Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
BAG. Journal of basic and applied genetics
versión On-line ISSN 1852-6233
BAG, J. basic appl. genet. vol.24 no.2 Ciudad Autónoma de Buenos Aires dic. 2013
ARTÍCULOS ORIGINALES
Embryogenic Callus induction on the scutellum and regeneration of plants as basis for genetic transformation of spring wheath (Triticum Aestivum L.) cultivars from Argentina
Souza Canada E. D., Beck E.
Department of Plant Physiology, University of Bayreuth, D 95440 Bayreuth, Germany++49-921-553038; fax number: ++49-921-552642
daniel.souza-canada@uni-bayreuth.de
ABSTRACT
An effective tissue culture is an indispensable basis for the production of transgenic plants. In this study, we investigated the induction of embryogenic callus production from the scutellum of spring wheat and subsequent regeneration of plants. Twenty-two Argentine spring wheat varieties from three breeders were screened to select promising genotypes for the optimization of the procedure. Experimental variables were the developmental stage of the immature embryos and the concentration of the growth regulator 2,4-dichlorophenoxyacetic acid (2,4-D). Two developmental stages, termed H and W, respectively (according to He et al., 1986), were selected, and their performance in culture media containing high and low concentrations of 2,4-D was examined. Parameters for the screening were: callus induction, callus proliferation on the scutellum, precocious germination of the immature embryo (as negative trait), regeneration and shoot formation and total efficiency of the in vitro culture. The best results were achieved with a combination of developmental stage H and a low concentration of 2,4-D. Scutellar callus induction and callus proliferation on the scutellum surface were positively correlated. However, scutellar callus induction, regeneration and number of shoots were independent of each other. Considerable genotype differences were found in the suitability of the varieties investigated for spring wheat propagation via in vitro culture. The cultivar Klein Brujo exhibited the highest overall culture efficiency (84.4 %) related to the number of immature embryos used and the number of plants regenerated from one embryo (9.5).
Key words: In vitro culture; Immature embryos; Embryogenic scutellar callus; Callus proliferation.
RESUMEN
La base indispensable para la producción de plantas transgénicas es un eficaz cultivo de tejidos. En este estudio se investigó la inducción de callo embriogénico y su posterior regeneración en trigo. Se analizaron veintidós variedades argentinas de trigo de primavera de tres criadores, para seleccionar los genotipos adecuados para la optimización del procedimiento. Las variables experimentales fueron la etapa de desarrollo de embriones inmaduros y la concentración de ácido 2,4-diclorofenoxiacético (2,4-D), un regulador del crecimiento. Se seleccionaron dos etapas del desarrollo denominadas H y W, respectivamente (de acuerdo a He et al., 1986) y se examinaron sus comportamientos en los medios de cultivo con concentraciones altas y bajas de 2,4-D. Los parámetros del análisis fueron: inducción de callo, proliferación de callo escutelar, germinación precoz del embrión (rasgo negativo), regeneración y formación de plántulas y eficiencia del cultivo in vitro. Los mejores resultados se obtuvieron con una combinación de la etapa de desarrollo H y una concentración baja de 2,4-D. Hubo una correlación positiva entre la inducción de callo y su proliferación escutelar. Sin embargo, la inducción de callo, la regeneración y el número de plántulas fueron independientes entre sí. Las variedades de trigo investigadas mostraron considerables diferencias genotípicas en su capacidad de propagación en cultivo in vitro. La variedad Klein Brujo exhibió la eficiencia más alta de cultivo (84,4%), en relación con el número de embriones utilizados y de plantas regeneradas (9,5) a partir de un embrión.
Palabras clave: Cultivo in vitro; Embriones inmaduros; Callo embriogénico escutelar; Proliferación de callo.
