Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
BAG. Journal of basic and applied genetics
On-line version ISSN 1852-6233
BAG, J. basic appl. genet. vol.19 no.2 Ciudad Autónoma de Buenos Aires July/Dec. 2008
Effect of zinc on DNA integrity of cumulus cells during oocyte in Vitro maturation
Picco SJ1,2,3; Seoane A1,3; Anchordoquy JM1,3; Anchordoquy JP1,3; Rosa D2; Fazzio LE2; Mattioli GA2; Furnus CC1,2,3.
1Instituto de Genética Veterinaria (IGEVET) UNLP/CONICET. 2 Laboratorio de Nutrición Mineral y Fisiología Reproductiva. FCV-UNLP. 60 y 118 s/n. B1900AVW La Plata. 3CONICET.
ABSTRACT
Zinc is an essential trace element. It has been known that a non adequate level of zinc can alter not only gene expression but also a variety of cellular functions with severe consequences on animal health. Little is known about the role of zinc on the reproductive performance of bovines, especially in oocyte maturation. The objective of the present study was to evaluate the impact of different zinc concentrations added to culture medium, on the DNA integrity of bovine cumulus cells during in vitro maturation of oocytes. Cumulus-oocyte coplexes obtained from an abattoir were cultured in IVM mediums containing 30 (G1), 70 (G2), 110 (G3) and 150 (G4) μg/dl. At the end of IVM, cumulus cells were processed for Comet Assay. Result obtained with the comet assay shows a significant decrease in the percentage of cell with DNA migration from around 40% in G1 and G2 to 20% in G3 and G4 (p< 0,05). The intensity of DNA damage (DDV) was higher in G1 and G2 and significant different with G3 and G4 (p< 0,05). Taken into account, the relationship between in vitro maturation of cumulus cells oocytes complexes (COCs) and subsequent embryo development, it might be possible that different levels on Zinc concentration during IVM have consequences on early embryo development.
Key Word: Zinc; Cumulus cells; Comet assay
RESUMEN
El zinc es un micronutriente cuya deficiencia afecta no solo la expresión génica, sino también una variedad de funciones celulares con consecuencias directas en la salud animal. Poco se conoce acerca del rol del zinc en la performance reproductiva de los bovinos, especialmente en relación con la maduración de los ovocitos. El objetivo del presente trabajo fue evaluar la integridad del ADN de en células del cúmulus de bovinos, cultivadas con diferentes concentraciones de zinc adicionadas a el medio de cultivo de maduración in Vitro de ovocitos. Para ello, los complejos cúmulos-ovocitos obtenidos en frigoríficos fueron cultivados en Medios para Maduración in Vitro, conteniendo 30 (G1), 70 (G2), 110 (G3) y 150 (G4) μg/dl de zinc. Al finalizar la maduración, las células del cúmulos fueron procesadas para realizar el ensayo cometa. Los resultados obtenidos con dicho ensayo mostraron una disminución significativa en el porcentaje de células con migración del ADN, de alrededor del 40% en G1 y G2 hasta alrededor de 20% en G3 y G4 (p< 0,05). La intensidad del daño en el ADN fue significativamente mayor en G1 y G2 que en G3 y G4 (p< 0,05). Considerando la estrecha relación existente entre los complejos ovocito-células del cúmulus y el subsecuente desarrollo embrionario, es posible inferir que diferentes niveles de Zinc durante la MIV pueden tener consecuencias en el desarrollo embrionario temprano.
Palabras claves: Zinc; Células del cúmulus; ensayo cometa.
INTRODUCTION
Zinc is an essential trace element found in small amounts in a variety of cells and tissues. It is a cofactor of more than 300 enzymes Tapeiro and Tew, 2003), and is involved in several cell functions including signal transduction, transcription and replication (Cousins et al, 2006). About three to ten percent of all proteins in mammalian genomes are considered to bind zinc for holding, activity and conformational changes (Selker et al 2007).
It has been known that a non adequate level of zinc can alter not only gene expression but also a variety of cellular functions with severe consequences on animal health (Selker et al 2007). Recently, major efforts have been made to determine the effect of trace elements on reproduction impact in mammals, but little is known about the role of zinc on the reproductive performance of bovines, especially in oocyte maturation.
During oocyte maturation, the mammalian oocytes are surrounded by cumulus cells that are coupled through gap junctions to the oocyte. Also, these gap junctions connect one cumulus cell with another one arround the oocyte. The function of this comunication is evident in the transporting of relatively low molecular weight molecules, ions, and amino acids to the oocyte. Consequently, this implicate a metabolic support for the oocyte. Moreover, Kim et al. (1996) demonstrated that although the presence of cumulus cells coupled to bovine oocytes was not necessary for nuclear maturation, they play an important role on the subsequent capacity of the oocytes for developing to blastocyst stage (Lonergan P and Fair T; 2008)
The objective of the present study was to evaluate the impact of different zinc concentrations added to culture medium, on the DNA integrity of bovine cumulus cells during in vitro maturation of oocytes.
