Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
BAG. Journal of basic and applied genetics
versión On-line ISSN 1852-6233
BAG, J. basic appl. genet. vol.23 no.1 Ciudad Autónoma de Buenos Aires ene./jun. 2012
ARTÍCULOS ORIGINALES
Introgression of cultivated sunflower in exotic Helianthus petiolaris populations
Gutiérrez A.*, Cantamutto M., Poverene M.
Centro de Recursos Naturales de la Zona Semiárida (CERZOS), CONICET-UNS y Departamento de Agronomía, Universidad Nacional del Sur, Bahía Blanca, Argentina
*Corresponding Author: aguti@criba.edu.ar
ABSTRACT
Cultivated sunflower Helianthus annuus and the wild exotic H. petiolaris are sympatric species in an extensive region of central Argentina. Both species are sexually compatible, they overlap their flowering time and share pollinators in the region. Although there are important barriers to introgression between them, interspecific hybrids are occasionally found in contact zones. We present evidences of crop introgression in H. petiolaris populations under natural conditions and controlled and semi-controlled crosses in the experimental field using morphological traits. If crop genes persist in subsequent generations after hybridization, it could then be possible to find crop morphological traits and molecular markers in hybrid offsprings. Plants of H. petiolaris, sunflower and plants of intermediate morphology (IMP) were characterized in their natural habitats, and progenies of IMPs were grown in a common garden and compared to progenies of interspecific hybrids obtained by open pollination and backcrosses to H. petiolaris. Variability of IMPs in the wild was comparable to variability under experimental conditions, thus confirming introgression from sunflower in H. petiolaris populations. Molecular markers confirmed introgression. This has implications on the usage of herbicide tolerant sunflower varieties and the likely release of genetic modified varieties in agro-ecological regions invaded by H. petiolaris populations.
Key words: Crop-wild; Intermediate morphology; Interspecific hybridization; RAPD.
RESUMEN
El girasol cultivado Helianthus annuus y la especie silvestre exótica H. petiolaris son simpátricos en una extensa región central de Argentina. Ambas especies son sexualmente compatibles, superponen su floración y comparten polinizadores en las condiciones locales. Aunque hay importantes barreras a la introgresión entre ellas, en las zonas de contacto se encuentran ocasionalmente híbridos interespecíficos. En este estudio presentamos evidencias de la introgresión del cultivo en las poblaciones naturales de H. petiolaris y cruzamientos controlados y semi-controlados producidos en el campo experimental, utilizando caracteres morfológicos. Si los genes del cultivo persisten en las generaciones posteriores a la hibridación, sería posible encontrar rasgos morfológicos y marcadores moleculares del cultivo en la descendencia de los híbridos. Se caracterizaron plantas de H. petiolaris, girasol y plantas de morfología intermedia (IMP) en sus sitios naturales. Se criaron las progenies de las IMPs en un jardín común y se compararon con progenies de híbridos interespecíficos y sus retrocruzas con H. petiolaris producidas bajo condiciones controladas. La variabilidad fenotípica de las IMPs en el hábitat natural fue similar a la observada en la progenie de cruzas interespecíficas criadas bajo condiciones experimentales, lo cual confirma la introgresión del girasol en H. petiolaris. Los marcadores moleculares también confirmaron la introgresión. Este hallazgo tiene implicancias sobre el uso de variedades de girasol tolerantes a herbicida y la posible liberación de variedades genéticamente modificadas en regiones agro-ecológicas invadidas por poblaciones de H. petiolaris.
Palabras clave: Cultivo-silvestre; Morfología intermedia; Hibridación interespecífica; RAPD
INTRODUCTION
Sunflower, Helianthus annuus var. macrocarpus L. (crop, domesticated sunflower) and the wild exotic H. petiolaris Nutt. are sympatric species in an extensive region of central Argentina. Both species overlap their flowering time and share pollinators in the invaded landscape (Poverene et al., 2004). Because reproductive barriers between both species are incomplete (Rieseberg et al., 1999a) interspecific hybrids with intermediate morphological traits are occasionally found in contact zones (Heiser, 1947; Rieseberg et al., 1999b; Ureta et al., 2008). In Argentina plants with intermediate morphology (IMP) have been found for several consecutive years in wild H. petiolaris populations near sunflower fields in districts of Buenos Aires and La Pampa provinces (Poverene et al., 2006). This could be indicative of the natural occurrence of mating events between both species in the region (Poverene et al., 2008).