INTRODUCTION
Wheat is one of the world's major food crops. Unfortunately, stable transformation efficiency is rather erratic and not very high. For successful biotechnological work with wheat, methodological improvements, such as an efficient system with a high potential of regeneration, are required. Although in vitro techniques have been partly established for wheat, many of the factors that affect the development of embryogenic callus and subsequently regulate plant regeneration are not yet well understood. As compared to model genotypes like Florida and Bobwhite (Rasco-Gaunt et al., 2001), high-yield cultivars of wheat perform poorly in both processes (León et al., 2006). As starting material for tissue culture, wheat offers only a few sources of explants that are suitable for regeneration in tissue culture (Sparks and Jones, 2009), e.g. immature zygotic embryos and immature inflorescences. The scutellum surface of immature zygotic embryos is the target most commonly used for wheat genetic transformation with particle bombardment or gene transfer by Agrobacterium (Sparks and Jones, 2009). Calli proliferating on the scutellum surface are especially useful for biolistic gene transfer experiments (Viertel et al., 1998). Callus formation capacity and plant regeneration depend not only on the type and age of the explants (Özgen et al., 1998), but also on the genotype (Carman et al., 1987; Fennell et al., 1996; Özgen et al., 1998; Dağüstü, 2008), the culture medium (Carman et al., 1987; Fennell et al., 1996; Barro et al., 1999; Rasco-Gaunt et al., 2001), the plant growth regulators (PGRs) and carbon resource concentrations (Mathias, 1990; Rasco-Gaunt et al., 2001; Almouslem et al., 2005) and the culture conditions (He et al., 1988). The developmental stage of immature zygotic embryos plays an important role in the induction of embryogenic callus (Sears and Deckard, 1982; Maddock et al., 1983; He et al., 1988; Vasil, 1987; Özgen et al., 1998; Rasco-Gaunt et al., 2001). Very young or very old immature embryos usually do not form calli or do so only with low efficiency. Particularly with increasing age, immature embryos tend to germinate precociously (He et al., 1988), which is problematic for the subsequent cultivation. Development of a standardized protocol is influenced by the genotype, the age of the immature embryos and the supply of PGRs to the medium. Addition of the artificial auxin 2,4-dichlorophenoxyacetic acid (2,4-D) is indispensable for the induction of scutellar callus (Scott et al., 1990). Concentrations between 1 (Sears and Deckard, 1982; Dağüstü, 2008) and 2 mg/l 2,4-D (He et al., 1988; Redway et al., 1990; Özgen et al., 1998; Varshney and Altpeter, 2001) have been used for callus induction.
The objective of this work was to improve the vegetative propagation of wheat plants from immature embryos as a basis for genetic transformation. To this end, two steps were investigated in detail: the induction and propagation of scutellar calli and the regeneration of wheat plants from those calli. For callus induction and propagation, in addition to different genotypes, two developmental stages of immature zygotic embryos, and two concentrations of 2,4-D were used. The combination of both variables resulted in four different culture conditions. For a reliable statistical analysis, 22 commercial wheat genotypes from three different breeders were used. The varieties investigated are grown in Argentina, which is one of the main wheat exporting countries (FAO 2012).
MATERIALS AND METHODS
Seeds of 22 Argentine spring wheat cultivars were kindly supplied by three breeders from Buenos Aires province: José Buck S.A. (José Buck), Criadores Klein S.A. (Criadores Klein) and Instituto Nacional de Tecnología Agropecuaria (INTA) (Table 1). Donor plants were grown from these seeds in the greenhouse at 20°C and additional illumination was provided during the winter by mercury discharge lamps (180 μmol m-2s-1) to maintain a 16-h photoperiod. The substrate was 70 % compost and 30 % clay.
Table 1. Plant breeders and cultivars of the 22 Argentine spring wheat (Triticum aestivum L.) genotypes.

The developmental stage of immature zygotic embryos was determined from morphological features of the grains according to the Zadoks scale (Zadoks et al., 1974). Grains were surface-sterilized in 25 % (v/v) commercial bleach [10.5 % (v/v) NaOCl; 0.3 % (w/v) Na2CO3; 10 % (w/v) NaCl; 0.5 % (w/v) NaOH] for 15 min, followed by four washes with sterile distilled water. Immature embryos were aseptically excised under a stereomicroscope in a laminar flow hood and transferred to sterilized callus induction medium. Two developmental stages of immature zygotic embryos (H and W) were identified by two traits: scutellum and axis of the embryo (Table 2). Developmental stage H corresponded to stage II, whereas stage W corresponded to stage IV, as characterized by He et al. (1986) (Fig. 1).
Table 2. Experimental treatments. The combinations of the developmental stages of immature zygotic embryos with the two 2,4-D concentrations assayed were identified with the letters A to D.


Figure 1. Five different developmental stages of immature zygotic embryos of wheat. Letters a and b represent stage W and letters c-e represent stage H, according to He et al. (1986).
