Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
BAG. Journal of basic and applied genetics
versión On-line ISSN 1852-6233
BAG, J. basic appl. genet. vol.22 no.2 Ciudad Autónoma de Buenos Aires dic. 2011
ARTÍCULOS ORIGINALES
Diurnal cortisol slope in girls and adolescents with diagnosis of turner syndrome
López, M.C.1, Aguilar, M.J.2, Zabaleta, V.3, Bakker, L.4
1 Facultad de Psicología, Grupo de Investigación Comportamiento Humano, Genética y Ambiente, Centro de Investigación en Procesos Básicos, Metodología y Educación (CIMEPB), Universidad Nacional de Mar del Plata, Argentina
2Grupo de Investigación Comportamiento Humano, Genética y Ambiente, Centro de Investigación en Procesos Básicos, Metodología y Educación (CIMEPB),Universidad Nacional de Mar del Plata, Argentina
3Facultad de Psicología, Centro de Investigación en Procesos Básicos, Metodología y Educación (CIMEPB), Universidad Nacional de Mar del Plata, Argentina
4Universidad Agraria de la Habana (UNAH), San José a carretera Tapaste, Municipio San José, Mayabeque, Cuba.
ABSTRACT
Turner Syndrome is a non-inheritable chromosomal abnormality with highest incidence in female population. Women diagnosed with this condition present atypical brain morphology which includes hippocampal and amygdalar disorders. Both structures are key to the regulation of daily cortisol concentrations. This study analyzes diurnal cortisol slope in girls and adolescents diagnosed with Turner Syndrome. The aim of our study is to establish possible associations with the atypical brain morphology described for this population. A competitive radioimmunoassay was conducted on salivary cortisol samples obtained at 8 a.m. and 6:00 p.m. for two days, at a school and rest day. Descriptive statistical analyses were performed and their average values compared with a similar study in non-Turner girls. An alteration of diurnal cortisol slope was observed in all the participants, all evidencing high evening levels. The present study attempts to associate hypothalamic-pituitary-adrenal axis hyperactivity with anatomical and functional alterations of the hippocampus and amygdala, considering that they constitute the primary neural development defect for women diagnosed with this syndrome. This also entails great vulnerability to environmental stress factors. The goal of this study is to advance our understanding of the factors that favor the anatomical and functional reorganization and recovery of important neural structures related to cognitive and emotional development of women suffering from Turner Syndrome.
Key words: Turner syndrome; Diurnal cortisol slope; Salivary cortisol.
RESUMEN
El Síndrome de Turner es el trastorno cromosómico no heredable de mayor incidencia en la población femenina. Las mujeres con este diagnóstico presentan una morfología cerebral atípica que incluye alteraciones en hipocampo y amígdala. Ambas estructuras son claves en la regulación de las concentraciones diarias de cortisol. Este estudio analiza la pendiente diurna de cortisol en niñas y adolescentes con diagnóstico de Síndrome de Turner, con la finalidad de establecer posibles asociaciones con la morfología cerebral atípica descripta para esta población. Se realizó radioinmunoensayo competitivo en muestras de cortisol salival obtenidas a las 8:00 y 18:00 horas durante dos jornadas, escolar y de descanso. Se aplicaron análisis estadísticos descriptivos y los valores medios obtenidos fueron comparados con un estudio similar realizado con niñas y adolescentes sin diagnóstico de Síndrome de Turner. Se observó alteración de la pendiente diaria de cortisol en el total de las participantes, con altos niveles vespertinos. Este trabajo intenta vincular la hiper-reactividad del eje hipotálamo-hipofiso-adrenal con la alteración anatómica y funcional del hipocampo y la amígdala, considerando que las mismas son un defecto primario del desarrollo neural en las mujeres con diagnóstico de Síndrome de Turner, hecho que implica una mayor vulnerabilidad a estresores ambientales. Se propone profundizar el estudio de los factores que favorecen la recuperación y reorganización anatómica y funcional de estructuras neurales importantes en el desarrollo cognitivo y emocional de las mujeres con este diagnóstico.
Palabras clave: Síndrome de Turner; Pendiente diaria de cortisol; Cortisol salival.
