Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
BAG. Journal of basic and applied genetics
versión On-line ISSN 1852-6233
BAG, J. basic appl. genet. vol.22 no.2 Ciudad Autónoma de Buenos Aires dic. 2011
ARTÍCULOS ORIGINALES
In vitro apprach to the study of chronic exposure to low does of X-rays
Ponzinibbio M.V.1,2, Peral-García P.1,2, Seoane A.1,2,*
1IGEVET (UNLP-CONICET), La Plata, Argentina
2CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Argentina
*Corresponding Author: aseoane@fcv.unlp.edu.ar
ABSTRACT
The present research was undertaken in order to study the effect of repeated low doses of radiation on two different cell lines from the same species. An in vitro model test was developed to avoid the influence of the confounding factors affecting epidemiological studies and to simulate a chronic exposure (50 mSv of x-rays during ten consecutive days). Comet assay and early apoptosis were analyzed immediately after exposure and after chronic irradiation. Sequential irradiation induced an increase of cells showing DNA damage. Index Damage was higher than that of the controls in both cell lines immediately after exposure and after chronic irradiation. These differences between exposed and control cells were statistically significant only for the transformed cell line after chronic irradiation (p<0.001). Significantly higher levels of apoptosis were scored after chronic exposure in both cells lines (p<0.001). The induction of DNA damage and apoptosis in hamster cells by chronic exposure to low dose ionizing radiation was demonstrated. Cell types reacted differently to chronic exposure. Though further investigation is needed to understand the mechanisms of radiation effects on chronic low-dose-exposed cell populations, cellular type should be taken into account in the design of in vitro experiments to understand low-dose-irradiation effects.
Key words: Apoptosis; Comet assay; In vitro; Ionizing radiation; Low dose.
RESUMEN
El presente trabajo fue realizado con el fin de estudiar el efecto de dosis bajas y repetidas de radiación sobre dos líneas celulares de la misma especie. Se desarrolló un modelo in vitro para evitar la influencia de los factores de confusión que afectan a los estudios epidemiológicos y para simular una exposición crónica. Las técnicas utilizadas fueron el ensayo cometa y el análisis de apoptosis temprana; estas se llevaron a cabo inmediatamente después de la exposición y luego de la irradiación crónica. La irradiación secuencial indujo un aumento de células con daño en el ADN. El índice de daño fue mayor que el de los controles en ambas líneas celulares, tanto inmediatamente después de la exposición como luego de la irradiación crónica. Este aumento fue estadísticamente significativo solamente para la línea celular transformada luego de la irradiación crónica (p<0,001). El análisis de apoptosis arrojó niveles significativamente mayores al control para ambas líneas celulares luego de la exposición crónica (p<0.001). Se demostró que la exposición crónica a radiación ionizante de dosis bajas indujo daño en el ADN y apoptosis en células de hámster chino cultivadas in vitro. Las respuestas de ambos tipos celulares fueron algo diferentes. Evidentemente, el tipo celular debe ser tenido en cuenta a la hora de diseñar experimentos in vitro para entender los efectos de la radiación crónica de dosis baja en las poblaciones celulares.
Palabras clave: Apoptosis; Ensayo cometa; In vitro; Radiación ionizante; Dosis bajas.
INTRODUCTION
Living beings are permanently exposed to ionizing radiation emitted either from natural or anthropogenic sources. Although for public health purposes the overall risks of cancer are assumed to increase in proportion to the dose of ionizing radiations (Upton, 2002), the effects caused by low-dose irradiation differ from those caused by high-dose irradiation. Assessment of risks that might be attributable to low level acute, or even chronic, radiation exposure entails the use of models that enable a better understanding of the underlying mechanisms of such exposures. In this sense, there is increasing evidence that low doses of radiation might induce other phenomena such as bystander effects and adaptive responses (Azzam et al., 1998; Venkat et al., 2001; Sawant et al., 2002; Ballarini et al., 2002; Little et al., 2002).
