Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
BAG. Journal of basic and applied genetics
versão On-line ISSN 1852-6233
BAG, J. basic appl. genet. vol.24 no.2 Ciudad Autónoma de Buenos Aires dez. 2013
ARTÍCULOS ORIGINALES
Respiratory function in healthy first-degree relatives of asmathic adolescents
Pereira C.1, Veiga N.2, Barros H.3
1 CI&DETS. Polytechnic Institute of Viseu, Portugal.
2 Department of Health Sciences - Portuguese Catholic University, Viseu, Portugal.
3 Medical School of the University of Porto, Portugal.
carlospereiraviseu@gmail.com
ABSTRACT
Asthma is a complex disease associated with biological and physiological phenotypes. The objective of this study was to compare the respiratory function in healthy first-degree relatives of asthmatic and non-asthmatic adolescents. We used a cross-sectional approach to assess 101 family cases (presence of one or more adolescents classified as asthmatic) and 275 stable families (families without adolescents classified as asthmatic). We obtained a final sample of 822 relatives. The respiratory function was evaluated by the forced expiratory volume in 1 second (FEV), and the forced vital capacity (FVC), using the Microlab 3300® Spirometer. A self-administered questionnaire was used to record upper and lower airway symptoms, allergic symptoms and medical history. To compare continuous variables, the Mann-Whitney and Kruskal-Wallis tests were applied when variables did not follow a Gaussian distribution and the variances were not homogeneous. A linear regression adjusted for age, gender and height was also applied to compare the lung function between asthmatic and non-asthmatic relatives. Parents of asthmatic adolescents had significantly lower values of the lung function than both parents together and mothers of non-asthmatics (84.6 vs. 97.6, p<0.01, for FEV and 84.3 vs. 97.9, p<0.01, for FVC, and 97.3 vs. 109.7, p <0.01, for FEV and 89.5 vs. 105.5, p<0.01, for FVC, respectively). Also, siblings of asthmatic adolescents had lower FEV (98.6 vs. 109.4, p<0.01) and FVC values (85.9 vs. 102.7, p<0.01). The healthy first-degree relatives of asthmatic adolescents have worse respiratory function than those of non-asthmatic adolescents. Asymptomatic relatives of asthmatics can have physiological characteristics that may reveal the phenotypic pattern of the disease.
Key words: Asthma; Respiratory function; Genetic factors; Environmental factors; Phenotype.
INTRODUCTION
The familial aggregation of asthma has been documented in several studies (Bedolla-Barajas et al., 2013; Lima et al., 2012; Webster et al., 2011; Wechsler et al., 2002; Chen et al., 2001; Jenkins et al., 1997). However, most of the attention has been focused on the diagnosis and phenotypic characterization of the disease (Thomsen et al., 2010). Despite the attention received, deciphering the relative importance of genetic factors in the origin of asthma has been proved to be a very complex task. Segregation studies have shown that there are genes that can explain the development and outcome of the disease (Koppelman et al., 2002; Chen et al., 2001; Los et al., 1999). Studies made with twins and analyzing genealogical trees have allowed the observation of the clear input of genetic factors in the disease (Fagnani et al. 2008; Rasanen et al., 2000). In a research involving 6,996 pairs of twins, the concordance for asthma was 19% in monozygotic pairs and 4.8% in dizygotic ones (Nolan, 1994).
The wide phenotypic variability found among asthma patients suggests an important etiological heterogeneity, strengthened by environmental influences (Abdou et al., 2013; Ober et al., 2011; Thomsen et al., 2010).
A specific gene located on chromosome 11 seems to be responsible for the transmission of atopy, which occurs through the maternal line (Holberg et al., 1998; Nolan, 1994; Cookson, 1992). However, the development of asthma is more dependent on environmental factors, which act in individuals with genetic susceptibility (Colilla et al., 2003; Howard et al., 2003; Palmer et al., 2000). This is supported by the fact that people from regions with low prevalence of asthma, such as isolated rural environments, when set in urban areas, end up expressing an increased frequency of the disease, possibly due to factors such as exposure to certain new fungi, infectious agents, air pollution and changes in the eating patterns (Gonzalez-Barcala et al., 2012; Ober et al., 2011; Delfino et al., 2003; Custovic et al., 1996; Nicolai et al., 1997). Studies carried out after the reunification of Germany have shown that the prevalence of asthma and other respiratory problems tends to reach values close to those of western cities (Heinrich et al., 1998).