INTRODUCCIÓN
The study of the features that characterize people with genetic disorders opens up new lines of research that further show the convergence of biological and environmental factors in the development of cognitive processes (Plomin et al., 2002). Turner Syndrome (TS) is a genetic disorder that involves either total or partial X-monosomy in females. This non-inheritable chromosomal abnormality occurs in approximately 1 out of 1900 female live births (Murphy et al., 2006). As to the neurocognitive profile, previous studies have revealed that verbal and intellectual abilities remain intact (Doswell et al., 2006; Kesler et al., 2004b). Notwithstanding this, selective deficits of certain domains, such as visual-spatial abilities, including spatial perception processes, visual-motor integration, left/right orientation and non-verbal memory have been reported. Difficulties in working memory and attention tasks requiring control of impulsivity and self-monitoring have also been documented (Zinn et al., 2007; Hart et al., 2006; Mazzocco, 2006; Murphy et al., 2006; Ross et al., 2006; Schmidt et al., 2006; Haberecht et al., 2001).
Research on brain development is therefore key to learn about the above-mentioned neurocognitive deficits. The main findings within this line of research reveal the presence of a cortical organization and atypical brain morphology, particularly in the right brain hemisphere with deficit in the frontotemporal circuits (Tamm et al., 2003). Furthermore, magnetic resonance images have revealed a decrease in the gray substance volume of the right occipital and temporal lobes and left and right frontal lobes (Doswell et al., 2006; Hart et al., 2006; Ross et al., 2006; Danielewicz & Pisula, 2005; Brown et al., 2004; Kesler et al., 2004b). Alterations have also been reported in the cerebellum and pons as well as in subcortical structures, such as the thalamus, amygdala and hippocampus. On the other hand, McCauley and Sybert (2006) and Kesler et al. (2004a) observed an enlarged left amygdala, involving the gray substance and a reduction in the right hippocampus, including the gray and white substance.
The hippocampus, which is located in the medial temporal lobe, is key to cognitive processes and it is involved in the consolidation of episodic and spatial memory. Kesler et al. (2004a) reported an association between structural alterations of the hippocampus and memory deficit in TS which affect primarily short and long-term memory as well as visual-spatial memory. The amygdala, which is located in the ventral hippocampal region, regulates, in turn, emotional events as well as learning and attention processes, assigning an emotional load to memory to the extent that it increases its consolidation and evocation. It has also been demonstrated that the electric stimulation of this structure elicits behavioral characteristics connected with feelings of fear and anxiety (Joseph-Bravo & Gortari, 2007), thus accomplishing a fundamental role in understanding social interactions. On the other hand, Kesler et al. (2004b) related amygdala hyperactivity to high social anxiety in TS, which is expressed as shyness, social isolation, concern for keeping control and flexibility deficit in terms of routine changes (McCauley & Sybert 2006; Russell et al., 2006).
The amygdala and hippocampus are connected through the cingulate cortex and closely related to the prefrontal cortex. In this respect, Maldonado et al. (2008) claimed that the ventromedial prefrontal and dorsolateral cortex play a central role in the development of cognitive functions, such as memory and attention. Furthermore, together with the frontal cortex and the hypothalamus, they are key brain structures for the stress circuit. The hippocampus, whose neurons carry the largest concentration of corticosteroid receptors in mammal brain, is one of the targets of cortisol action. One of its neuroendocrine functions is to regulate the concentration of circulating cortisol through processes of negative feedback, thus inhibiting the hypothalamic-pituitary-adrenal axis (HPA). By means of cortisol secretion, this axis operates on several organs and tissues, including the central nervous system, and particularly the limbic system and the prefrontal regions involved in central cognitive processes for the acquisition of new abilities and knowledge. Moderate cortisol levels permit the use of glucose in the brain, mediating neuron plasticity, which exerts a facilitating effect on memory and favors the learning process. In contrast, high cortisol levels interrupt neuron plasticity mechanisms, obstructing the favorable development of these cognitive processes (Jimerson et al., 2006).
On the other hand, when fear and anxiety are involved, the amygdala secrets a neurotransmitter that activates HPA axis (corticotropin-releasing hormone, CRH), whose effects stimulate and modulate several brain regions involved in emotions and stress. Baseline concentrations of CRH result in increased alertness, thus favoring learning, while when in excess, CRH leads to attention deficits (Joseph-Bravo & Gortari, 2007).