Most of the studies of radiation exposures and mutagenesis have been carried out either in vivo with exposed human populations or in vitro with cell lines irradiated with single radiation doses. An in vitro model test was developed to avoid the influence of the confounding factors affecting epidemiological studies and to simulate a chronic exposure. This model allowed the preservation of controlled conditions by using cultured cells exposed daily to x-rays. Induction of chromosomal aberrations and DNA damage by repeated exposure to very low doses of x-rays were described in our laboratory by means of this approach (Güerci et al., 2003; 2004; 2005). However, these findings may be limited to the cell line employed and they may, consequently, not be a generalized phenomenon caused by repeated low doses of radiation.
The present research was undertaken in order to study the genetic effect of repeated low doses of radiation on two different cell lines from the same species: one is the transformed, epithelial-like and aneuploid Chinese Hamster Ovary (CHO) and the other, which originated from a primary culture, is the fibroblastic, diploid and non-transformed Chinese Hamster Embryo Diploid (CHED).
The single cell gel electrophoresis assay, a sensitive and rapid method to determine the level of DNA damage in terms of strand breaks and alkaline labile sites (Wojewodzka et al., 1998; MØller et al., 2000; Collins, 2004) was used. In addition, apoptosis induction was evaluated by means of Annexin V-FITC marked.
MATERIAL AND METHODS
Cells
CHO cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and CHED cells were kindly provided by Professor A.T. Natarajan. They were cultured in Ham's F10 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Natocor Laboratories, Province of Cordoba, Argentina) and antibiotics (50 IU penicillin and 50 mg/ml streptomycin) (Bagó Laboratories, Buenos Aires, Argentina) in a humidified atmosphere with 5% CO2. Cells were cultured in Falcon T-25 (Nunc, Denmark).
Experimental design
Two flasks for each cell line were cultured for ten days. One of them was employed as negative control and the other was irradiated daily at the same hour. Control and irradiated populations were assayed immediately after the first and the tenth irradiations. Following the first irradiation, cells were tripsinized, resuspended and divided into various fractions. One was kept in culture to obtain the repeatedly exposed population and aliquots were separated for comet assay and apoptosis analysis. After ten days, cells were tested again for the same assays. The doubling time of CHO and CHED cells under these culture conditions was periodically checked in the laboratory by the bromodeoxyuridine technique (BrdU); it varies between 12-15 h. Each experiment was repeated twice. Average values are shown in the tables below. Analysis was carried out blinded by one investigator.
Irradiation conditions
The radiation dose employed (50 milisievert=mSv) (≈50 mGy) was determined taking into account previous experience in our laboratory (Güerci et al., 2003; 2004; Seoane et al., 2007) and the dosimetry reported in other epidemiological studies (Barquinero et al., 1993; Paz y Miño et al., 1995; Balakrishnan and Rao, 1999; He et al., 2000; Cardoso et al., 2001; Maluf and Erdtmann, 2000; Cavallo et al., 2002). The x-ray apparatus was supplied by Dental San Justo Company (Buenos Aires, Argentina) and operated at 65kV and 5 mA. Doses were determined by using a dosimeter Keithley Digital 35617 EBS microchamber PTW N 2336/414 (C-Com Industries, Robertville, MO, USA) which was employed at a dose rate of 50 mSv/min. Radiation was administered from above through the medium at exposure times of 60 seconds. For the irradiation treatment, 10 ml of fresh medium were placed on the attached cells to prevent cell detachment from occurring.