ML3 basal medium (Viertel and Hess, 1996), consisting of L3 medium (Jähne et al., 1991) supplemented with 3 % (w/v) maltose and two concentrations of 2,4-D (1 or 2 mg/l, respectively), was used for induction and maintenance of the calli (callus induction medium). The medium was solidified with 0.2 % Gelrite. The combination of the two developmental stages of immature embryos and two concentrations of 2,4-D resulted in four culture conditions (A, B, C and D) (Table 2). Twenty-five immature zygotic embryos of each variety and treatment were cultured in petri-dishes (90 x 15 mm) with the scutellum up and the embryo axis kept in contact with the solidified callus induction medium (30 ml). This procedure was repeated five times. After three weeks of incubation at 25°C in total darkness, embryogenic scutellar callus formation, callus proliferation on the scutellum and precocious germination were evaluated in all embryos, under a stereomicroscope.
For regeneration of plants, embryogenic calli were transferred to petri dishes containing modified MSB medium (Viertel and Hess, 1996), consisting of MS salts and B5 vitamins (Gamborg et al., 1968), supplemented with 0.5 mg/l 6-benzylaminopurine (BA) and 0.05 mg/l 1-naphthalene acetic acid (NAA) (Ahuja et al., 1982) and solidified with 0.2 % Gelrite.
Cultivation was at 25°C under 16/8h light/dark periods (ca. 54 μmol m-2s-1. Plant regeneration capacity and number of regenerated plants were evaluated after 3-4 weeks under a stereomicroscope. To stimulate root growth, plants were transferred to the root-promoting 190-2 medium, as described by Viertel and Hess (1996) without plant growth regulators, supplemented with 3 % (w/v) sucrose, 1 % (w/v) activated charcoal and 0.2 % Gelrite instead of agar.
Plants with well-developed root systems were transferred into autoclaved soil (see above). After acclimation in a culture room, cultivation was continued in the greenhouse (Viertel and Hess, 1996).
Data were analyzed by a chi-square test of independence (contingency table analysis), determination of variance analysis, standard deviation and correlation. If necessary, the post hoc test was carried out between the samples from the four culture conditions (A vs. B; C vs. D; A vs. C and B vs. D). Subsequently, the significance calculated was compared with the manually corrected value (Bonferroni-Correction according to Holm). The statistical software package used was SPSS 13.0 (SPSS 2004).
RESULTS
Induction of scutellar callus
A total of 10091 immature wheat embryos were used as starting material. Explants were able to produce both embryogenic and non-embryogenic calli. Embryogenic calli were nodular and solid, with a white to pale yellow surface, whereas non-embryogenic calli were soft, watery, and translucent (Fig. 2). After 3 weeks of culture on induction medium, all the spring wheat cultivars used, except Prointa Elite, produced embryogenic scutellar calli. Induction of scutellar embryogenic calli was significantly dependent on the culture conditions (Chi-square-test X2 = 1017.5; p<0.001), as revealed by the rates of callus formation. The capacity of embryogenic callus formation varied widely among genotypes, ranging from 20.5 % to 85.7 % under culture condition A, from 0.0 % (five genotypes) to 46.8 % under culture condition B, from 10.1 % to 86.4 % under culture condition C, and from 0.0 % (two genotypes) to 89.3 % under culture condition D (Table 3). Under culture condition A, production of embryogenic calli was higher than 75 % with five genotypes (Klein Brujo, Klein Pegaso, Klein Dragón, Prointa Puntual, Prointa Federal), under culture condition C, with two of the genotypes (Klein Dragón, Prointa Imperial), and under condition D with one genotype (Prointa Federal). Under culture condition B, none of the genotypes reached 50% (Table 3).

Figure 2. Scutellar callus three weeks after induction of immature embryos. Callus showing embryogenic
(e) and nonembryogenic (ne) structures. Estimated percentage of callus proliferation on the scutellum surface: a) 20%; b) 45%; c) 70% and d) 100%.
Table 3. Effect of the combination of the two developmental stages of immature zygotic embryos with the two 2,4-D concentrations (culture conditions A, B, C and D) on the frequency of scutellar callus induction, callus proliferation on the scutellum and precocious germination from twenty two Argentine wheat genotypes.

1 Number of immature embryos forming embryogenic callus / number of immature embryos X 100
2 Number of immature embryos with germinating zygotic embryo / number of immature embryos X 100
3 a-h Means followed by the same letter are not significantly different at 1 % level.