An increasing number of studies as of 2000 have documented gene flow from crops to wild relatives, thus indicating the potential risk of gene or transgene escape via hybridization (Jenczewski et al., 2003; Hails and Morley, 2005). However, the impact of allele release depends on the likelihood of its persistence in wild populations, which, in turn, depends on the fitness of plants bearing the trait, which is difficult to prove beyond the first hybrid generation (Vacher et al., 2004). Most studies on crop-wild hybridization have documented first generation hybrids but little progress has been made on generations after hybridization (Kirkpatrick and Wilson, 1988; Langevin et al., 1990; Robert et al., 1991; Klinger et al., 1991; Santoni and Bervillé, 1992; Arias and Rieseberg, 1994; Brubaker and Wendel, 1994; Arriola and Ellstrand, 1996). The bare observation of first generation hybrids does not prove gene flow from the crop into wild or weedy populations on account of the fact that interspecific hybrids might be almost completely sterile. Demonstration of crop gene introgression into wild populations is essential since the persistence of either genes or transgenes to confer adaptive advantages in wild populations is an ecological risk (Linder et al., 1998). For example, plants could acquire herbicide tolerance through crop pollen flow, rendering wild or weedy populations more difficult to control in agricultural lands. The herbicide-resistant sunflower varieties and the experimental assays with genetically modified varieties (MAGYP, 2012) that could be released in the future are of concern, sunflower being a species of high-risk category in terms of gene escape probability after crop-wild hybridization according to Ahl Goy and Duesing (1996). On the other hand, as wild populations are more tolerant to adverse conditions than crops, a transgene conferring resistance to a pest or an herbicide may not necessarily represent the same advantage as for the crop. On the contrary, it could have a fitness cost due to pleiotropic effects and the impact of a transgene could result in both beneficial and cost effects. This might represent an adaptive cost in a new environment (Vila-Aiub et al., 2009).
In view of the above, the purpose of the present study was to account for interspecific introgression of sunflower in H. petiolaris invasive populations under natural conditions. Particular attention was paid to whether or not morphological variability in H. petiolaris populations at different sites was a consequence of crop introgression following natural hybridization. Two H. petiolaris populations located in the sunflower cropping area of Argentina were studied focusing on the following queries: 1) do controlled crosses between hybrid plants and wild species in the experimental field induce morphological variability similar to that observed under natural conditions?, and 2) can this comparison help explain hybridization under natural conditions? In an attempt to answer these queries, IMPs, which were suspected to have arisen from interspecific hybridization and were found under natural conditions, and IMPs obtained in the experimental field through controlled and semi-controlled crosses, were studied. Different plant materials were analyzed in three experiments, namely i) plants of sunflower crop, H. petiolaris, and IMPs under natural conditions; ii) progenies of the latter from a common garden; and iii) progenies of interspecific hybrids obtained in a common garden. Plants were characterized taking into account several morphological traits for their comparison among the different experiments. The underlying hypothesis was that if crop genes persist in subsequent generations after hybridization, it seems likely that there will be crop morphological traits or molecular markers in the offspring of hybrids or their backcrosses to the wild species.
MATERIALS AND METHODS
Experiment #1
We studied IMP individuals in two regions of Argentina where H. petiolaris (HP) has naturalized in the sunflower cropping area. Both species overlap in flowering time and the presence of IMPs is frequent. Off-type plants showing atypical records of morphological traits within H. petiolaris populations were considered as IMPs. Large H. petiolaris populations grow along roadsides and among sown fields, in a patchy distribution. A total of 10 IMPs were analyzed in Trenque Lauquen (approximately S35˚49.5', W62˚49.5'), and 10 IMPs in Catriló (approximately S36˚30.1',W63˚44.7'). Data were collected from 49 H. petiolaris plants from both locations and 15 sunflower plants from a crop lot in the former site were used as controls.
Experiment #2
Progenies of another set of 10 IMPs collected from Trenque Lauquen, following the same criteria as in experiment #1, were analyzed in a common garden (IMPp-TL). Seeds were placed in plastic trays on wet paper and kept at 4ºC for one week to break dormancy. They were sown in the greenhouse and they grew with appropriate watering and temperature until seedlings had 4-6 leaves. At this stage, they were transplanted to the experimental field in a completely randomized design, each plot representing the progeny of a single plant. Plants from six HP populations and one sunflower hybrid cultivar (cv. Dekalb 3881) were included in adjacent plots. A total of 91 plants were analyzed.