Comet Assay
Single cell gel electrophoresis was performed by employing the alkaline version described by Singh and coworkers (1988) with some modifications (Tice and Strauss, 1995). Briefly, slides were covered with a first layer of 180 μl of 0.5% normal agarose (Carlsbad, CA, USA). An amount of 75 μl of 0.5% low melting point agarose (Carlsbad, CA, USA) was mixed with approximately 15,000 cells suspended in 15 μl and layered onto the slides, which were then immediately covered with coverslips. After agarose solidification at 4°C for 10 min, coverslips were removed and slides were immersed in fresh lysis solution at 4°C overnight. The slides were equilibrated in alkaline solution for 20 min. Electrophoresis was carried out for 30 min at 25 V and 300 mA (1.25 V/cm). The slides were subsequently neutralized by washing them three times with Tris buffer (pH 7.5) every 5 min and then in distilled water. The slides were stained with 1/1000 SYBR Green I (Molecular Probes, Eugene, OR, USA) solution (Olive, 1999). A total of 400 randomly selected comet images were analyzed per treatment. Data were statistically analyzed with the X2-test with the Statgraphics® 5.1 software.
Scoring was made at 400x magnification by means of a fluorescent microscope (Olympus BX40 provided with a 100 W high pressure mercury lamp (USHIO USH 102 D) and a 515-560 nm excitation filter. Images were captured with a Sony 3 CCD-IRIS Color Video Camera and saved by using Image Pro Plus® software. The comet assay was carried out blinded on coded slides.
Based on the extent of strand breakage, cells were classified according to their tail length in five categories, ranging from 0 (no visible tail) to 4 (still a detectable head of the comet but most of the DNA in the tail) (Olive, 1999; Collins, 2004) (Fig. 1). In order to quantify DNA damage from the comets, the measuring method of Collins (2004) was used. Index Damage (ID) was obtained: if 100 comets are scored and each comet is assigned a value of 0 to 4 according to its category, the total score for the sample gel will be between 0 to 400 "arbitrary units". Visual scoring (arbitrary units) is rapid as well as simple and there is a very close agreement between this method and computer image analysis (percentage of DNA in tail) (Collins, 2004).
ID = (Degree1x1)+ (Degree2x2)+ (Degree3x3)+ (Degree4x4) x 100
Total
Analysis of apoptosis by Annexin V /PI staining assay
Early apoptosis as evaluated by membrane redistribution of phosphatidylserine was examined by using an Annexin V-FITC. Externalization of phosphatidylserine (PS) to the outer side of the plasma membrane is one of the earliest features of cells undergoing apoptosis, which can be marked by Annexin V, a calcium-dependent phospholipid-binding protein with a very high affinity for PS (Todd Allen et al., 1997; van Engeland et al., 1998). By conjugating fluorescein isothiocyanate to Annexin V, it is possible to identify and quantify apoptotic cells. Briefly, cells were washed twice with binding buffer, resuspended in 0.1 ml of FITC-Annexin V (1 mg/ml final concentration), incubated for 10 min in the dark at room temperature, washed twice in binding buffer, added to 0.1 ml of PI solution (1 mg/ml final concentration) and analyzed under a fluorescence microscope. A total of 1000 cells were scored per experimental point. Data were statistically analyzed with the X2-test with the Statgraphics® 5.1 software.
RESULTS
Tables 1 to 4 summarize the results derived from the present study. Sequential irradiation induced an increase of cells showing DNA damage. Index Damage was higher than that of the controls in both cell lines, not only immediately after exposure (50 mSv) but also after chronic irradiation (500 mSv); these differences between exposed and control cells were statistically significant only for CHED cells after chronic irradiation (p<0.001). In addition, differences between cell exposed one time and those exposed 10 times were evaluated, these differences were statistically significant only for CHED cells (p=0.05).
Table I. DNA damage CHO cells analyzed immediately after exposure to 50 mSv x-rays. Standard error of the mean is indicated between parentheses.

Table II. DNA damage in CHO cells analyzed after ten days of chronic exposure to 50 mSv x-rays. Standard error of the mean is indicated between parentheses.

Table III. DNA damage in CHED cells analyzed immediately after exposure to 50 mSv x-rays. Standard error of the mean is indicated between parentheses.