4 a-f Means followed by the same letter are not significantly different at 5 % level
5 a-h Means followed by the same letter are not significantly different at 0.1 % level
Other factors influencing the formation of embryogenic calli from immature embryos were the stage of development and the concentration of 2,4-D. Immature donor embryos of developmental stage H and 1 mg 2,4-D/l medium (culture condition A) produced the best results, whereas embryos of the same stage and 2 mg 2,4-D (culture condition C) produced the second best results, being the difference between the two conditions statistically significant (Table 3). In contrast, increasing the concentration of 2,4-D from 1 to 2 mg/l medium had consistently positive effects when immature donor embryos of stage W were used (Table 3).
Capacity of callus proliferation of the cells of the scutellum surface
Callus proliferation of the cells of the scutellum surface was estimated and given in percent of the total scutellum surface (Fig. 2). The capacity of scutellum surface cells to form calli varied with the culture conditions and the genotype: from 30.0 % to 84.4 % under culture condition A, from 0.0 % to 63.3 % in culture condition B, from 25.0 % to 71.2 % in culture condition C, and from 25.0 % to 67.0 % in culture condition D (Table 3). The differences between the individual conditions were highly significant (Chi-square-test X2 = 16.5; p<0.01). However, callus proliferation capacity varied less with the culture conditions than callus formation capacity as such (Table 3). The developmental stage of the immature embryos, however, was more crucial, being stage H significantly better than stage W (Table 3). On the other hand, the concentration of 2,4-D was less important for callus proliferation. Like the capacity of scutellar embryogenic callus formation, culture condition A produced the highest callus proliferation (two genotypes above 75%, Klein Brujo, Klein Dragón, and 11 above 50%), whereas culture condition C produced the second highest callus proliferation (11 genotypes above 50%, but none above 75%). A significant correlation between scutellar embryogenic callus formation and callus proliferation on the scutellum surface was observed under culture conditions A (r = 0.56; p = 0.006) and C (r = 0.47; p = 0.030).
Precocious germination of immature embryos
Precocious germination of the immature zygotic donor embryos varied among genotypes, ranging from 0.0 % to 32.1 % in culture condition A, from 0.0 % to 78.4 % in culture condition B, from 0.0 % to 31.4 % in culture condition C, and from 0.0 % to 97.7 % in culture condition D (Table 3). Even the otherwise not responding immature embryos of Prointa Elite showed precocious germination. Most failures of precocious germination were observed under the conditions C (11 out of 22 genotypes) and D (5 out of 22 genotypes) (Table 3). Both the culture conditions (Chi-square-test X2 = 985.7; p<0.001) and the genotype influenced the extent of precocious germination (Table 3). Developmental stage H and 2 mg 2,4-D/l medium (culture condition C) was optimal for the inhibition of precocious germination. Differences with the other conditions were highly significant (Table 3). The frequency of precocious germination was highest in culture condition B.
Plant regeneration
The potential of scutellar embryogenic calli to regenerate whole plants after transfer to modified MSB medium (Fig. 3a and b) was investigated. Plant regeneration from scutellar embryogenic calli ranged from 46.4 % to 100 % under culture condition A, from 0.0 % to 100 % under culture condition B, from 30.0 % to 95.2 % in culture condition C, and from 0.0 % to 100 % in culture condition D (Table 4). Remarkably, under conditions A and C, all genotypes (but not all calli) produced plants. The composition of the callus induction medium and the developmental stage of the immature donor embryos influenced plant regeneration (Chi-square-test X2 = 139.8; p<0.001). As expected, the genotype was also important. Culture condition A, i.e. immature embryos of stage H in a medium with 1 mg 2,4-D/l, was the best combination for the subsequent regeneration of plants (14 out of 21 = 66.6 %), which was triggered by transfer of the calli to regeneration medium. Developmental stage W in the same induction medium (culture condition B, 3 out of 21 = 14.3 %), as well as stage H cultivated in induction medium with 2 mg 2,4-D/l (culture condition C, 4 out of 21 = 19.0 %) were significantly less efficient (Table 4). For culture condition A, 20 genotypes showed regeneration efficiency over 50%, three of which (Klein Brujo, Inta Huenpan, and Prointa B. Remodon) reached 100%. For culture condition C, 19 genotypes showed regeneration efficiency over 50%, but none reached 100 % (Table 4). We found no relationship between scutellar embryogenic callus induction and plant regeneration capacity of the callus. We observed that genotypes exhibiting relatively poor callus induction frequency did not necessarily produce fewer regenerable calli (Table 3 and 4).