Cortisol circadian rhythm is used as a biological marker to assess the proper functioning of HPA axis. According to this rhythm, cortisol levels begin to increase in the last hour of sleep, reach their peak about thirty minutes after waking up, and quickly decrease during the following thirty-to-sixty minutes, to keep on decreasing steadily during the rest of the day, and reaching their lowest level at the end of diurnal activity phase (López-Mato, 2004). Deregulation of HPA axis leads to a circadian rhythm disruption which manifests itself in changes in the daily decrease of cortisol concentrations with either absence of or decrease in the decline. Furthermore, although Oskis et al. (2009) described a positive correlation between girls´ pubertal stage and cortisol awakening response, the daily decline of cortisol concentration curve remains significantly robust, not being affected by age, sex, developmental stage or body composition (Rosmalen et al., 2005).
In view of the above, the purpose of the present study was to analyze diurnal cortisol slope in girls and adolescents diagnosed with TS. Findings from this research are expected to contribute to determining the bases of a hypothesis that could help explain the relationships between circadian rhythm and the anomalies reported for this population by the literature available on the hippocampus and amygdala.
METHODS
Design
A simple group and a retrospective ex-post facto design were adopted for this study, in agreement with Montero and León´s classification (2007).
Participants
Subjects in this study were girls and adolescents diagnosed with TS (N = 6). Their ages ranged from 7 to 16 years. They were recruited following specific healthcare protocols for the treatment of this syndrome in Mar del Plata city, Republic of Argentina. Girls and/or adolescents were excluded from our study if they had a BMI higher than 35kg/m2, diabetes, chronic liver or renal failure, had been under corticoid treatment in the last year, or had suffered from an acute illness in the last two weeks.
Description of participants
The families surveyed belonged to middle class and the girls and adolescents included in this study attended either public or private schools in Mar del Plata city. All participants had received growth hormone and had started, in most of the cases, their treatment at an early age, which had allowed them to reach height percentiles within the normal range. Only one participant received estrogen therapy to achieve puberty. Taking into account the impact that these hormone therapies have on psychosocial development, it is important to note that both are crucial to optimizing life quality in the studied population.
Table I describes the most relevant biological parameters for each participant.
Table I. Clinical information of girls and teenagers with diagnosis of Turner's Syndrome (N= 6).
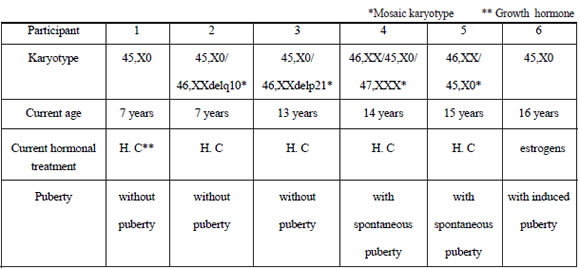
Materials
Salivary-free cortisol determination was carried out. This method offers great advantages over serum or urinary measurements on account of the fact that it is painless and non-invasive and, above all, it reduces the stress derived from blood collection. It thus prevents bias which may arise from the application of this procedure. On the other hand, it has been demonstrated that salivary cortisol levels represent plasma levels properly which remain stable for several weeks, thus permitting to accurately and efficiently quantify biologically active cortisol (Mirasoli et al., 2002).
Salivary cortisol was measured by means of a competitive radioimmunoassay, using the commercial Coat-a-Count kit with serum standards diluted 1:10. Assay analytical sensitivity was of 8.28 nmol/l, whereas intra- and interassay sensitivity was under 6.1% and 7.1%, respectively. Our study was carried out at the Laboratory of Clinical and Endocrine Analyses LACYE (90240) in Mar del Plata city (Republic of Argentina) and accredited by the CONEA. Official permission has been granted to this laboratory to use in vitro radioactive tracers in human beings.
Procedure
Healthcare centers and professionals treating girls and adolescents diagnosed with TS were firstly contacted and through them, families were subsequently contacted. Details on the objectives and main characteristics of this research were given to them. Families were further asked to sign an informed consent form and girls and adolescents were asked to provide their assent, whereby it was explicitly stated that cooperation was voluntary and anonymous. With previous parental consent secured, the clinical records of girls and adolescents participating in our study were collected. Salivary sample collection protocol was further distributed with the following indications: i) not to brush their teeth before collecting saliva sample to avoid bleeding gums, and ii) not to drink water or eat food, or use steroid inhalers. Approximately 1ml of saliva was obtained, collected after direct salivation in a sterile glassware tube without preservatives and stored at 4º C. Two daily measurements were taken, one right after waking up (at 8 a.m.) and the other in the evening (at 6 p.m.). Ethical approval was provided by the Ethical Committees of the participating heath care centers.