Experiment #3
Progenies of eight interspecific H. petiolaris - sunflower crop natural hybrids from seven different HP populations from La Pampa and Buenos Aires provinces, already described in Gutierrez et al. (2010) were analyzed. Hybrid offsprings were obtained in the experimental field either by open-pollination (OP) or backcrosses (BC) to the wild parent. Male parents of OP were not identified, being comprised of the pollen community of the experimental field, which consisted in H. petiolaris, cultivated sunflower and products of interspecific crosses. Backcrosses (BC) were obtained from previously bagged H. petiolaris heads, applying pollen from crop-wild hybrids with a cotton brush. The experiment included OP and BC plants derived from natural hybrids and pure H. petiolaris plants from seven locations in La Pampa, Buenos Aires and San Luis provinces (Table 1). Seeds were placed in plastic trays on wet paper and kept at 4ºC for one week to break dormancy. They were grown in the greenhouse as described above and transplanted to the experimental field in a completely randomized design. A sunflower hybrid cultivar (cv. Dekalb 3881) was included in adjacent rows. A total of 44 HP, 49 BC, 83 OP, and 10 sunflower plants were analyzed.
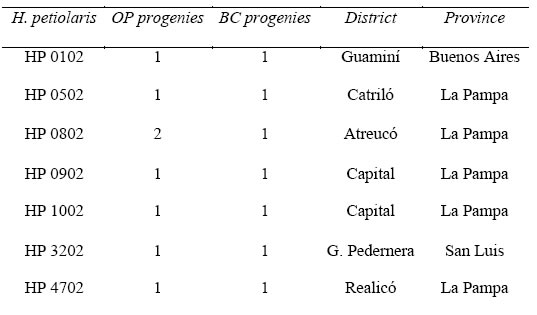
Table 1. Materials studied of H. petiolaris (HP), open-pollinated crop-wild hybrid progenies (OP) and backcrosses to the wild parent (BC). Numbers indicate the sites from where seeds were collected. Two hybrids from 0802 population yielded one OP progeny each.
Morphological characterization and data analysis
The quantitative and qualitative morphological traits analyzed in each experiment are shown in Table 2. In experiment #2 (IMPp in a common garden) four metric traits difficult to measure in natural populations because of wild fruit shattering, namely filled seed number (FS), empty seed number (ES), fertility (F) measured as viable seeds (FS/FS+ES), and seed length (SL), were added. Metric data were subjected to Principal Component Analysis (PCA, bi-plot) from a correlation matrix. Means were compared using the non-parametric Kruskal-Wallis test. A hybrid index for categorical traits was calculated using a scale for each variable (Table 3). The highest and lowest values in the scale correspond to pure parental species and intermediate values correspond to plants showing intermediate morphology (Briggs and Walters, 1997). By means of discriminant analysis of morphological data, IMPs from experiment #1 were classified into one of the groups including the two stabilized species (HA and HP) and the progeny of their interspecific hybrids (BC or OP) characterized in experiment #3. Data analyses were performed with InfoStat (2006).
Table 2. Morphological traits taken into account for plant description following Rieseberg and Morefield (1995). Variable units are in cm except for head, bract, and seed number.

Table 3. Categories assigned to morphological traits for hybrid index analysis.

Molecular characterization
Molecular analysis was carried out in progenies of some crosses in the common garden to evaluate the frequency of crop specific markers in advanced generations of interspecific hybrids. The selected biotypes were descendants of previously confirmed crop-wild hybrids, in which crop had been the pollinating parent (Gutierrez et al., 2010). They included three crop-wild interspecific hybrids (HI0802, HI0902, and HI1002), 21 open-pollinated descendants (seven individuals per interspecific hybrid, OP0802, OP0902, OP1002), 21 backcrosses to the wild parent (seven individual per interspecific hybrid BC0802, BC0902, BC1002) and sunflower inbred lines previously studied by Gutierrez et al. (2010), namely HA89, HA369, HAR2, HAR3, HAR5, and RHA274. Young leaves were lyophilized and DNA was isolated with CTAB method (Hoisington et al., 1994). RAPD markers were amplified using two primers (A2 and B5, Operon Technologies) showing three crop-specific markers found in the crop-wild interspecific hybrids analyzed (Gutierrez et al., 2010). Amplifications were carried out in a total volume of 25μl with 50 ng of purified DNA as template, 30 ng of primer, 1U of Taq DNA polymerase, and a final concentration of 2 mM MgCl2, 10X buffer, and 50 mM of dNTP. Reactions were placed in a PTC-100 MJ Research Thermal Cycler programmed for a 6-min cycle at 94ºC, 40 15-sec cycles at 94ºC, a 45-sec cycle at 40ºC, a 1-min cycle at 72ºC, and a 7-min final extension at 72ºC. Amplification products were separated by electrophoresis in 1.5% TAE agarose gels and detected by staining with ethidium bromide.