Although the presence of atopy as a familial antecedent is more common among asthmatics, it has been suggested that the genetic determinants of atopy and asthma are different (Kurzius-Spencer, et al. 2012).
Children whose family members have a history of asthma are more likely to have the disease, particularly if both parents are asthmatic (Burke et al., 2003; Kreeger, 2003; Fernández-Espinar et al., 2001; Barros et al., 1999; Arshad et al., 1993). Beyond the demonstration of the familial aggregation of asthma, familial aggregation of the disease indicators and of the measured and predicted values of respiratory function parameters for patients and their descendants has also been observed (Bedolla-Barajas et al., 2013; Holberg et al., 1998). This aggregation can also be explained by genetic and environmental factors, as well as by lifestyle habits, including smoking (Lima, et al., 2012; Lewitter et al., 1984). However, the analysis of this aggregation implies greater complexity due to the simultaneous influence of these factors, whose relative contribution is difficult to quantify.
The objective of this study was to compare the respiratory function in healthy fist-degree relatives of asthmatic and non-asthmatic adolescents.
MATERIALS AND METHODS
Using the school population of the district of Viseu (Portugal), a randomly selected sample of 101 families with adolescents with asthma criteria (presence of episodes of dyspnea, with wheezing in the absence of respiratory infection) was selected. For each family with adolescents with asthma, two or more families with adolescents without asthma criteria and living in the same street or locality as the asthmatic families were selected. We assessed a total of 101 family cases (presence of one or more asthmatic adolescents) and 275 stable families (families without asthmatic adolescents), obtaining a final sample of 822 individuals. The criteria for inclusion of adolescents in the analysis were: to be aged between 12 and 18 years old, not having smoked for 6 months straight and absence of history of asthma or bronchitis.
A phone call was made to explain the objectives of the study and to book the day and time of the interviews. The adolescents and first-degree relatives living under the same roof (father, mother and siblings over twelve years of age) were interviewed by home visit. When any of these individuals with criteria were not at home at the time of this visit, we agreed a new date for a second home visit. Their not being home in the second visit determined their exclusion of participation in the study. A self-administered questionnaire was filled out by the adolescents and their relatives to collect information regarding upper and lower airway symptoms, allergic symptoms and medical history and then the forced expiratory volume in 1 second (FEV) and the forced vital capacity (FVC) were evaluated with the Microlab 3300® Spirometer. FEV was calculated using the formula (100x FEV observed / expected FEV) and FVC by the formula (100x FVC observed / predicted FVC). The respiratory function tests were performed in a sitting position, repeated at least three times and no more than five times, considering the most correct and highest value. Before each assessment, data regarding sex, age, height and ethnicity were introduced into the spirometer. The spirometer was calibrated daily.
All the participants were weighed barefoot, without coats and with the same digital scale. Height was measured using a tape measure. Asthma and bronchitis were assessed through the question "Has your doctor ever told that you that you had asthma or bronchitis?". Smoking habits were assessed through questions concerning the status of "smoker", "non-smoker" and "ex-smoker." Every participant who smoked at least one cigarette a day and had not stopped smoking in the last six months was considered a smoker.
Data were processed and analyzed using the Epi-Info 6.04® and the Statistical Program for the Social Studies (SPSS 11.5®). Continuous variables were described by the mean value and the standard deviation. To compare continuous variables, we decided to apply the Mann-Whitney and Kruskal-Wallis tests when the variables did not present a Gaussian distribution and the variances were not homogeneous. A linear regression adjusted for age, gender and height was also applied to compare the lung function between asthmatic and non-asthmatic relatives. To analyze the variation of a quantity depending on another, we used the Pearson correlation coefficient.