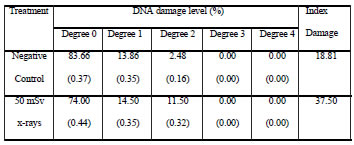
Table IV. DNA damage in CHED cells analyzed after ten days of chronic exposure to 50 mSv x-rays. Standard error of the mean is indicated between parentheses.

When comparison was made between cell lines, CHO and CHED cells showed similar damage level upon being scored immediately after the first exposure (no significant differences). Following chronic irradiation, the responses from both cellular types were quite different. Index Damage scored in CHED cells was significantly higher than that scored in CHO cells (p<0.01).
Early apoptosis results are summarized in Table 5. Both cell lines showed significant levels of apoptosis when they were scored immediately after exposure and at the end of the experiment (p<0.0001). Significantly higher frequency of apoptosis was also observed at the end than at the beginning of the experiment (p<0.0001, CHO; p<0.01 CHED). When comparison was made between cell lines, CHED cells showed significantly more apoptosis (p<0.0001) than CHO cells at both sample times.
Table V. Frequency of FITC-Annexin V positive cells analyzed after ten days of chronic exposure to 50 mSv x-rays. Standard error of the mean is indicated between parentheses.
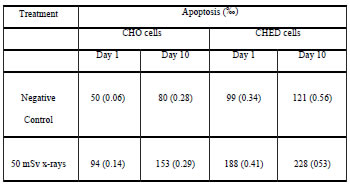

Figure 1. Cell classification according to their tail length. Categories: 0 (no visible tail) to 4 (still a detectable head of the comet but most of the DNA in the tail).
DISCUSSION
When cells are exposed to low doses of ionizing radiation, one of the most important kinds of damage is double-strand breaks (Gollin, 2005). In response to the introduction of DSB into DNA, eukaryotic cells trigger a cascade of mechanisms that lead first to the cell cycle arrest and then to DNA repair if possible. When repair is wrong or not possible, a cell should die or remains damaged (Morgan, 2003; Mothersill and Seymour, 2003). Single cell gel electrophoresis (SCGE) or comet assay is a rapid and sensitive fluorescent microscopic method that allows measurement of DNA strand breaks in individual cells (Singh, 2000). In its alkaline version, it detects DNA damage as single-stranded and/or double-stranded DNA breaks in eukaryotic genome, as well as alkali-labile sites, incomplete DNA repair sites, and changes in structural chromosome configuration (Tice et al., 2000). Our results indicate that continuous x-ray exposure for 10 days at 50 mSv/day induces DNA-damage and apoptosis in these cell lines. In this context, these results are consistent with our previous findings with lower doses of X rays (2.5 to 10 mSv) (Güerci et al., 2004; 2005) and those of other authors (Vijayalaxmi et al., 1993; Wojewodzka et al., 1998; Sudprasert et al., 2006) who found DNA damage after exposure to doses of 50 mGy (≈ 50mSv) of ionizing radiation in other cellular types.
Based on direct epidemiological evidence of human populations, Brenner and colleagues (2003) claim that when exposure occurs at doses above 50-100 mSv (protracted exposure) or 10-50 mSv (acute exposure) the risk of some types of cancer increases. According to Mothersill and Seymour (2003), the results obtained could be explained by assuming that cells do not recover well after the first exposure. Genetic changes could be accumulated during the experiment as a result of the direct effect of radiation, genomic instability or other non-targeted phenomena. Some authors have reported the relationship between radiation induced genetic instability and the events of spontaneous apoptosis and increased reproductive cell death (Limoli et al., 1998; Mendonca et al., 1999; Chang and Little, 1992; Jamali and Trott, 1996).