RESULTS
Morphological characterization
Experiment #1
PCA (bi-plot) based on 11 metric morphological traits collected in the field explained 87.5% of the variability recorded in the first two axes. It showed two different groups: one consisting of cultivated sunflower plants and the other consisting of H. petiolaris and IMPs. The latter scattered between both parental species (Fig. 1). Most IMPs from Trenque Lauquen (IMPs-TL) were observed to have an intermediate position among stabilized species whereas several of them from Catriló (IMPs-C) were closer to H. petiolaris plants. Variability was observed to be higher in the plants from Trenque Lauquen than in those from Catriló.

Figure 1. Principal component analysis (bi-plot) based on field data of 11 metric morphological traits. Each point represents an individual of H. petiolaris (black circles), domesticated sunflower (white circles), Catrilo IMP (white squares) and Trenque Lauquen IMP (gray squares) in field conditions. For bi-plot references see Table 2. N=84 plants.
Metric morphological traits analyzed by Kruskal-Wallis test showed differences between H. petiolaris and IMPs-C for five out of 11 of the traits analyzed. Highly significant differences were found between IMPs-TL and H. petiolaris for every trait, except bract number (Table 4). The hybrid index showed that IMPs-C and IMPs-TL were intermediate between H. petiolaris and sunflower although some of them showed no differences with respect to pure wild plants (Fig. 2).
Table 4. Morphological traits (means ± SD) of H. petiolaris (HP), domesticated sunflower (HA), intermediate plants of Catrilo (IMP-C) and Trenque Lauquen (IMP-TL) in field conditions. Variable units are cm, except for ligule and bract number. Means followed by different letters indicate significant differences in Kruskal-Wallis test (P<0.05) N=84 plants.


Figure 2. Hybrid index based on 11 categorical morphology traits of H. petiolaris (HP), cultivated sunflower (HA), intermediate morphology plants of Catrilo (IMP-C) and Trenque Lauquen (IMP-TL) in field conditions. N=84.
Experiment #2
Many seeds from IMP individuals were inviable or seedlings died before the reproductive stage. The PCA (bi-plot) of morphological traits of 103 viable plants (including HP and sunflower plants) explained 73% of variation in the first two axes and showed a clear separation of the pure species along PC1. Progenies of IMP-TL (IMPp-TL) were more related to H. petiolaris plants (Fig. 3). Highly significant differences in Kruskal Wallis test were found between IMPp and the pure species, H. petiolaris and sunflower (Table 4). The hybrid index showed that IMPp were intermediate between H. petiolaris and sunflower although some of them showed no differences with respect to pure wild plants (Fig. 4).

Figure 3. Principal component analysis (bi-plot) based on 16 metric morphological traits. Each point represents a descendant of a single HP (black circles), cultivated sunflower (open circles) and IMPp (gray diamonds) grown in common garden conditions. For bi-plot references see Table 2. N = 103 plants.

Figure 4. Hybrid index based on 11 categorical morphology traits of H. petiolaris (HP), cultivated sunflower (HA) and progenies of intermediate morphology plants (IMPp). N=103.
The PCA (bi-plot) based on 12 metric morphological traits measured in a common garden explained 75% of variability in the first two axes (Fig. 5). The OP plants were found scattered between both parental species although some plants were closer to the wild species. Many, though not all, BC plants were similar to the female parent, H. petiolaris. A higher variability was observed among OP compared with BC.

Figure 5. Principal component analysis (bi-plot) based on 12 metric morphological traits of Helianthus annuus (HA), H. petiolaris (HP), their advanced generations from open-pollinated hybrids (OP) and backcrosses (BC) obtained in the experimental field. Each point represents an individual of HP (black circles), BC (open triangles), OP (gray triangles) and sunflower, HA (open circles) biotypes. For bi-plot references see Table 2. N=186 plants.