RESULTS
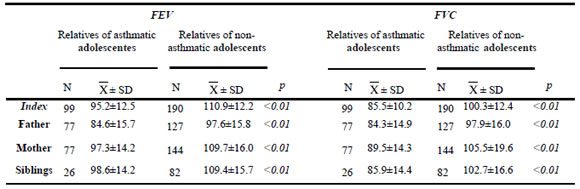
The asthmatic adolescents showed lower mean values of FEV than non-asthmatic ones (95.2 vs. 110.9 p<0.01). The same was observed for FVC values (85.5 vs. 100.3, p<0.01). Also, the parents of asthmatic adolescents had significantly lower values of lung function than both parents together and mothers of non-asthmatics (84.6 vs. 97.6, p<0.01, for FEV and 84.3 vs. 97.9, p<0.01, for FVC, and 97.3 vs. 109.7, p <0.01, for FEV and 89.5 vs. 105.5, p<0.01, for FVC, respectively). Also, siblings of asthmatic adolescents had lower FEV (98.6 vs. 109.4, p<0.01) and FVC (85.9 vs. 102.7, p<0.01) (Table 1).
Table 1. Forced vital capacity (FVC) and forced expiratory volume (FEV) in relatives of asthmatic and non-asthmatic adolescents.
In families with asthmatic adolescents, the FVC values of adolescents were significantly correlated with those of their siblings (r = 0.3) and those of their mothers (r = 0.4). In families without cases of asthma, the FVC values of adolescents were significantly correlated with those of their siblings (r = 0.4), mothers (r = 0.5) and fathers (r = 0.5) and the FEV values were significantly correlated with those of their siblings (r = 0.3), mothers (r = 0.4) and parents together (r = 0.5). In both the families with and without asthmatic cases, there was no correlation between the FEV or FVC values of both parents and those of the mothers.
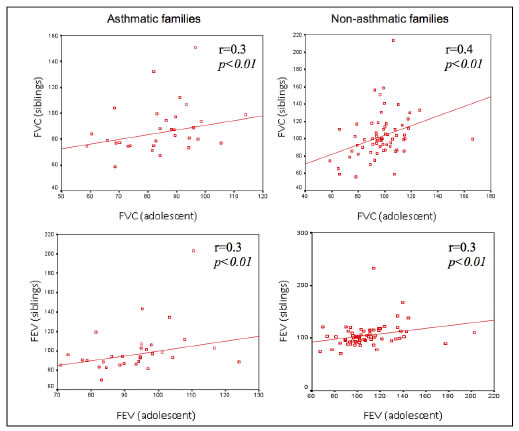
Figures 1 to 4. FVC and FEV of adolescents and siblings in asthmatic and non-asthmatic families.
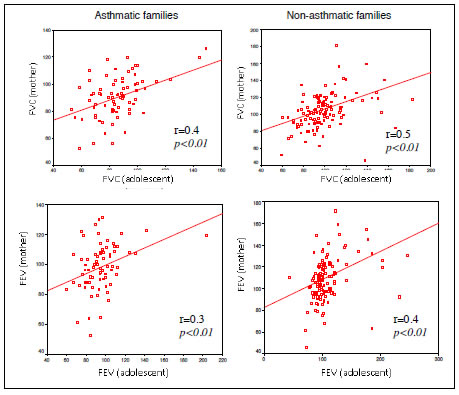
Figures 5 to 7. FVC and FEV of adolescents and mothers in asthmatic and non-asthmatic families.

Figures 8 to 11. FVC and FEV of adolescents and parents in asthmatic and non-asthmatic families.
DISCUSSION
The results of the present study showed lower respiratory parameters (FEV and FVC) among adolescents with history of asthma as well as in their first-degree relatives (parents and siblings) regardless of their age, sex and height. On the other hand, the FEV and FVC values of adolescents were positively correlated with those of the different members of the family, in both the families with and without asthmatic cases, except those of adolescents and parents in asthmatic families.