To assess the effect of possible environmental demands, and considering that within the multiple contexts in which children are reared, school environment may become a potential stress factor as a result of its daily demands, four salivary samples were obtained from each participant. Two of them were collected during a regular school day and the other two during a rest day, the latter being considered as baseline in accordance with Moya-Albiol et al. (2005).
Daily cortisol slope analysis was carried out by means of descriptive tests and mean difference tests, contrasting morning and evening values collected on both days with those reported by Gröschl et al. (2003).
Ethical considerations
Ethical research principles concerning human beings were followed and data were confidentially kept.
Cortisol levels in the girls and teenagers participating in this study as well as central trend measurements and salivary cortisol concentration variability between morning (8 a.m.) and evening (6 p.m.) are shown in Table II.
Table II. Levels of morning and evening cortisol (nmol/l) of the population approached in both days.
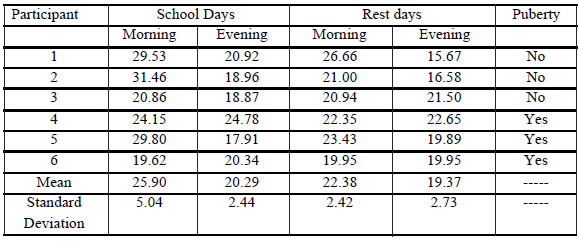
RESULTS
The comparison of the values corresponding to both days via Wilcoxon test showed no significant differences between morning and evening cortisol levels during both days. Based on the absence of variability, both morning and evening values in Table III correspond to an average of the values retrieved during school and rest day. A comparison of the descriptive statistical values of our study population with those reported by Gröschl (2003) on healthy girls and adolescents ranging from 2 to 15 years is shown in Table III.
Table III. Univariate descriptive statistics of girls and teenagers with diagnosis of Turner's Syndrome, comparison with population information

DISCUSSION
Although sample size could be a limiting factor, findings from our research lead us to conclude that, based on normal morning values (Table V), diurnal cortisol decline in girls and adolescents diagnosed with TS evidences a significantly flattened slope with respect to high evening cortisol values . The same was observed when the values collected in our study were compared with cortisol reference ranges recorded in post-menarchal girls´ saliva by Aardal and Holm (1995).
The fact that all participants evidenced daily cortisol slope dysfunction, regardless of the stage of pubertal development (pre-and post- menarchal girls), karyotype (pure line and mosaic) and hormone treatments received at the time of the study (growth hormone and estrogen hormone), lead us also to conclude that this daily dysfunction could be interpreted as a characteristic of syndrome expression. It would therefore be interesting to verify these results by means of a case-control study to ensure that high evening cortisol levels are pathognomonic in ST population.
On the other hand, as no significant differences were recorded between school and rest days, this could not only minimize the impact of environmental factors but also strengthen the hypothesis according to which changes in the daily decline of cortisol concentration curve can be considered a characteristic of TS expression. Therefore, diurnal cortisol slope dysfunction could be analyzed in terms of the assumption that anatomical and functional alterations in the hippocampus and amygdala are the primary defect of neural development in women with TS. Hippocampal hypodevelopment seems to induce a deregulation of HPA axis as a result of a deficit in the negative feedback on CRH release by the hypothalamus. In addition, as amygdalar volume gets larger, this could contribute to axis overexcitement, becoming sensitized to the action of possible stress factors. As a result, diminished hippocampal volumes along with larger amygdalar volume could lead to HPA axis hyper-reactivity with sustained cortisol increase.
Concomitantly, previous research has shown that high chronic cortisol concentrations are linked to neurotoxicity processes which reduce hippocampal volume due to neurosynaptogenesis decrease in the CA-3 zone (Meraz and Bañuelos, 2009). This, in turn, increases hippocampal impairment, thus hindering neuron regeneration and reinforcing non-adaptation processes that end in cognitive and affective disorders whose physiopathological feature is hippocampal atrophy.
