nikon E-800 microscope. Metaphase images were taken using a 100X objective lens with a CoolSNAP-Pro Monochrome Digital camera (Media Cybernetics, Silver Spring, USA). The measurements were carried out on digital images using the Image Pro Plus software by Media Cybernetics. Each fragment was measured five consecutive times and these measures were averaged for minimizing observer errors. These relative values were taken from five metaphases and were also averaged (see appendix).
A chi-square test with Yates' correction was applied for analyzing the distribution of break points along the biarmed chromosomes (BTAXs and rob1;29) and the monobraquial BTA1 and BTA29.
RESULTS
Three-hundred ten metaphases were analyzed from both control (0uM) and APC treated (0.3uM) culture tubes. Fifty metaphases was scored for each sample and only 3% of the total control metaphases showed break points. Twohundred ten metaphases was registered in the experimental tubes scoring 84% with cromatin damage. Our observations focused on the trivalent rob(1;29), its homologous (BTA1 and BTA29) and the BTAXs. The localization of break point was achieved using RBG, GTG-bandings. A new one, with relative length of chromosome fragments measurements, was found (Fig1, 2, 3, Table 1).

Figure1. APC-effects (0,3uM) in partial metaphases of female heterozygous Robertosonian translocation 1;29 and Rbanding in control metaphase (0uM). A and B giemsa stained (pH: 6.8). C) R-banding in auto somes, and early/late replication BTAX. Arrows indicate fragile site in: BTArob1;29, BTA1 and BTAX. Bar=10μ.
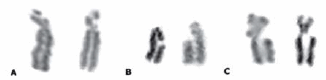
Figure 2. Location of APC-inducted break point. A) rob1;29p13; B) BTA1q13/21; C) BTAXq12.

Figure 3. a) Diagram of the four fragments measured in rob1;29. b) ideogram of BTA29. Arrow signs the break point found in the short arms of the Robertsonian translocation (rob1;29p13/21) (ISSCNDB, 2001)
Table 1. Mean values and standard deviations of the 4 fragments and the relative distance measure in all Metaphases.

The BTA29 did not show break points in any examined induction-metaphases, while BTA1, rob(1;29) and BTAXs showed fragile regions located proximal to centromere (1q13/21, rob(1:29)q13/21, Xq12) in late replication region, and proximal to telomere (1q43, rob(1;29)q43, Xq31) in early replication region. These APC-effects were then used to score into homology region and intra RBG+/RBG- regions. Twenty eight break points corresponded to q13/21 and 14 of the rob(1;29) break points corresponded to q43 (Table 2).
Table 2. Comparison of APC effect between the biarmed chromosomes: BTAX, rob1;29, and BTA1: proximal to centromere (Xq12, rob1:29q13/21, 1q13/21); proximal to telomere (Xq31, rob1;29q43, 1q43).