Considering that asthma is a disease with an important genetic component, exacerbated in a complex manner by environmental factors, the magnitude of the relative influence of each factor is difficult to measure (Ober et al., 2011; Burke et al., 2003; Arshad et al., 1993). Since the aggregation of the disease and its indicators has been verified, one is tempted to infer its familial transmission. However, the familial aggregation of this disease does not necessarily imply genetic transmission, because it may result from environmental exposure shared by the various members of the family. The magnitude of the risk associated with "exposure" depends on the methodology used in the research, which raises additional difficulties in generalizing the findings. Nevertheless, several studies have shown that children and adolescents with asthmatic first-degree relatives have a higher risk of getting the disease than those who have no family history of asthma (Bedolla-Barajas et al., 2013; Gu et al., 2012; Lima et al., 2012; Burke et al., 2003; Litonjua et al., 1998; Jenkins et al., 1997; Burrows et al., 1995).
The results of the present study support the conclusions of other studies that have shown the existence of familial aggregation of lung function (Koppelman et al., 2002; Palmer et al., 2001; Chen et al., 1999; Xu et al., 1999; Givelber et al., 1998; Chen et al., 1997; Chen et al., 1996). Considering that the comparisons made in the present study were restricted to the family members with no history of asthma or bronchitis, the aggregation found, even under the medical threshold (i.e. without respiratory disease symptoms), may result from the fact that these members share constitutional characteristics. Therefore, beyond the expression of asthma with obvious physical symptoms, there may be a pattern of deficits among asthmatic relatives. This aggregation can be explained by vertical transmission, since the values of the parameters studied were correlated among family members. This removes the consistency of the hypothesis that the aggregation may result from environmental exposure.
Some studies have reported an increased risk of developing atopic diseases when transmission occurs through the maternal line (Burrows et al., 1995; Dol et al., 1992). However, population-based studies have found no significant differences in the relative risk of transmission through the mother's or father's family line (Burrows et al., 1995; Dol et al., 1992). In the present study, the values of respiratory function of asthmatic adolescents were correlated only with the mother's values, suggesting that the standard functional respiratory deficit is more influenced by the maternal line.
Over recent years, several chromosomal regions have been identified and genetic variants in genes have been associated with the outcome of asthma (De Wan et al., 2012; Koppelman et al., 2002; Heinzmann et al., 2001). However, although a high proportion of this disease could be explained by genetic factors, its development is modulated by environmental factors, whose effects are expressed individually (Thomsen et al., 2010; Skadhauge et al., 1999). Moreover, the increased frequency of asthma recorded in the past decades cannot be explained by the genetic changes that occur too slowly. Rather, it is likely that genetic factors, together with the environment, which has suffered dramatic changes in the last decades, have made a great number of people susceptible to the disease.
The present study does not allow excluding environmental factors as possible causes of the lower respiratory function parameters found in the relatives of asthmatic adolescents (Abdou et al., 2013; Ober et al., 2011). Thus, we may conclude that the familial aggregation found in lung function may result from genetic factors, environmental factors or both. In addition, this aggregation can exist even without translation into obvious symptoms of asthma. If we consider that this deficient pattern can affect in a lesser or greater degree the ability to perform multiple activities, apparently healthy relatives may express the consequences of this disease, even in an almost imperceptible way.