MATERIALS AND METHODS
Reagents and Media
Tissue culture medium (TCM) 199, fetal calf serum (FCS), sodium pyruvate, L-glutamine, fatty acid-free BSA, kanamycin, polyvinylpyrrolidone (PVP; average Mr 40 000), and antibiotics- antimycotics were purchased from Sigma Chemical Co. (St.Louis, MO). All reagents were of cell culture-tested quality. FSH was purchased from Biotay (Canadá). IVM medium was bicarbonate- buffered TCM-199 supplemented with 10% (v:v) FCS, 0.2 mM sodium pyruvate, 1 mM glutamine, 1 μg/ml porcine FSH (pFSH), 1 μg/ml estradiol-17beta , and 50 g/mlkanamycin. From comet assay reagements used were low melting point agarose (Gibco BRL, NY), normal melting point agarose (Promega), EDTA (Gibco BRL, NY), NaCl (Gibco BRL, NY), Tris (USBiological, MA), Triton X-100 (Sigma, St Louis, MO), NaOH (Carlo Erlsa, Milano, Italy), and SYBR Green I (Molecular Prubes, Eugene, OR).
Cumulus-Oocytes Complexes
Bovine ovaries were obtained from an abattoir and transported to the laboratory in sterile NaCl solution (9 g/L) with antibiotics at 37°C within 3 h of slaughter. The ovaries were pooled regardless of stage of the estrous cycle of donors, and cumulus-oocyte complexes (COCs) were aspirated from follicles from 2 to 8 mm with a 18 G syringe. Only cumulus-intact oocytes with evenly granulated cytoplasm were selected, using a lowpower (x20-30) stereomicroscope, for IVM.
IVM
Cumulus cell complexes were washed in TCM-199 buffered with 15 mM Hepes containing 10% (v:v) FCS, after which COCs were washed twice in the same medium and twice in IVM medium. Groups of 10 oocytes were transferred into 50 μl of IVM medium under mineral oil (Squibb, Princeton, NJ) pre-equilibrated in a CO2 incubator. The COCs were cultured in IVM medium at 39°C in an atmosphere of 5% CO2 in air with saturated humidity for 24 h.
Comet Assay
At the end of IVM, the oocytes were assessed for cumulus expansion; only oocytes with good expansion of the cumulus cells were processed for Comet Assay. Oocytes were stripped of surrounding cumulus cells by repeated pipetting with a narrow-bore glass pipette in TCM 199 buffered with HEPES and washed 3 times in calcium- and magnesium-free PBS containing 1 mg/ml PVP. For each replicate, pools of > 50 oocytes in 10 μl of PBS from each treatment were placed in microtubes, frozen at -200C, and thawed at room temperature. This procedure was repeated three times. Complete cell disruption was achieved by repeated aspiration using a narrowbore pipette. Samples were then mixed with agarose low melting point and treated as describe in Picco et al (2004) with minor modifications. The data for comet assay was recorded blind on coded slides. A total of 100 cells per slide were scored. Cells were classified in with or without DNA damages. The DNA damage was classified according to Kobayashi et al. (1995) as follows: Type 0 (comets without a tail), Type 1 (comets with tiny tail), Type 2 (comets with a dim tail), Type 3 (comets with a clear tail), and Type 4 (comets with a clear decrease in the diameter of the head and a clear tail). Arbitrary units of DNA damage value was established according to Collins (2004).
Zinc determination
Culture media samples were centrifuged and then was treated with 10% (w/v) trichloroacetic acid, separating the supernatant for Zn analysis. Zinc concentration was analysed by flame atomic absorption spectrophotometry (double beam atomic absorption spectrophotometer GBC 902) using internal quality control (Piper and Higgins, 1967).
Experimental Design
In all experiments, oocytes were in vitro-matured, as described above, for 24 h. After this period, cumulus cell complex were processed to obtain granulose cells for comet assay. COCs and DOs were matured in IVM medium supplemented with zinc sulphate to obtain a final concentration of 30 μg/dl(experiment 1-baseline concentration of media), 70 μg/dl (experiment 2), 110 μg/dl (experiment 3), or 150 μg/dl (experiment 4). Four Replicates of experiments were performed on different days with different batches of oocytes.
Data Analysis
The number of cells with or without DNA damage among experiments was analyzed using Square Chi test. Differences among cell types and differences in DDV in each experiment were analizad using Student t test. Results are expressed as a mean ± SD.
RESULTS
Result obtained with the comet assay shows a significant decrease in the percentage of cell with DNA migration from around 40% in groups 1 and 2 to 20% in 3 and 4 (Table 1). Diferent degree of DNA damaged cells founded are in table 2. The most important diferences were found in types 1, 2 and 3. In these cases, treatment 1 and 2 were similar and significant different with treatment 3 and 4 (p< 0,05). The intensity of DNA damage (DDV) founded with different zinc concentrations are shown in figure 1. DDV was higher in treatments 1 and 2 and significant different with treatment 3 and 4 (p< 0,05).
Table 1. Number and percentage of cells with or without DNA damage according with the level of zinc concentration in the culture media.