According to findings from previous research, atypical neural development of the hippocampus and amygdala could be due to estrogen deficiency in women with TS (Kesler et al., 2004a; Monereo-Megias and Peñalver-Talavera, 2003). Estrogens are responsible for the regulation of diverse neuron mechanisms including synaptogenesis, synaptic plasticity, neuron density, long-term potentiation effects and excitatory postsynaptic potentials. Furthermore, although accounting for volume differences between diminished hippocampus and enlarged amygdala is not an easy task, they could be considered as resulting from dysfunctional processes in cell migration, neuroplasticity and synaptic and dendritic prunings. Such dysfunction could lead to an abnormal distribution and organization of nerve tissues, thus inducing disproportionate cell density increase and decrease in certain brain regions. Furthermore, if the genetic expression of the syndrome involves diminished hippocampal volume and increased amygdalar volume, this atypical brain morphology could be a risk factor as of birth stage for women with TS.
A more comprehensive study of the relationship between atypical brain morphology and diurnal cortisol slope in this population via simultaneous comparative study including magnetic resonance imaging, will contribute not only to comparing and contrasting the hypothesis herein presented but also to accounting for high biological environmental sensitivity observed in women diagnosed with TS on account of the fact that persistent activation of HPA axis is associated with emotional and cognitive biases that affect the development of key learning processes. The study on the relationships between genetic and environmental factors and their concomitant expression in the psychoneuroendocrine system in vulnerable populations, will help develop strategies encouraging both the recovery and anatomical and functional reorganization of fundamental neural structures for the cognitive and emotional development of women with TS. Finally, in view of the fact that some women with ST were found to evidence distinctive features of pubertal development, further studies on the awakening response will contribute to determining intrapopulation differences, such as those identified by Oskis et al. (2008).
REFERENCES
1. Aardal, E., Holm, A.C. (1995). Cortisol in saliva. Reference ranges and relation to cortisol in serum. Eur. J. Clin. Chem. Clin. Biochem. 33:927-932. [ Links ]
2. Brown, W., Kesler, S., Eliez, S., Warsofsky, I., Haberecht, M., Reiss, A. (2004). A volumetric study of parietal lobe subregions in Turner Syndrome. Dev. Med. Child Neurol. 46 (9):607-609. [ Links ]
3. Danielewicz, D., Pisula, E. (2005). Self-esteem evaluation of girls of Turner Syndrome. Ann. Univ. Mariae Curie-Skłodowska 60 (16/72):329-332. [ Links ]
4. Doswell, B.H., Visootsak, J., Brady, A.N., Graham, J.M. (2006). Turner Syndrome: An update and review for the primary pediatrician. Clin. Pediatr. 45(4):301-313. [ Links ]
5. Gröschl, M., Rauh, M., Dörr, H.G. (2003). Circadian rhythm of salivary cortisol, 17 a-hydroxyprogesterone, and progesterone in healthy children. Clin. Chem. 49(10):1688-1691. [ Links ]
6. Haberecht, M., Menon, V., Warsofsky, I., White, C., Dyer Friedman, J., Glover, G., Nelly, K., Reiss, A. (2001). Functional neuroanatomy of visuo-spatial working memory in Turner Síndrome. Hum. Brain Mapp. 14(2):96-107. [ Links ]
7. Hart, S., Davenport, M., Hooper S., Belger A. (2006). Visuospatial executive function in Turner Syndrome: Functional Mri and neurocognitive findings. Brain 129 (5):1125-1136. [ Links ]
8. Jimerson, S.R., Durbrow, E.H., Adam, E., Gunnar, M., Bozoky, I.K. (2006). Associations among academic achievement, attention, and adrenocortical reactivity in caribbean village children. Can. J. School Psychol. 21(1/2):120-138. [ Links ]