BIBLIOGRAPHY
1. Abdou CM, Dominguez TP, Myers HF. Maternal familism predicts birthweight and asthma symptoms three years later. Soc Sci Med. 2013 Jan;76(1):28-38. [ Links ]
2. Arshad S, Stevens M, Hide D. The effect of genetic and environmental factors on the prevalence of allergic disorders at age of two years. Clin Exp Allergy 1993; 23:504-11. [ Links ]
3. Barros H, Pereira C, Mateus P. Asma em crianças dos 6 aos 9 anos. Um estudo populacional em duas cidades portuguesas (Porto e Viseu). Rev Port Imunoalergol 1999; 7:9-18. [ Links ]
4. Bedolla-Barajas M, Morales-Romero J, Robles-Figueroa M, Fregoso-Fregoso M. Asthma in late adolescents of Western Mexico: prevalence and associated factors. Arch Bronconeumol. 2013 Feb;49(2):47-53. [ Links ]
5. Burrows B, Martinez F, Cline M, Lebowitz M. The relationship between parental and children's serum IgE and asthma. Am J Respir Crit Care Med 1995; 152:1497-500. [ Links ]
6. Burke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. Am J Prev Med 2003; 24:160-9. [ Links ]
7. Chen Y, Schnell A, Rennie D, Elston R, Lockinger L, Dosman J. Segregation analyses of asthma and respiratory allergy: the Humboldt family study. Am J Med Genet 2001; 104:23-30. [ Links ]
8. Chen Y, Dosman J, Rennie D, Lockinger L. Major genetic effects on airway-parenchymal dysanapsis of the lung: the Humboldt family study. Genet Epidemiol 1999; 16:95-110. [ Links ]
9. Chen Y, Rennie D, Lockinger L, Dosman J. Major genetic effect on forced vital capacity: the Humboldt Family Study. Genet Epidemiol 1997; 14:63-76. [ Links ]
10. Chen Y, Horne S, Rennie D, Dosman J. Segregation analysis of two lung function indices in a random sample of young families: the Humboldt Family Study. Genet Epidemiol 1996; 13:35-47. [ Links ]
11. Colilla S, Nicolae D, Pluzhnikov A, Blumenthal M, Beaty T, Bleecker E et al. Evidence for gene‑environment interactions in a linkage study of asthma and smoking exposure. Collaborative Study for the Genetics of Asthma. J Allergy Clin Immunol 2003; 111:840‑6. [ Links ]
12. Cookson W, Young R, Sandford A, Moffatt M, Shirakawa T, Sharp P et al. Maternal inheritance of atopic IgE responsiveness on chromosome 11q. Lancet 1992; 340:381-4. [ Links ]
13. Custovic A, Taggart S, Francis H. Exposure to house dust mite allergens and the clinical activity of asthma. J Allergy Clin 1996; 98:64-72. [ Links ]
14. De Wan A, Egan K, Hellenbrand K, Sorrentino K, Pizzoferrato N, Walsh K, Bracken B. Whole-exome sequencing of a pedigree segregating asthma. BMC Medical Genetics 2012; 13:95. [ Links ]
15. Delfino R, Gong H, Linn W, Pellizzari E, Hu Y. Asthma symptoms in Hispanic children and daily ambient exposures to toxic and criteria air pollutants. Environ Health Perspect. 2003; 111:647-56. [ Links ]
16. Dol S, Wjst M, von Mutius E, Reitmer P, Stiepel E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch Dis Child 1992; 67:1018-22. [ Links ]
17. Fagnani C, Annesi-Maesano I, Brescianini S, D'Ippolito C, Medda E, Nisticò L, Patriarca V, Rotondi D, Toccaceli V, Stazi MA. Heritability and shared genetic effects of asthma and hay fever: an Italian study of young twins. Twin Res Hum Genet. 2008 Apr;11(2):121-31. [ Links ]
18. Fernández-Espinar J, Rubio J, Pomar C, Martinez C, Cortez V, Pérez-Yarza. Factores de riesgo de asma, alergia e hiperreactividad bronquial en niños de 6 a 8 años. An Esp Pediatr 2001; 55:205-12. [ Links ]
19. Gonzalez-Barcala FJ, Pertega S, Garnelo L, Castro TP, Sampedro M, Lastres JS, San Jose Gonzalez MA, Bamonde L, Valdes L, Carreira JM, Silvarrey AL. Truck traffic related air pollution associated with asthma symptoms in young boys: a cross-sectional study. Public Health. 2012 Mar;127(3):275-81. [ Links ]
20. Givelber R, Couropmitree N, Gottlieb D, Evans J, Levy D, et al. Segregation analysis of pulmonary function among families in the Framingham Study. Am J Respir Crit Care Med 1998; 157:1445-51. [ Links ]