Figure 3. In vitro regeneration of wheat: (a) Regeneration on MSB-medium, (b) Continuation of the regeneration in small glass (c) Root formation in 190-2 medium. Greenhouse phase: (d) Mature plant derived from embryogenic scutellar callus and (e) seed-derived control plant of the cultivar Klein Brujo.
Table 4. Frequency of plant regeneration capacity, culture efficiency and number of plants regenerated per embryo (precultured under condition A, B, C and D) cultured on MSB-medium.
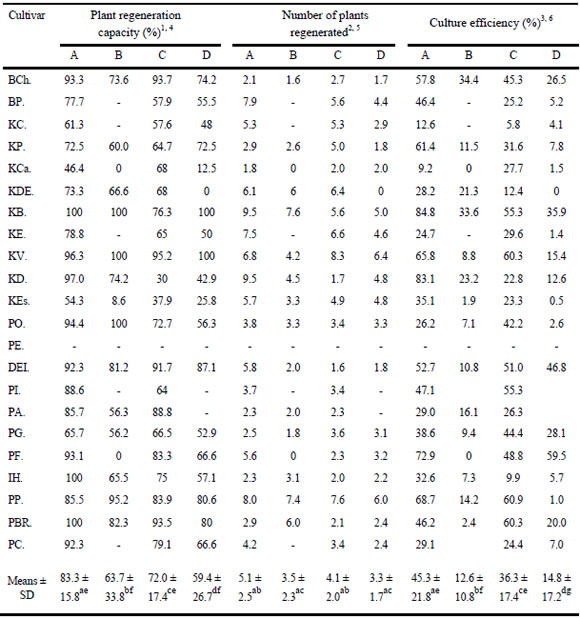
1 Number of regenerable calli / number of calli induced X 100.
2 Number of regenerants per embryo cultured gave as mean numbers
3 Number of regenerable calli / number of embryos cultured X 100 (regenerable callus = nodular callus with green spots).
4 a-f Means followed by the same letter are not significantly different at 0.1 % level
5 a-c Means followed by the same letter are not significantly different at 5 % level
6 a-g Means followed by the same letter are not significantly different at 1 % level
Number of plants arising from scutellar embryogenic callus
Because usually more than one embryogenic callus was formed on the surface of the scutellum, differing numbers of plants were expected from one immature embryo (Fig. 3a). Effects of the genotype as well as of the culture conditions during scutellar callus induction were investigated. Culture condition A (1.8 - 9.5, average 5.1 plants per donor embryo) again proved better than the other three conditions, which gave rather similar results (B: 1.6 - 7.6, average 3.5; C: 1.6 - 7.6, average 4.1; D: 1.7 - 6.4, average 3.3) (Table 4). However, the differences were not statistically significant (Kruskal-Wallis-test: X² = 57.4 p = 7.3). The number of plants regenerated and the plant regeneration capacity of scutellar embryogenic calli were not significantly correlated.
Because of the high number of plants, plant samples were taken randomly from each cultivar and culture condition. Each plant regenerated was transferred into soil in greenhouse conditions (ex vitro) after in vitro root promotion (Fig. 3c). In that phase, neither the cultivar nor the culture condition influenced the morphogenetic development (Fig. 3d). All plants matured normally and set fertile seeds after approximately three months.
Overall efficiency of the four culture conditions
Given the wide range of embryogenic callus formation displayed by the 22 cultivars investigated and their differing capabilities for plant regeneration, the significant differences in overall efficiencies in plant regeneration were not unexpected (Chi-square-test X2 = 31.9; p<0.001). Comparison of the influence of culture conditions A - D showed that younger immature donor embryos (stage H) were better than more advanced ones (stage W), irrespective of the concentration of 2,4-D in the medium (Table 4). With donor embryos of stage H, the lower concentration of 2,4-D (1 mg 2,4-D/l) was better than the higher one (2 mg 2,4-D/l), whereas with donor embryos of stage W, the higher concentration was slightly more effective. The influence of the genotype was overridden by the culture conditions, i.e. the values of overall efficiency displayed by the 22 cultivars were not identical after the four culture conditions, not even when the same developmental stage H or W was considered: while Klein Brujo (84.8 %), Klein Dragón (83.1 %) and Prointa Federal (72.9 %) were the best after preculture under condition A, Prointa Puntal (60.9 %), Prointa B. Remodon (60.3 %) and Klein Volcán (60.3 %) were the best after preculture under condition C. When using developmental stage W, the three best varieties were Prointa Federal (59.5 %), Don Ernesto Inta (46.8 %) and Klein Brujo (35.9 %) after preculture under condition D, and Buck Charrua (34.4 %), Klein Brujo (33.6 %) and Klein Dragón (23.2 %) after preculture under condition B.