The response of CHED cells was quite different from that of CHO cells. Different probabilities of DSB conversion into lethal lesions or different repair capacities could be suggested to explain this. In this respect, McLachlan-Burgess and coworkers (2006) reported differences in the response to low dose ionizing radiation-induced DNA damage and increased reactive oxygen species between normal human fibroblasts and cancerous cells while other authors observed an adaptive response in normal cells but not in tumor ones (Jiang et al., 2008). The differences between both cell lines may indicate that in the low dose region, the response to radiation depends as much on the characteristics of the cell population as on the dose employed (Little et al., 1989; Crompton et al., 2002).
On the other hand, Index Damage appears to be a sensitive parameter as also found by some authors in different cell types (Collins et al., 1995; da Silva et al., 2000). Its use as a parameter of the comet assay was reported by Maluf and coworkers (2001) and Martino-Roth and coworkers (2003) in human lymphocytes exposed to radiation doses similar to those used in the present study. In the latter, Index Damage turned out to be useful to detect DNA damage in CHO and CHED cells exposed to low-dose X-rays. This index not only allows the observation of significant differences between control and treated cells but also between the two different cell types.
The induction of DNA damage and apoptosis in hamster cells by chronic exposure to low-dose- ionizing radiation was demonstrated. Transformed and non-transformed cell types react differently to direct and indirect damage when using low-dose repeated exposures to ionizing radiation. Though further investigation is needed to understand the mechanisms of radiation effects on chronic low-dose-exposed cell populations, cellular type should be taken into account in the design of in vitro experiments to understand low-dose-irradiation effects.
ACKNOWLEDGEMENTS
This work was supported by grant PIP 5583 from CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), grant PICT 14329 from ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica) and grant 11-V138 from UNLP (Universidad Nacional de La Plata), Argentina. Authors are grateful to Prof. Juan Andrieu for the calibration of the irradiation equipment and to Professor of English, Ms. Ana Insausti, for the style revision of this manuscript
BIBLIOGRAPHY
1. Azzam E.I., de Toledo S.M., Gooding T., Little J. (1998). Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Rad. Res. 150:497-504. [ Links ]
2. Balakrishnan S. and Rao S. (1999). Cytogenetic analysis of peripheral blood lymphocytes of occupational workers exposed to low levels of ionizing radiaton. Mutat. Res. 442:37-42. [ Links ]
3. Ballarini F., Biaggi M., Ottolenghi A., Sapora O. (2002). Cellular communication and bystander effects: a critical review for modelling low dose radiation action. Mutat. Res. 501:1-12. [ Links ]
4. Barquinero J.F., Barrios L., Caballin M., Miró R., Ribas M., Subias A., Egozcue J. (1993). Cytogenetic analysis of lymphocytes from hospital workers occupationally exposed to low low levels of ionizing radiaton. Mutat. Res. 286:275-279. [ Links ]
5. Brenner D.J., Doll R., Goodhead D., Hall E., Land C., Little J., Lubin J., Preston D., Preston R., Puskin J., Ron E., Sachs R., Samet J., Setlow R., Zaider M. (2003). Cancer risk attributable to low doses of ionizing radiation: assessing what we really know. Proc. Nat. Acad. Scs. (USA) 25:13761-13766. [ Links ]
6. Cardoso R., Takahashi-Hyodo S., Peitl P. Jr., Ghilardi-Neto T., Sakamoto-Hojo E. (2001). Evaluation of chromosomal aberrations, micronuclei and sister chromatid exchanges in hospital workers chronically exposed to ionizing radiation. Teratog. Carcinog. Mutagen. 21:431-439. [ Links ]