Kruskal-Wallis test showed that eight metric morphological traits differentiated BC and OP individuals from H. petiolaris and sunflower. Highly significant differences were found between OP and BC progeny of the first generation hybrids and cultivated sunflower except for bract (phyllary) number. No differences in ligule length and width were found between H. petiolaris and BC (Table 5). Furthermore, the hybrid index showed that BC and OP plants were intermediate between H. petiolaris and sunflower although some of them showed no differences with respect to pure wild plants (Fig. 6).
Table 5. Morphological traits (means ± SD) of H. petiolaris (HP), sunflower (HA), and progeny of their interspecific hybrids produced by controlled backcrosses to the wild parent (BC) or under open-pollination in the experimental field (OP). Variable units are in cm except for ligule and bract number. Means followed by different letters indicate significant differences in Kruskal-Wallis test (P<0.05).


Figure 6. Hybrid index based on 11 categorical morphology traits of Helianthus petiolaris (HP), domestic sunflower, H. annuus (HA), backcrosses (BC), and open- pollinated progeny (OP) in the experimental field. N=186.
Descriptive discriminant analysis of materials from experiments #1 and #3 confirmed, in general, PCA results in each experiment (Fig. 7). Prediction ellipses showed the probability of finding a plant within a given group with 95% confidence level. Cross-classification table showed group membership prediction of 20 IMP plants (Table 6). Five and seven IMPs from Catriló and Trenque Lauquen were classified as interspecific hybrid descendants (OP or BC). Some HP plants from Trenque Lauquen site (not identified in Table 6) were found and classified as BC and OP.

Figure 7. Discriminant análisis based on metric morphological traits of Helianthus annuus (HA, open circles), H. petiolaris (HP, black circles), their advanced generations from open-pollinated hybrids (OP, gray triangles), and backcrosses (BC, open triangles). Each point represents an individual. Prediction ellipses correspond to 95% confidence level.
Table 6. Cross-classification of Helianthus petiolaris (HP), domestic sunflower, H. annuus (HA), backcrosses (BC), open-pollinated plants (OP), and 20 Catrilo (C) and Trenque Lauquen (TL) IMP considered as unknown individuals.

Molecular characterization
Two out of three crop-specific markers (A2720, A2820 and B5680) were found in the progeny of interspecific hybrids in experiment #3. Band B5680 was not found in any of the plants analyzed. Four out of 21 (19%) 0802 OP plants derived from one interspecific hybrid plant showed specific markers of cultivated sunflower. Two of them were found to have band A2720 and two were observed to have band A2820. Two out of 21 (9.5%) backcrosses of the same population (0802 BC) showed the presence of crop-specific marker A2720.
DISCUSSION
This is a comparative analysis through which experimental data are compared with data collected under natural conditions, this being the procedure recommended to to assess gene flow (Ellstrand, 1995; Kareiva et al., 1996). In the three experiments conducted, PCA, mean differences and hybrid indexes showed morphological differences between the parental species sunflower H. annuus and the wild exotic H. petiolaris. Polygenic (metric) traits tended to be intermediate in hybrids as well as in many cases of F1 and advanced-generation interspecific hybrids in Helianthus (Rieseberg and Ellstrand, 1993). In the present study, advanced generations from open-pollinated hybrids between these two species were intermediate and similar to H. petiolaris when hybrids backcrossed to wild plants. The intermediate morphology of the IMPs collected in Catriló and Trenque Lauquen showed similar variability, thus indicating that crop introgression is likely to occur naturally in agro-ecosystems where both species are sympatric. Data from progenies of controlled crosses in the common garden confirmed this although variability was not as high as in progenies of natural crosses. This was attributed to a higher variability under natural conditions, which led to carry out experiment #2 in order to standardize experimental conditions and highlight genotypic differences. Segregation within plots also confirmed the hybrid origin of intermediate plants. On the other hand, experiment #2 showed a straight relationship between H. petiolaris populations and fertility and between cultivated sunflower plants and filled seeds. Both are characteristics of stabilized species, in contrast to the association between intermediate morphology and empty seeds as a consequence of low gamete viability in interspecific hybrids (Rieseberg et al., 1998). This confirms that while barriers to hybridization between H. annuus and H. petiolaris are not complete (Rieseberg et al., 1999a), there is a chromosomal incompatibility causing a low production of fertile seeds in hybrid plants. Chromosomal differences consist of at least 11 rearrangements affecting 10 out of 17 pairs of chromosomes in both Helianthus species (Burke et al., 2004). This could greatly affect introgression because recombination rates are reduced in non-collinear portions of the genome (Rieseberg et al., 1995). However, fecundity parameters recover fast in advanced-generation hybrids in agreement with previous field experiments (Gutierrez et al., 2011).