In decreasing order, the four cultivars with highest scutellar embryogenic callus were Prointa Federal (89.3 %) under culture condition D, Prointa Imperial (86.4 %) under culture condition C, and Klein Dragón (85.7 %) and Klein Brujo (84.8 %) under culture condition A. Nevertheless, this order changed when the culture efficiency and the number of plants regenerated were considered. The first place was occupied by Klein Brujo with 84.8 % and 9.5 respectively, followed by Klein Dragón (83.1 %, 9.5), Prointa Federal (59.5 %, 3.2) and Prointa Imperial (55.3 %, 3.4).
We may conclude that among the 22 spring wheat varieties investigated, Klein Brujo was the best when pre-cultured under condition A, but also yielded moderate to good results under the other three conditions. Klein Dragón was the second best, with similar good results under condition A but not under the other three conditions.
DISCUSSION
Variables and their interactions in callus formation and plant regeneration
The data presented show that induction of scutellar callus, precocious germination of immature embryos, capacity of scutellar embryogenic callus to grow into plants and the entire culture efficiency, all depended on the genotype, the stage of development of the immature embryo, the concentration of 2,4-D in the medium, and the interaction(s) between them. While the capacity of scutellar calli to develop new shoots under the experimental conditions applied depended only on the genotype, callus proliferation on the scutellum also depended on the developmental stage of the immature embryos.
The significant influence of the genotype on both processes has also been observed in other studies (Carman et al., 1987; Fennell et al., 1996; Özgen et al., 1998; Viertel et al., 1998; Barro et al., 1999; Dağüstü, 2008). In this work, the importance of the developmental stage was given special attention in combination with the concentration of the hormone in the medium. In agreement with that reported by León et al. (2006), the younger stage of the immature embryo was more effective and the effect of maturation of the embryo could not be counteracted by doubling the 2,4-D concentration of the medium. The higher 2,4-D concentration was more effective in the promotion of callus formation only if older embryos (stage W) were used, a finding which is line with that reported by Almouslem et al. (2005). Using young immature embryos (stage H), the higher concentration of the auxin in the medium usually inhibited both morphogenetic processes, as well as the precocious germination of the embryo. This finding was conflicting because callus formation and propagation and regeneration of plants from the calli were more effective with the lower concentration of auxin in the medium, which also promoted precocious germination. Either a slight or no inhibitory effect of a higher 2,4-D concentration on embryogenic callus formation and plant regeneration was reported by Viertel et al. (1998) for 18 German spring wheat varieties. In agreement with our results, Barro et al. (1999) found that the concentration of 2,4-D played a crucial role and that the lower concentration was better for scutellar embryogenic callus formation and regeneration. A loss of responsiveness to the hormone with the advance of maturation of the embryo cannot be ruled out as an explanation of the phenomena observed. Inhibition of the increase in precocious germination as an indicator of the advancing maturation required higher auxin concentration in the medium.
Is there a correlation between the potentials of callus induction and plant regeneration from the calli?
Vegetative propagation of wheat varieties by tissue culture methods depends on both a high potential for embryogenic callus production and a high capacity of plant regeneration from the calli. In the Argentine spring wheat cultivars studied, the regeneration rate of plants from calli was high, but the scutellar callus formation rate of the immature embryos was rather low. Not the entire surface of the wheat-scutellum is capable of forming embryogenic callus (Scott et al., 1990). In that respect, callus proliferation on the scutellum surface is an important parameter for biolistic transformation of wheat. However, only Viertel et al. (1998) studied this issue. These authors found an increase in the portion of the callus producing scutellar surface when 1 mg/l instead of 2 mg/l of 2,4-D was used in the medium. Like in our present work, Viertel et al. (1998) also reported that there was a positive correlation among the frequency of immature embryos forming embryogenic callus and the callus proliferation on the scutellum surface. In the present study, with a substantial collection of Argentine spring wheat cultivars, the young developmental stage of the immature embryo produced a higher percentage of callus proliferation, although the variation between the genotypes was considerable.