9. Joseph-Bravo, P., Gortari, P. (2007). El estrés y sus efectos en el metabolismo y el aprendizje. Biotecnología 14 (3):65-76. [ Links ]
10. Kesler, S., Garrett, A., Bender, B., Yankowitz, J., Zeng, S.M., Reiss, A. (2004a). Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologia 42:1971-1978. [ Links ]
11. Kesler, S., Haberecht, M., Menon, V., Warsofsky, I., Dyer-Friedman, J., Neely, J., Reiss, A. (2004b). Functional neuroanatomy of spatial orientation processing in Turner Síndrome. Cereb. Cortex 14 (2):174-180. [ Links ]
12. López-Mato, A.M. (2004). Psiconeuroinmunoendocrinología II. Buenos Aires: Polemos. [ Links ]
13. Maldonado, E.F., Fernández, F.J., Trianes, M.V., Wesnes, K., Petrini, O., Zangara, A., Enguix, A., Ambrosetti, L. (2008). Cognitive performance and morning levels of salivary cortisol and amylase in children reporting high vs. low daily stress perception. Span. J. Psychol. 11(1):3-15. [ Links ]
14. Mazzocco, M. (2006). The cognitive phenotype of Turner Syndrome: Specific learning disabilities. Int. Cong. Ser. 1298:83-92. [ Links ]
15. McCauley, E., Sybert, V. (2006). Social and behavioral development of girls and women with Turner Syndrome. Int. Cong. Ser 1298:93-99. [ Links ]
16. Meraz, M.T., Bañuelos P.J. (2009). Efecto del estrés crónico sobre la remodelación dendrítica en la región CA3 del hipocampo. e-Gnosis 7(3):1-21. [ Links ]
17. Mirasoli, M., Deo, S.K., Lewis, J.C., Roda, A., Daunert, S. (2002) Bioluminescence inmunoassay for cortisol using recombinant aequorin as a label. Anal. Biochem. 306:204-11. [ Links ]
18. Monereo-Megias, S., Peñalver-Talavera, D. (2003). La mujer adulta con Síndrome de Turner: algunas consideraciones. En: Sociedad Española de Endocrinología Pediátrica (Org.), Síndrome de Turner (pp. 147-165). Barcelona: J& C Ediciones Médicas S.L. [ Links ]
19. Montero, I., León, O. (2007). A guide for naming research studies in Psychology. Int. J. Clin. Health Psychol. 7(3):847-862. [ Links ]
20. Moya-Albiol, L., Serrano, M. A., González-Bono, E., Rodríguez-Alarcón, G. and Salvador, A. (2005). Respuesta psicofisiológica de estrés en una jornada laboral. Psicothema, 17 (2), 205-211. [ Links ]
21. Murphy, M., Mazzocco, M., Gerner, G., Henry, A. (2006). Mathematics learning disability in girls with Turner Syndrome or fragile X Syndrome. Brain Cogn. 61(2):195-210. [ Links ]
22. Oskis, A., Loveday, C., Hucklebridge, F., Thorn, L., Clow, A (2009). Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinol. 34:307-16. [ Links ]
23. Plomin, R., De Fries, J., McClearn, G., Guffin, P. (2002). Genética de la Conducta. Buenos Aires: Ariel Ciencia. [ Links ]
24. Rosmalen, J., Oldehinkel, A., Ormel, J., de Winter, A., Buitelaar, J., Verhulst, F. (2005). Determinants of salivary cortisol levels in 10-12 year old children; a population-based study of individual differences. Psychoneuroendocrinol. 30:483-495. [ Links ]
25. Ross, J., Roeltgen, D., Zinn, A. (2006). Cognition and the sex chromosomes: Studies in Turner Syndrome. Horm. Res. 65:47-56. [ Links ]
26. Russell, H., Wallis, D., Mazzocco, M., Moshang, T., Zackai, E., Zinn, A., Ross, J., Muenke, M. (2006). Increased prevalence of ADHD in Turner Syndrome with no evidence of imprinting effects. J. Pediatr. Psychol. 31(9):945-955. [ Links ]
27. Schmidt, P., Rubinow, D., Bondy, C. (2006). Adult women with Turner Syndrome: A systematic evaluation of current and past psychiatric illness, social functioning, and self-esteem. Int. J. Clin. Health Psychol. 1298:100-107. [ Links ]
28. Tamm, L., Menon, V., Reiss, A.L. (2003). Abnormal prefrontal cortex function during response inhibition in Turner Syndrome: Functional magnetic resonance imaging evidence. Biol. Psychiatry 53(2):107-111. [ Links ]
29. Zinn, A., Roeltgen, D., Stefanatos, G., Ramos, P., Elder, F., Kushner, H., Kowal, K., Ross, J. (2007). A Turner Syndrome neurocognitive phenotype maps to Xp22.3. Behav. & Brain Funct. 3(24):1-14. [ Links ]