Discriminant analysis classified 60% of the IMPs as progenies of interspecific hybrids between H. petiolaris (as female parent) and sunflower (as male parent) and no IMP seemed to relate with the sunflower group. We therefore hypothesized that introgression was different in each site. In Trenque Lauquen hybridization and introgression occur repetitively due to extensive sunflower cultivation every year, determining a stable frequency of IMP individuals. In contrast, in Catriló hybridization occurs occasionally and advanced-generation hybrids resemble more HP plants. In addition, cross-classification table predicting membership of 20 unknown plants also showed some HP plants collected in Trenque Lauquen classified as BC or OP. This reveals introgression traits in such HP plants although these traits were not observed during data collection. The BC and OP plants inaccurately classified in Table 5 show that these groups are phenotypically heterogeneous due to the recombination and segregation of interspecific traits.
Plants of hybrid origin are difficult to identify in wild populations after the second generation following hybridization. This is clearly shown by hybrid index and PCAs, through which it could be observed that BC and IMP-C, and OP and IMP-TL individuals (though in lower proportion) overlap with the wild species. Furthermore, progeny test carried out on 10 IMPs-TL in the common garden showed segregation, the latter being characteristic of a hybrid progeny. In both cases, IMPs were interspecific hybrids of different generations. Furthermore, Trenque Lauquen IMP individuals could be interpreted as corresponding to advanced back-cross generations receiving pollen from both H. petiolaris and sunflower. Catriló IMP individuals could be interpreted as corresponding mostly to backcrosses to H. petiolaris. Trenque Lauquen is a district with the largest area of sunflower crop production in Buenos Aires province, having, in fact, reached a 50% higher of the total production in the province in the last five years (MAGPyA, 2012). Although IMPs have been found in every season for the last 10 years, H. petiolaris plants are similar to those of the centre of origin (Poverene et al., 2008) and no "intermediate biotypes" have been established as populations in those sites. Hybrid biotype stabilization is likely to require ecological divergence either to co-exist with parent species or to occupy a different ecological niche, as occurred in North America where three homoploid annual species were found to have arisen from hybridization between H. annuus and H. petiolaris (Rieseberg, 1997; Rieseberg et al., 2003, Karrenberg et al., 2007). These species exhibit several transgressive traits (significantly exceeding parental trait values) while results from the present study showed no transgressive traits in advanced-generation hybrids, thus not accounting for ecological divergence. In contrast, IMPs were found to evidence gene flow preventing reproductive isolation from occurring. In addition, no discrete or different ecological niches associated to the sampled sites were found, both of which are located in the Pampean ecological region exhibiting similar micro-habitat parameters (Cantamutto et al., 2008).
In the cases in which intermediate morphological characters are not evident, it is necessary to use additional techniques such as molecular markers. In H. petiolaris populations growing adjacent to cultivated sunflower plots in North America, plants were observed to show no morphological evidence of hybridization but revealed crop gene introgression when surveyed with molecular markers (Rieseberg et al., 1999b). Also, crop alleles were found to persist in wild H. annuus populations for five generations at moderate frequencies (Whitton et al., 1997). In line with this, and based on the specific markers of sunflower inbred lines found in progenies of interspecific hybrids, results from the present study demonstrate that sunflower crop alleles may also persist in H. petiolaris populations after hybridization.
The morphological traits analyzed in the present study as well as the evidence of gene flow after the first generation of hybridization confirm introgression from sunflower into H. petiolaris (Heiser, 1947; Rogers et al., 1982; Rieseberg et al., 1999b). Gene spread to related wild populations cannot be prevented from occurring because crop pollen can reach long distances by insect and other pollinator transportation (Arias and Rieseberg, 1994; Ureta et al., 2008). Furthermore, although sunflower crop introgression reduces hybrid plant fitness, the fast recovery of fecundity parameters in the generations following hybridization allows predicting that traits conferring an ecological advantage, such as herbicide tolerance or disease resistance, are likely to diffuse into wild populations (Gutierrez et al., 2011). Herbicide tolerance introgression in wild annual Helianthus was confirmed (Massinga et al., 2005; Presotto et al., 2012). This suggests that sunflower gene or eventually transgene escape to wild relative populations in Argentina could not be discarded.
ACKNOWLEDGMENTS
Authors thank the National Research Council of Argentina (CONICET) for a fellowship granted to GA. This research was supported by grants ANPCYT PICT 2286 and PAE-PICT-2007-00020.