Even with the best of our protocols (culture condition A), the overall efficiency of the entire tissue culture approach did not exceed the values reported for South American wheat cultivars (Chowdhury et al., 1991).
In wheat, like in other plants, the variation in tissue culture response is assumed to be controlled by the interaction of a large number of genes (polygenes) (Fennell et al., 1996; Varshney and Altpeter, 2001). With the 22 Argentine spring wheat varieties investigated in this work, we found no relationship between embryogenic callus formation and regeneration capacity. This is in agreement with observations by Sears and Deckard (1982), Chowdhury et al. (1991) and Özgen et al. (1996, 1998). It appears that both phenomena are controlled by different genes or gene combinations. However, Viertel et al. (1998), Barro et al. (1999) and Dağüstü (2008) reported a close correlation between both processes and, at the current state of knowledge, there is no explanation for this contradiction.
Reports about the numbers of plants regenerated per cultured explant differ significantly, a fact that may be explained by the genotypic variation and the culture conditions. In the Argentine spring wheat cultivars studied, the influence of the culture conditions appeared to be restricted to the early stages of the tissue culture protocol. Once shoot regeneration from calli was induced, neither the developmental stage of the immature embryo nor the auxin concentration in the medium played a particular role in the number of plants regenerated.
The Argentine wheat genotypes analyzed regenerated a low number of plants per embryo (1.6 to 9.5) in comparison with other reports (Fennell et al., 1996: 6 to 42; Viertel et al., 1998: 1 to 19; León et al., 2006: 2.3 to 16.8). Only the genotypes examined by Varshney and Altpeter (2001) showed results (1.01 to 9.2) similar to those of the present study.
The present study aimed to gain knowledge on the best conditions for successful genetic transformation of spring wheat. Embryogenic scutellar calli are the most widely used target tissue for gene transfer in wheat. As the medium is important for embryogenesis in these calli, it could also be used as selection medium for transgenic plants. Among the 22 Argentine spring wheat genotypes studied, Klein Brujo and Klein Dragón seemed to be the most promising. The present study also showed that standard culture conditions cannot be applied for all genotypes. This result concurs with the hypothesis of Redway et al. (1990), which establishes that the differences in genotype response are physiological in nature and thus require adjustment of the in vitro culture conditions and media.
ACKNOWLEDGEMENTS
We are grateful to Dr. Sebastian Fettig for his scientific input at the beginning of the study and to G. Blaich and his gardeners for growing the plants. Part of the work has been supported by the Deutsche Akademischer Austauschdienst (DAAD), Bonn, Germany.
BIBLIOGRAPHY
1. Ahuja P.S., Pental D., Cocking E.C. (1982) Plant regeneration from leaf base callus and cell suspensions of Triticum aestivum. Z Pflanzenzücht 89: 139-144. [ Links ]
2. Almouslem A.B., Amleh N., Najjar M.A. (2005) Callus induction and plant regeneration of wheat via isolated immature scutella culture: technology and applications. http://ipac.kacst.edu.sa/edoc/2005/146250_1.PDF. (accessed May 2013). [ Links ]
3. Barro F., Martin A., Lazzeri P.A., Barcelo P. (1999) Medium optimization for efficient somatic embryogenesis and plant regeneration from immature inflorescences and immature scutella of elite cultivars of wheat, barley and tritordeum. Euphytica, 108:161-167. [ Links ]
4. Carman J., Jefferson N. E., Campbell W. F. (1987) Induction of embryogenic Triticum aestivum L. calli. I. Quantification of genotype and culture medium effects. Plant Cell Tissue Org Cult 10: 101-113. [ Links ]
5. Chowdhury S.H., Kato K., Yamamoto Y., Hayashi K. (1991) Varietal variation in plant regeneration capacity from immature embryo among common wheat cultivars. Jpn J Breed 41: 443-450. [ Links ]
6. Dağüstü N., (2008) Comparison of callus formation and plant regeneration capacity from immature embryo culture of wheat (Triticum aestivum L.) genotypes. Biotechnol Biotec Eq 22 (3): 778-781. [ Links ]