Localization of a new APC-inducted break point in rob(1;29). Chromosome measurements procedure.
A break point was identified in the short arm (p) of the rob(1;29) in 5 metaphases (3%) (Fig.1A, 2A). Measurements of four chromosome fragments generated by the aphidicolin expression were done. Fragments were identified as follows: p1=short arm; p2t= fragment proximal to telomere; p2c= fragment proximal to centromere; q=large arm (Fig 3). Mean values generated the following ratios: p2c/p1=0.45; p2t/p1=0,61; locating the break point in rob(1;29)p13/21. This region corresponds to a GBG+ and the reverse RBGbanding according to Di Berardino et al. (2001) (Fig 3, Table 1) (see appendix).
Statistical analyses
The frequency of common fragile sites (c-fra) observed after the APC treatment in the heterochromatic regions of BTA1q13/21 and rob(1:29)q13/21 were compared to quantify the probability of homozygosis of APC effects. In rob(1:29)q13/21, 28 c-fra (57%) were observed, in comparison with 21 c-fra (43%) in BTA1q13/ 21. This inter-chromosomal quantification demonstrates a clear heterozygosis (75%) in this heterochromatic region.
To estimate the incidence of APC expression in biarmed chromosomes (BTAXs, rob1;29) and BTA1, two groups of c-fra were tested. One group showed c-fra in an RBG- band: Xq12, rob1:29q13/21, 1q13/21, and the other showed cfra in an RBG+ band: Xq31, rob1;29q43, 1q43. The major APC effect was observed in RBGbands, proximal to centromere of the biarmed chromosomes, while BTA1 showed in both group, similar results (Fig 2; Table 2).
DISCUSSION
Clastogenic agents (aphidicolin, 5-azacytidine- C, 5-bromodeoxyuridine) have been selected as useful tools to study alterations in chromatin structure (Di Berardino et al, 1983; Verma and is identified as rob(1;29)p13 (relative distance: p2c/p1=0.45) (Table 1, see appendix). This break point was neither observed in homologous (BTA29) of the individual carriers nor in normal karyotype of Creole cattle (Postiglioni et al., 2001, 2002). It is interesting to point out that this new break point, and the fragile site rob1;29q13/21 -both proximal to the complex rearrangements region around of the monocentric rob1;29-, are located in late replication regions, where APC induction selected dCTP nucleotides.
Undercondensation of late replicating X chromosome was evident with high concentration of 5-azacytidine-C induction in 2 hrs (G2), due to DNA demethylation (Haaf and Schmid, 2000). So, we could find a similar effect of decondensation if hypomethylated DNA was found in this important heterochromatic region (rob1;29q13/21), after 2 hrs of lymphocytes induction with high concentration of 5-aza-C (10mM). These events will contribute to support our hypothesis that proximal to centromere of rob1;29 exists dynamic heterochromatic regions, where multiple microrearrangements could have occurred during chromosome evolution, possibly affecting gene expression.
Schibler et al., (1998) presented the collagen typeVI-a1 gene (Col6A1) location at BTA1q12/14, a region of late replication (RBG- band) which is proximal to our fragile site: rob1;29q13/21. In agreement to King (1991) we suggested that this chromosomal alteration could affect this gene expression, delaying the embryo balanced carrier development, changing the theoretical ratio of normal:balanced carrier (1:1) to 2:1. King (1991) referred to inbred strains of mice where failures of mesoderm, notochord formation and collagen production, as possible cause of the delay on embryo development. In human, loss of stromal type VI collagen contributes to remodeling the maternal extracellular matrix of pregnancy (Mylonae et al., 1995). This idea could be related to the major transcriptional activity of bovine embryos that occur up to the 8-cells stage (Kawarsky et al., 1996) and to specific tissue genes occasionally expressed in G-positive region (Holmquist and Ashley, 2006).
According previous reports (Postiglioni et al., 2001; 2002; Rodriguez et al., 2002) aphidicolin (0,3uM) generates alterations in chromatin structure of BTArob(1;29), BTA1 and the biarmed BTAX chromosome, with a high incidence in late replication regions after 24 hours of treatment (one cell cycle). Statistical comparison between c-fra located in early and late replication bands of the biarmed chromosomes; rob(1;29) and BTAX, showed a high level of c-fra located in late replication region proximal to the centromere (see Table 2). These regions could be more disposed to suffer chromosome rearrangements, where transpositions, translocation and inversions could occur (Robinson et al., 1998; Schiber et al., 1998). However, BTA1 shows similar APC effect either in late (q13/21) or early (q43) replication region, suggesting a differential chromatin stage between homologous region, as proposed Wang (2006).
Mayr et al. (1998) described an heterozygous reciprocal translocation 60, XX, t(X;1)(42;13) in a high number of cells of a cattle female chimera born with a male twin. This result is related to the fragile site rob1;29q13/21 here described. This instable region could have been repeatedly reorganized, resulting in chromatin structure changes.
Reorganization of repetitive sequences in chromatin of rob1;29 have been previously reported (Di Meo et al., 2006). For instance, the INRA143 is a cosmid that normally hybridize at BTA29q12, GBG+ band with a relative position in 0 (Shibler et al., 1998; Taylor et al., 1998). Chromosome transposition of this repetitive segment to proximal q-arm of the translocated chromosome was showed with FISH methodology. On the other hand BMS4015 is located in BTA1q21 (31cM), and its polymorphism is associated to rob1;29 (Joerg et al., 1998). In this paper, we showed the high incidence of APC-effects in rob1;29q13/21 over its homologous, in agreement to a previous report (Tellechea et al., 2004). So, this particular region of late replication has been transformed in an heterozygous segregation region (Sutherland and Hecht, 1985).
Now, if we associate this observation, -where alterations in a specific region of chromatin structure is evidenced with the delayed development in cattle embryos carriers of rob1;29-, we propose that further research focuses on chromatin transformation caused by an epigenetic effect (Holmquist and Ashley, 2006).
Appendix: Original data to obtain Table 1