BIBLIOGRAPHY
1. Ahl Goy P., Duesing J.H. (1996) Assessing the environmental impact of gene transfer to wild relatives. Biotechnol. 11:39-40. [ Links ]
2. Arias D.M., Rieseberg L.H. (1994) Gene flow between cultivated and wild sunflowers. Theor. Appl. Genet. 89:655-660. [ Links ]
3. Arriola P.E., Ellstrand N.C. (1996) Crop-to-weed gene flow in the genus Sorghum (Poaceae): spontaneous interspecific hybridization between johnsongrass, Sorghum halepense and crop sorghum, S. bicolor. Am. J. Bot. 83:1153-1160. [ Links ]
4. Briggs D., Walter S.M. (1997) Plant Variation and Evolution. Cambridge University Press, UK. [ Links ]
5. Brubaker C.L., Wendel J.F. (1994) Reevaluating the origin of domesticated cotton (Gossypium hirsutum; Malvaceae) using nuclear restriction fragment length polymorphisms (RFLPs) Am. J. Bot. 81:1309-1326. [ Links ]
6. Burke J.M., Lai Z., Salmaso M., Nakazato T., Tang S., Heesacker A., Knapp S.J., Rieseberg L.H. (2004) Comparative mapping and rapid karyotypic evolution in the genus Helianthus. Genetics 167:449-457. [ Links ]
7. Cantamutto M., Poverene M., Peinemann N. (2008) Multi-scale analysis of two annual Helianthus species naturalization in Argentina. Agric. Ecosys. Envir. 123:69-74. [ Links ]
8. Ellstrand N. (1995) Evaluación de los riesgos del flujo transgénico de los cultivos a las especies silvestres. Memorias del Foro CIMMYT, pp. 86-89. [ Links ]
9. Gutierrez A., Cantamutto M., Poverene M. (2011) Persistence of sunflower crop traits and fitness in Helianthus petiolaris populations. Plant Biol. 13:821-830. [ Links ]
10. Gutierrez A., Carrera A., Basualdo J., Rodriguez R., Cantamutto M., Poverene M. (2010) Gene flow between cultivated sunflower and Helianthus petiolaris (Asteraceae). Euphytica 172: 67-76. [ Links ]
11. Hails R.S., Morley K. (2005) Genes invading new populations: a risk assessment perspective. Trends Ecol. Evol. 20:245-252. [ Links ]
12. Heiser C.B. (1947) Hybridizations between the sunflower species Helianthus annuus and H. petiolaris. Evolution 1:249-262. [ Links ]
13. Hoisington D., Khairallah M., Gonzalez de Leon D. (1994) Laboratory Protocols, CIMMYT, 2nd ed., CIMMYT, Mexico. [ Links ]
14. Infostat (2006) InfoStat version 2006. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. [ Links ]
15. Jenczewski E., Ronfort J., Chèvre A.M. (2003) Crop-to-wild gene flow, introgression and possible fitness effects of transgenes. Environ. Biosafety Res. 2:9-24. [ Links ]
16. Kareiva P., Parker I.M., Pascual M. (1996) Can we use experiments and models in predicting the invasiveness of genetically engineered organisms? Ecology 77:1670-1675. [ Links ]
17. Karrenberg S., Lexer C., Rieseberg L.H. (2007) Reconstructing the history of selection during homoploid hybrid speciation. Am. Nat. 169:725-737. [ Links ]
18. Kirkpatrick K.J., Wilson H.D. (1988) Interspecific gene flow in Cucurbita: C. texana vs. C. pepo. Am. J. Bot. 75:519-527. [ Links ]
19. Klinger T., Elam D.R., Ellstrand N.C. (1991) Radish as a model system for the study of engineered gene escape rates via crop-weed mating. Conserv. Biol. 5:531-535. [ Links ]
20. Langevin S.A., Clay K., Grace J.B. (1990) The incidence and effects of hybridization between cultivated rice and its related weed red rice (Oryza sativa L.). Evolution 44:1000-1008. [ Links ]
21. Linder C.R., Taha I., Seiler G., Snow A., Rieseberg L.H. (1998) Long-term introgression of crop genes into wild sunflower populations. Theor. Appl. Genet. 96:339-347. [ Links ]
22. MAGYP (2012) http://www.minagri.gob.ar/site/agricultura/biotecnologia/50-evaluaciones/historica/index.php (accessed March 9, 2012). [ Links ]