7. Fennell S., Bohorova N., van Ginkel M., Crossa J., Hoisington D. (1996) Plant regeneration from immature embryos of 48 Elite CIMMYT bread wheats. Theor Appl Genet 92: 163-169. [ Links ]
8. Food and Agriculture Organization of the United Nations (FAO) (2012) Food Outlook. Global Market Analysis. Trade and Markets Division. Information, Analysis and Forecasts. http://www.fao.org/giews/. (accessed May 2013). [ Links ]
9. Gamborg O.L., Miller R.A., Ojima, K. (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151-158. [ Links ]
10. He D.G., Tanner G., Scott K.J. (1986) Somatic embryogenesis and morphogenesis in callus derived from the epiblast of immature embryos of wheat (Triticum aestivum). Plant Sci 45: 119-124. [ Links ]
11. He D.G., Yang Y.M., Scott, K.J. (1988) A comparison of scutellum callus and epiblast callus induction in wheat: the effect of genotype embryo age and medium. Plant Sci 57 (3): 225-234. [ Links ]
12. Jähne A., Lazzeri P. A., Jagergussen M., Lorz, H. (1991) Plant regeneration from embryogenic cell suspensions derived from anther cultures of barley (Hordeum vulgare L). Theor Appl Genet 82: 74-80. [ Links ]
13. León E., Marin S., Barro F. (2006) Improvement of in vitro culture response of elite wheat cultivars by selecting the source spike, the scutellum size and the temperature for the induction of embryogenesis. Plant Breed 125: 580-583. [ Links ]
14. Maddock S.E., Lancaster V.A., Risiott R., Franklin J. (1983) Plant regeneration from cultured immature embryos and inflorescences of 25 cultivars of wheat (Triticum aestivum L.). J Exp Bot 34: 915-926. [ Links ]
15. Mathias R.J. (1990) Factors affecting the establishment of callus cultures in wheat. In: Bajaj, Y.P.S. (Ed.) Biotechnology in agriculture and forestry. Springer-Verlag, Berlin Heidelberg, 13 pp. 25-45. [ Links ]
16. Özgen M., Turet M., Altinok S., Sancak C. (1998) Efficient callus induction and plant regeneration from mature embryo culture of winter wheat (Triticum aestivum L.) genotypes. Plant Cell Rep 18: 331-335. [ Links ]
17. Özgen M., Turet M., Ozcan S., Sancak C. (1996) Callus induction and plant regeneration from immature and mature embryos of winter durum wheat genotypes. Plant Breed 115: 455-458. [ Links ]
18. Rasco-Gaunt S., Riley A., Cannell M., Barcelo P., Lazzeri P.A. (2001) Procedures allowing the transformation of a range of European elite wheat (Triticum aestivum L.) varieties via particle bombardment. J Exp Bot 52: 865-874. [ Links ]
19. Redway F.A., Vasil V., Lu D., Vasil I.K. (1990) Identification of callus types for long-term maintenance and regeneration from commercial cultivars of wheat (Triticum aestivum L). Theor Appl Genet 79: 609-617. [ Links ]
20. Scott K.J., He D.G., Yang Y.M. (1990) Somatic embryogenesis in wheat. In: Bajaj, Y.P.S. (Ed.) Biotechnology in agriculture and forestry. Springer-Verlag, Berlin Heidelberg, 13 pp. 45-67. [ Links ]
21. Sears R.G., Deckard E.L. (1982) Tissue culture variability in wheat callus induction and plant regeneration. Crop Sci 22: 546-550. [ Links ]
22. Sparks C.A., Jones H.D. (2009) Biolistics transformation of wheat. Methods in Molecular Biology, Transgenic Wheat, Barley and Oats. In: Huw D. Jones and Peter R. Shewry (Eds.) Humana Press, a part of Springer Science + Business Media 478 pp.71-92. [ Links ]
23. Varshney A., Altpeter F. (2001) Stable transformation and tissue culture response in current European winter wheats (Triticum aestivum L.). Mol Breed 8: 295-309. [ Links ]
24. Vasil I.K. (1987) Developing cell and tissue culture systems for the improvement of cereal and grass crops. J Plant Physiol 128: 193-218. [ Links ]
25. Viertel K., Hess D. (1996) Shoot tips of wheat as an alternative source for regenerable embryogenic callus cultures. Plant Cell Tissue Org Cult 44: 183-188. [ Links ]
26. Viertel K., Schmid A., Iser M., Hess D. (1998) Regeneration of German spring wheat varieties from embryogenic scutellar callus. J Plant Physiol 152: 167-172. [ Links ]
27. Zadoks J.C., Chang T.T., Konzak C.F. (1974) Decimal code for growth stages of cereals. Weed Res 14: 415-421. [ Links ]