Each fragment was measured five consecutive times (from measure1 to 5) in order to avoid observer errors. p2c/p1 and p2t/p1 represent relative values (see Figure 3 for diagram). Averages and standard deviation were calculated for each metaphase and in total.
ACKNOWLEDGMENTS
The authors thank Eileen Armstrong for the English language correction. Rosa Gagliardi and Mónica Martinez to blood extraction and Iris Hernández for the technical assistance. This work was supported by grants from PEDECIBA (Programa de Desarrollo de las Ciencias Básicas), CSIC (I + D Proyect, Universidad de la República) and CIDEC (Facultad de Veterinaria). 30:305-308.
REFERENCES
1. Britten RJ. Kohne D. (1968). Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science161:529-540. [ Links ]
2. Carme F. Rosa M. Miro R. Josep E. Egozcue J. (1999). Chromosome aberrations induced by aphidicolin. Mutation Research 430:47-53. [ Links ]
3. Di Berardino D. Iannuzzi L. Di Meo G. (1983). Localization of BrdU-induced break sites in bovine chromosomes. Caryologia 36(3)285- 292. [ Links ]
4. Di Berardino D, Di Meo G, Gallagher D. (2001). ISCNDB International System for Chromosome Nomenclature of Domestic Bovids. Cytogenet Cell Genet 92: 283-299. [ Links ]
5. Di Meo A, Perucatti R, Chaves F. (2006). Cattle rob(1;29) originating from complex chromosome rearrangements as revealed by both banding and FISH-mapping techniques. Chromosome Research 14:649-655. [ Links ]
6. Gustavsson I. (1969). Cytogenetics, distribution and phenotypic effects of a translocation in Swedish cattle. Hereditas 63:68-169. [ Links ]
7. Haaf T, Schmid M. (2000). Experimental condensation inhibition in constitutive and facultative heterochromatin of mammalian chromosomes. Cytogenet Cell Genet 91:113- 123. [ Links ]
8. Holmquist G, Ashley T. (2006). Chromosome organization and chromatin modification: influence on genome function and evolution. Cytogenet & Genome Res 114:96-125. [ Links ]
9. Joerg H, Garner D, Rieder S, Suwattana D, Stranzinger G. (1998). Molecular genetic characterization of Robertsonian translocations in cattle. J Anim Breed Genet 118:371-377. [ Links ]
10. Kawarsky SJ, Basrur P, Stubbings RB, Hansen PJ, King WA. (1996). Chromosomal abnormalities in bovine embryos and their influence on development. Biology of Reproduction 54:53-59. [ Links ]
11. King WA. (1991). Embryo-mediated pregnancy failure in cattle. Can Vet J 32:99-103. [ Links ]
12. Mayr B, Korb H, Kiendler S. (1998). Reciprocal X;1 translocation in a calf. Genet Sel Evol 30:305-308. [ Links ]
13. Mylona P, Kielty CM, Hoyland JA, Aplin JD. (1995). Expression of type VI collagen mRNAs in human endometrium during the menstrual cycle and first trimester of pregnancy. J.Reprod Fertil Jan:103(1):159-67. [ Links ]
14. Postiglioni A, Llambí S, Gagliardi R. De Bethencourt M. (1996). Genetic characterization of Uruguayan Creole cattle. I.Cytogenetic Characterization of a sample of Uruguayan Creole cattle. Archivos de Zootecnia 45:(170-171)209-213. [ Links ]
15. Postiglioni,A., Llambí,S., Arruga,MV. (2001). Fragilidad cromosómica inducida por afidicolina en reordenamientos cromosómicos (1/29) bovinos. Journal of Basic and Applied Genetics (BAG). 2:91 [ Links ]
16. Postiglioni,A., Llambí,S., Guevara,K. Rincón,G., Arruga,MV. (2002). Common fragile sites expresion in rob(1;29) and their relation with chromosome rearrangements. 15th European Colloquium on Cytogenetics of Domestic Animals and gene Mapping. . Sorrento, Italia. Chromosome Research 10: (1), 5. [ Links ]
17. Robinson T, Harrison W, Ponce de León F. (1998). A molecular cytogenetic analysis of X chromosome repatterning in the Bovidae: transpositions, inversion, and phylogenetic inference. Cytogenet Cell Genet. 80:179-184. [ Links ]
18. Rodríguez V, Llambí S, Postiglioni A, Guevara K, Rincón G, Fernández G, Mernies B, Arruga MV. (2002). Localization of aphidicolininduced break points in Holstein-Friesian cattle (Bos taurus) using RBG-banding. Genet Sel. Evol 34:649-656. [ Links ]
19. Shibler L, Vaiman D, Oustry A, Giraud-Delville C, Cribiu EP. (1998). Comparative gene mapping: A fine-scale survey of chromosome rearrangements between ruminants and humans. Genome Research 901-915. [ Links ]
20. Sutherland G, Hecht F (1985). Fragile sites on Human chromosomes. Oxford University Press [ Links ]
21. Sutherland G, Richard R. (1995). The molecular basis of fragile sites in human chromosomes. Current Opinion in Genetics and Development 5:323-327 [ Links ]
22. Taylor JF, Eggen A, Aleyasin A, Armitage SM, Barendse W, Beever JE, Bishop MD, Brenneman RA, Burns BM, Davis SK, Elo K, Harlizius B, Kappes SM, Keele JW, Kemp SJ, Kirkpatrick BW, Lewin HA, Ma RZ, McGraw RA, Pomp D, Stone RT, Sugimoto Y, Teale AJ, Vaiman D, Zanotti MC, et al. (1998). Report of the first workshop Aon the genetic map of bovine chromosome 1. Anim Genet.29(3):228-35. [ Links ]
23. Tellechea B., Llambí S., de Bethencourt M., Rincón G., Postiglioni A (2004). Avances en el estudio de la heterocromatina en rob 1;29. Un reordenamiento cromosómico que produce mortalidad embrionaria temprana. XXXII Jornadas Uruguayas de Buiatría. 228-231. 2004. [ Links ]
24. Vaiman D. (1999). The Molecular Genetics of cattle. In:The Genetics of cattle. 123-160. CAB International. [ Links ]
25. Verma, R, Babu, A. (1995). Human chromosomes. Principles and techniques. Mc Graw-Hill. [ Links ]
26. Wang, Y-H. (2006). Chromatin structure of human chromosomal fragile sites.Cancer Letters 232:70-78. [ Links ]