23. Massinga R.A., Al Khatib K., Stamand P., Miller J.F. (2005) Relative fitness of imazamox resistant common sunflower and prairie sunflower. Weed Sci. 53:160-174. [ Links ]
24. Poverene M., Carrera A., Ureta S., Cantamutto M. (2004) Wild Helianthus species and wild sunflower hybridization in Argentina. Helia 27:133-142. [ Links ]
25. Poverene M., Cantamutto M.A., Carrera A., Ureta S., Alvarez D., Alonso Roldán V., Presotto A., Gutiérrez A., Luis S., Hernández A. (2006) Wild sunflowers research in Argentina, Helia 29: 65-76. [ Links ]
26. Poverene M., Cantamutto M., Seiler G.J. (2008) Ecological characterization of wild Helianthus annuus and H. petiolaris germplasm in Argentina. Plant Genet. Resour. 7:42-49. [ Links ]
27. Presotto A., Ureta M.S., Cantamutto M., Poverene M. (2012) Effects of gene flow from IMI resistant sunflower crop to wild Helianthus annuus populations. Agric. Ecosys. Envir. 146:153-161. [ Links ]
28. Rieseberg L.H. (1997) Hybrid origins of plant species. Annu. Rev. Ecol. Syst. 28:359-389. [ Links ]
29. Rieseberg L.H., Ellstrand N.C. (1993) What can morphological and molecular markers tell us about plant hybridization? Crit. Rev. Plant Sci. 12:213-241. [ Links ]
30. Rieseberg, L.H., Baird S., Desrochers A. (1998) Patterns of mating in wild sunflower hybrid zones. Evolution 52:713-726. [ Links ]
31. Rieseberg L.H., Linder C.R., Seiler G. (1995) Chromosomal and genic barriers to introgression in Helianthus. Genetics 141:1163-1171. [ Links ]
32. Rieseberg L.H., Morefield J.D. (1995) Character expression, phylogenetic reconstruction, and the detection of reticulate evolution. In: Hoch P.C., Stephenson A.G. (Eds.). Experimental and Molecular Approaches to Plant Biosystematics. Monographs in Systematic Botany from the Missouri Botanical Garden 53:333-354. [ Links ]
33. Rieseberg L.H., Raymond O., Rosenthal D.M., Lai Z., Livingstone K., Nakazato T., Durphy J.L., Schwarzbach A.E., Donovan L.A., Lexer C. (2003) Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301:1211-1216. [ Links ]
34. Rieseberg L.H., Whitton J., Gardner K. (1999a) Hybrid zones and the genetic architecture of a barrier to gene flow between two sunflower species. Genetics 152:713-727. [ Links ]
35. Rieseberg L.H., Kim M.J., Seiler G. (1999b) Introgression between the cultivated sunflower and a sympatric relative, Helianthus petiolaris (Asteraceae). Int. J. Plant Sci. 160:102-108. [ Links ]
36. Robert T., Lespinasse R., Pernes J., Sarr S. (1991) Gametophytic competition as influencing gene flow between wild and cultivated forms of pearl millet (Pennisetum typhoides). Genome 34:195-200. [ Links ]
37. Rogers C.E., Thompson T.E., Seiler G.J. (1982) Sunflower Species of the United States. National Sunflower Association, Fargo, ND, pp. 75. [ Links ]
38. Santoni S., Bervillé A. (1992) Evidence for gene exchanges between sugar beet (Beta vulgaris L.) and wild beets: consequences for transgenic sugar beets. Plant Mol. Biol. 20:578-580. [ Links ]
39. Ureta S., Cantamutto M., Carrera A., Delucchi C., Poverene M. (2008) Natural hybrids between cultivated and wild sunflowers (Helianthus spp.) in Argentina. Genet. Resour. Crop Evol. 55:1267-1277. [ Links ]
40. Vacher C., Weis A.E., Hermann D., Kossler T., Young C., Hochberg M.E. (2004) Impact of ecological factors on the initial invasion of Bt transgenes into wild populations of birdseed rape (Brassica rapa). Theor. Appl. Genet. 109:806-814. [ Links ]
41. Vila-Aiub M.M., Neve P., Powles S.B. (2009). Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol. 184:751-767. [ Links ]
42. Whitton J., Wolf D.E., Arias D.M., Snow A.A., Rieseberg L.H. (1997) The persistence of cultivar alleles in wild populations of sunflowers five generations after hybridization. Theor. Appl. Genet. 95:33-40. [ Links ]