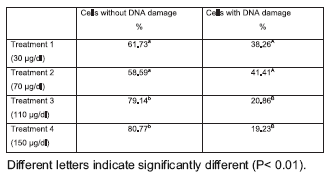
Table 2. DNA damage measured by the comet assay
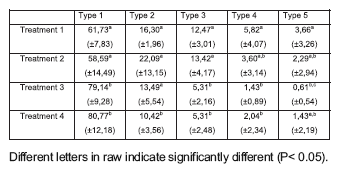
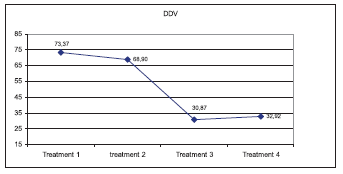
Figure 1. DNA damage intensity estimated according with Collins (2004)
DISCUSSION
The results obtained in the present study indicate that regular levels of zinc used in culture media for oocyte maturation in vitro are poor to prevent or reduce DNA damage in cumulus cells. Even, 70 μg/ dl Zinc showed a similar effect to basal concentration (30 μg/ dl Zn). However, Zn concentrations of 110 to 150 μg/ dl reduced significantly the number of cells with DNA damage, and the intensity of damage suggesting a positive effect of media supplemented with Zn during bovine oocyte in vitro maturation.
In recent years, several studies are focused on the implications of apoptosis and DNA fragmentation in cumulus cells, particularly on the features of this process. The question is, if this process are spontaneous and consequently physiological, or an artifact of culture conditions (Ikeda and Yamada, 2003). According with Luciano et al (2000), follicular atresia seems to be initiated with death of granulose cells. It has been demonstrated either in vivo and in vitro studies, that granulose cells death is characterized by DNA fragmentation, suggesting that these cells die through an active programmed process or apoptosis. However, at present the results obtained are controversial. Luciano et al. (2000) reported that cumulus cells of bovine Cumulus Enclosed Oocytes (CEOs) cultured in defined media did not undergo apoptosis after 24 h of IVM. In contrast, Ikeda et al (2003) demonstrated that the apoptotic cells and DNA fragmentation are markedly increased as the IVM culture progress. Moreover, using different culture conditions, they detect different induction periods of apoptosis or DNA fragmentation, suggesting that these differences may reflect the effects of culture conditions variations. Our study showed that, different zinc concentrations supplemented to IVM medium had a distinguish response.
According with Collins (2004), classic comet assay do not provide specific evidence of apoptosis. However, the increase of DNA fragmentation values obtained here (Treatment 1 and 2) may us speculate that zinc supplementation is at least insufficient. Taken into account, the relationship between in vitro maturation of cumulus cells oocytes complexes (COCs) and subsequent embryo development, it might be possible that different levels on Zinc concentration during IVM have consequences on early embryo development. Further studies are necessary to elucidate, the impact of the zinc levels during bovine IVM on in vitro fertilization and embryo culture until blastocyst stage.
REFERENCES
1. Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking and signals. Journal of Biologica Chemistry 281 (34): 24085- 24089 [ Links ]
2. Sekler I, Sensi SL, Hershfinkel M, Silverman WF. (2007). Mechanism and regulation of cellular zinc transport. Mol. Med. 13 (7-8): 337- 343. [ Links ]
3. Tapiero H, Tew KD.Trace elements in human physiology and pathology: zinc and metallothioneins (2003). Biomed Pharmacother. 57(9):399-411. [ Links ]
4. Kim SK, Minami N, Yamada M, Utsumi K. Functional role of cumulus cells during maturation in development of in vitro matured and fertilized bovine oocytes (1996). Theriogenology 45:278. [ Links ]
5. Lonergan P, Fair T. (2008). In vitro-produced bovine embryos: dealing with the warts. Theriogenology. 69(1):17-22. [ Links ]
6. Picco SJ, Abba MC, Mattioli GA, Fazzio LE, Rosa D, De Luca JC, Dulout FN. (2004). Association between copper deficiency and DNA damage in cattle. Mutagenesis 19 (6): 453-456. [ Links ]
7. Kobayashi,H., Sugiyama,C., Morikawa,Y., Hayashi, M. and Sofuni,T. (1995) A comparison between manual microscopic analysis and computerised image analysis in the single cell gel electrophoresis assay. MMS Commun., 3, 103--115. [ Links ]
8. Collins AR. The comet assay for DNA damage and repair. Principles, applications, and limitations. Mol Biotech 26: 249-261, 2004. [ Links ]
9. Piper,H.G. and Higgins,G. (1967) Estimation of trace metals in biological material by atomic absorption spectrophotometry. Proc. Assoc. Clin. Biochem., 7, 190--195. [ Links ]
10. Ikeda S, Imai H, Yamada M. (2003). Apoptosis in cumulus cells during in vitro maturation of bovine cumulus-enclosed oocytes. Reproduction 125(3):369-76. [ Links ]
11. Luciano AM, Modina S, Gandolfi F, Lauria A and Armstrong DT. (2000). Effect of cell-tocell contact on in Vitro deoxyribonucleic acid síntesis and apoptosis responses of bovine granulosa cells to insulin-like growth factor-I and epidermal growth factor Biology of Reproduction 63 1580-1585. [ Links ]