21. Heinrich J, Richter K, Magnussen H, Wichmann H. Is the prevalence of atopic diseases in East and West Germany already converging? Eur J Epidemiol 1998; 14:239-45. [ Links ]
22. Heinzmann A, Deichmann K. Genes for atopy and asthma. Curr Opin Allergy Clin Immunol 2001; 1:387-92. [ Links ]
23. Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Differences in familial segregation of FEV1 between asthmatic and nonasthmatic families. Role of a maternal component. Am J Respir Crit Care Med. 1998 Jul;158(1):162-9. [ Links ]
24. Howard T, Meyers D, Bleecker E. Mapping susceptibility genes for allergic diseases. Chest 2003; 123:363S-8S. [ Links ]
25. Jenkins M, Hopper J, Giles G. Regressive logistic modelling of familial aggregation for asthma in 7394 population-based nuclear families. Genet Epidemiol 1997; 14:317-32. [ Links ]
26. Koppelman GH, Stine OC, Xu J, Howard TD, Zheng SL, Kauffman HF, Bleecker ER, Meyers DA, Postma DS. Genome-wide search for atopy susceptibility genes in Dutch families with asthma. J Allergy Clin Immunol. 2002 Mar;109(3):498-506. [ Links ]
27. Kreeger K. Asthma, Genetics, and the Environment. The-Scientist 2003; 17:30-4. [ Links ]
28. Kurzius-Spencer M, Guerra S, Sherrill DL, Halonen M, Elston RC, Martinez FD. Familial aggregation of allergen-specific sensitization and asthma. Pediatr Allergy Immunol. 2012 Feb;23(1):21-7. [ Links ]
29. Lewitter F, Tager I, McGue M, Tishler P, Speizer F. Genetic and environmental determinants of level of pulmonary function. Am J Epidemiol 1984; 120:518-30. [ Links ]
30. Lima W, Lima E, Costa M, Santos A, Silva A, Costa E. Asthma and associated factors in students 13 and 14 years of age in São Luís, Maranhão State, Brazil. Cad. Saúde Pública 2012; 28(6):1046-56. [ Links ]
31. Litonjua A, Carey V, Burge H, Weiss S, Gold D. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med 1998; 158:176-81. [ Links ]
32. Los H, Koppelman G, Postma D. The importance of genetic influences in asthma. Eur Respir J 1999; 14: 1210-1227. [ Links ]
33. Nicolai T, von Mutius E. Pollution and the development of allergy: the East and West Germany story. Arch Toxicol Suppl 1997; 19:201-6. [ Links ]
34. Nolan T. Asthma. In: Pless I. The epidemiology of childhood disorders. New York: Oxford University Press, 1994: 415-38. [ Links ]
35. Ober C, Yao TC. The Genetics of Asthma and Allergic Disease: A 21st Century Perspective. Immunol Rev. 2011 July; 242(1): 10-30. [ Links ]
36. Palmer L, Knuiman M, Divitini M, Burton P, James A, Bartholomew H. Familial aggregation and heritability of adult lung function: results from the Busselton Health Study. Eur Respir J 2001; 17:696-702. [ Links ]
37. Palmer L, Burton P, James A, Musk A, Cookson W. Familial aggregation and heritability of asthma-associated quantitative traits in a population-based sample of nuclear families. Eur J Hum Genet. 2000 Nov;8(11):853-60. [ Links ]
38. Rasanen M, Kaprio J, Laitinen T, Winter T, Koskenvuo M, Laitinen L. Perinatal risk factors for asthma in Finnish adolescent twins. Thorax 2000; 55:25-31. [ Links ]
39. Ruiz R, Kemeny D, Price J. Higher risk of infantile atopic dermatitis from maternal atopy than from paternal atopy. Clin Exp Allergy 1992; 22:762-6. [ Links ]
40. Skadhauge L, Christensen K, Kyvik K, Sigsgaard T. Genetic and environmental influence on asthma: a population-based study of 11,688 Danish twin pairs. Eur Respir J 1999; 13:8-14. [ Links ]
41. Tager I, Rosner B, Tishler P, Speizer F, Kass E. Household aggregation of pulmonary function and chronic bronchitis. Am Rev Respir Dis 1976; 114(3):485-92. [ Links ]
42. Thomsen SF, Van der Sluis S, Kyvik K, Skytthe A, Backer V. Estimates of asthma heritability in a large twin sample. Clin Exp Allergy. 2010 Jul;40(7):1054-61. [ Links ]
43. Wechsler M, Israel E. The genetics of asthma. Semin Respir Crit Care Med. 2002 Aug;23(4):331-8. [ Links ]
44. Xu X, Yang J, Chen C, Wang B, Jin Y, Faang Z. Familial Aggregation of pulmonary function in a rural Chinese community. Am J Resp Crit Care Med 1999; 160:1928-33. [ Links ]