7. Cavallo D., Marinaccio A., Perniconi B., Tomao P., Pecoriello V., Moccaldi R., Iavicoli S. (2002). Chromosomal aberrations in long-haul air crew members. Mutat. Res. 513:11-15. [ Links ]
8. Chang W.P. and Little J.B. (1992). Persistently elevated frequency of spontaneous mutations in progeny of CHO clones surviving X-irradiation is associated with delayed reproductive death phenotype. Mutat. Res. 20:191-199. [ Links ]
9. Collins A. (2004). The comet assay for DNA damage repair. Mol. Biotechnol. 26:249-260. [ Links ]
10. Collins A., A1-Guo M., Duthie S. (1995). The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. 36:69-77. [ Links ]
11. Crompton N., Shi Y., Wuergler F., Blattmann H. (2002). A single dose of X-rays induces high frequencies of genetic instability (aneuploidy) and heritable damage (apoptosis), dependent on cell type and p53 status. Mutat. Res. 17:173-86. [ Links ]
12. da Silva J., de Freitas T., Heuser V., Marinho J., Erdtmann B. (2000). Genotoxicity biomonitoring in coal regions using wild rodent Ctenomys torquatus by Comet assay and micronucleus test. Environ. Mol. Mutagen. 35:270-278. [ Links ]
13. Gollin S.M. (2005). Mechanisms leading to chromosomal instability. Semin. Cancer Biol. 15:3-42. [ Links ]
14. Güerci A., Dulout F., Seoane A. (2003). Cytogenetic analysis in Chinese hamster cells chronically exposed to low doses of X-rays. Int. J. Radiat. Biol. 79:793-799. [ Links ]
15. Güerci A., Dulout F., Seoane A. (2004). DNA damage in Chinese hamster cells repeatedly exposed to low doses of X-rays. Cytogenet. Genome Res. 104:173-177. [ Links ]
16. Güerci A., Dulout F., Grillo C., Seoane A. (2005). Differential response of two cell lines sequentially irradiated with low X-ray doses. Int. J. Radiat. Biol. 81:367-372. [ Links ]
17. He J., Chen W., Jin L., Jin H. (2000). Comparative evaluation of the in vitro micronucleus test and the comet assay for the detection of genotoxic effects of X-ray radiation. Mutat. Res. 469:223-231. [ Links ]
18. Jamali M., Trott K.R. (1996). Persistent increase in the rates of apoptosis and dicentric chromosomes in surviving V79 cells after X-irradiation. Int. J. Radiat. Biol. 70:705-709. [ Links ]
19. Jiang H., Li W., Li X., Cai L., Wang G. (2008). Low-dose radiation induces adaptive response in normal cells, but not in tumor cells: in vitro and in vivo studies. J. Radiat. Res. 49:219-230. [ Links ]
20. Limoli C.L., Hartmann A., Shephard L., Yang C., Boothman D., Bartholomew J., Morgan W.F. (1998). Apoptosis, reproductive failure, and oxidative stress in Chinese hamster ovary cells with compromised genomic integrity. Cancer Res. 58:3712-3718. [ Links ]
21. Little J.B., Azzam E.I., de Toledo S.M., Nagasawa H. (2002). Bystander effects: intercellular transmission of radiation damage signals. Radiat. Prot. Dosimetry 99:159-162. [ Links ]
22. Little J.B., Ueno A., Dahlberg W. (1989). Differential response of human and rodent cell lines to chemical inhibition of the repair of potentially lethal damage. Radiat. Environ. Biophys. 28:193-202. [ Links ]
23. McLachlan-Burgess A., McCarthy S., Griffin C., Richer J., Cutler R., Pandey S. (2006). Differential response induced by exposure to low-dose ionizing radiation in SHSY-5Y and normal human fibroblast cells. Appl. Biochem. Biotechnol.135:159-178. [ Links ]
24. Maluf S., Passos D., Bacelar A., Speit G., Erdtmann B. (2001). Assessment of DNA damage in lymphocytes of workers exposed to X-radiation using the micronucleus test and the comet assay. Environ. Mol. Mutagen. 38:311-315. [ Links ]
25. Maluf S.W., Erdtmann B. (2000). Follow-up study of the genetic damage in lymphocytes of pharmacists and nurses handling antineoplastic drugs evaluated by cytokinesis block micronuclei analysis and single cell gel electrophoresis assay. Mutat. Res. 471:21-27. [ Links ]
26. Martino-Roth M., Viégas J., Roth D. (2003). Occupational genotoxicity risk evaluation through the comet assay and the micronucleus test. Genet. Mol. Res. 2:410-417. [ Links ]
27. Mendonca M., Howard K., Farrington D., Desmond L., Temples T., Mayhugh B., Pink J., Boothman D. (1999). Delayed apoptotic responses associated with radiation- induced neoplastic transformation of human hybrid cells. Cancer Res. 59:3972-3979. [ Links ]
28. MØller P., Knudsen L., Loft S., Wallin H. (2000). The comet assay as a rapid test in biomonitoring occupational exposure to DNA-damaging agents and effect of confounding factors. Cancer Epidemiol. Biomarkers Prev. 9:1005-1015. [ Links ]
29. Morgan W.F. (2003). Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 159:567-580. [ Links ]
30. Mothersill C., Seymour C. (2003). Low-dose radiation effects: experimental hematology and the changing paradigm. Exp. Hematol. 31:437-445. [ Links ]
31. Olive P. (1999). DNA damage and repair in individual cells: applications of the comet assay in radiobiology. Int. J. Radiat. Biol. 75:395-405. [ Links ]
32. Paz y Miño C., Dávalos M., Sanchez M., Arévalo M., Leone P. (1995). Should gaps be included in chromosomal aberration analysis? Evidence based on the comet assay. Mutat. Res. 516:57-61. [ Links ]
33. Sawant S.G., Zheng W., Hopkins K., Randers-Pehrson G., Lieberman H., Hall E. (2002). The radiation-induced bystander effect for clonogenic survival. Radiat. Res. 157:361-364. [ Links ]
34. Seoane A., Güerci A., Dulout F. (2007). Genetic instability induced by low doses of x-rays in hamster cells. Int. J. Radiat. Biol. 83:81-87. [ Links ]
35. Singh N., McCoy M., Tice R.R., Schneider E. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 175:184-191. [ Links ]
36. Singh N.P. (2000). Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat. Res 455:111-127. [ Links ]
37. Sudprasert W., Navasumrit P., Ruchirawat M. (2006). Effects of low-dose gamma radiation on DNA damage, chromosomal aberration and expression of repair genes in human blood cells. Int. J. Hyg. Environ. Health 209:503-511. [ Links ]
38. Tice R.R., Strauss G.H. (1995). The single cell gel electrophoresis/comet assay: a potential tool for detecting radiation-induced DNA damage in humans. Stem Cells 13:207-214. [ Links ]
39. Tice R.R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J.C., Sasaki Y. (2000). Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutag. 35:206-221. [ Links ]
40. Todd Allen R., Hunter W., Agrawal D. (1997). Morphological and biochemical characterization and analysis of apoptosis. J. Pharmacol. Toxicol. Methods 37:215-228. [ Links ]
41. Upton A.C. (2002). Carcinogenic effects of low-level ionizing radiation: problems and prospects. In Vivo 16:527-33. [ Links ]
42. van Engeland M., Nieland L., Ramaekers F., Schutte B., Reutelingsperger C. (1998). Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1-9. [ Links ]
43. Venkat S., Apte S.K., Chaubey R., Chauhan P. (2001). Radioadaptive response in human lymphocytes in vitro. J. Environ. Pathol. Toxicol. Oncol. 20:165-175. [ Links ]
44. Vijayalaxmi G., Struss R., Tice R. (1993). An analysis of gama-ray-induced DNA damage in human blood leukocytes, lymphocytes and granulocytes. Mutat. Res. 292:123-128. [ Links ]
45. Wojewodzka M., Kruszewski M., Iwaneñko T., Collins A., Szumiel I. (1998). Application of the comet assay for monitoring DNA damage in workers exposed to chronic low-dose irradiation I. Strand breakage. Mutat. Res 416:21-35. [ Links ]














