Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
BAG. Journal of basic and applied genetics
versión On-line ISSN 1852-6233
BAG, J. basic appl. genet. vol.25 no.2 Ciudad Autónoma de Buenos Aires dic. 2014
ARTÍCULOS ORIGINALES
Self-pollination and its implication in invasiveness of Helianthus annuus ssp. annuus AND H. Petiolaris
Autopolinización y su implicancia en la invasividad de Helianthus annuus ssp. annuus Y H. Petiolaris
Gutierrez A.1, Rueda F.2, Cantamutto M.1,2, Poverene M.1,2
1 CERZOS-CONICET, 8000 Bahía Blanca, Argentina.
2 Department of Agronomy, Universidad Nacional del Sur, 8000 Bahía Blanca, Argentina. aguti@criba.edu.ar
Fecha de recepción: 22/05/2014
Fecha de aceptación de versión final: 20/08/2014
ABSTRACT
Helianthus petiolaris and H. annuus are exotic invaders that have spread widely in central Argentina in the last 70 years. As other annuals of the same genus, these species are outcrossers bearing a sporophytic self-incompatible mechanism. We investigated the ability to self-pollinate in plants of five accessions of each species, its influence on some reproductive traits, and compared to the cultivated sunflower, in which breeding for self-compatibility has been very successful. Plants were allowed to self-pollinate in bagged heads, manually forced to self-pollinate or allowed to cross-pollinate. Although both invasive species were confirmed as self-incompatible, some plants within most accessions were able to self-pollinate. There was no significant interaction between accessions and pollination type for most traits, except for seed set which was attributed to interspecific introgression. The self-compatibility of some plants was transmitted to their progeny. No significant correlation between self-pollination and spread distance from the proposed entry point for the exotic species was found. The occurrence of self-compatible plants was demonstrated in both species and this fact could in part explain the high invasive ability of annual Helianthus species in Argentina.
Key words: Gene flow; Mating system; Seed set; Self-compatibility; Sporophytic SI.
RESUMEN
Helianthus petiolaris y H. annuus son invasores exóticos que se han extendido en el centro de Argentina en los últimos 70 años. Como otras anuales del mismo género, estas especies son de polinización cruzada y llevan un mecanismo de auto-incompatibilidad esporofítica. Se investigó la capacidad de auto-polinización en plantas de cinco colecciones de cada especie, su influencia sobre algunos aspectos reproductivos y en comparación con el girasol cultivado, en el que el mejoramiento para la auto-compatibilidad ha sido exitoso. Las plantas se auto-polinizaron con los capítulos tapados por bolsas, se auto-polinizaron de manera forzada manualmente o se permitió la polinización cruzada. Aunque ambas especies invasoras fueron confirmadas como auto- incompatibles, algunas plantas dentro de la mayoría de las colecciones fueron capaces de auto-polinizarse. No hubo interacción significativa entre las colecciones y el tipo de polinización para la mayoría de los rasgos, excepto para la producción de semillas que se debió a la introgresión interespecífica. La auto-compatibilidad de algunas plantas se transmitió a su descendencia. No se encontró una correlación significativa entre la auto-polinización y la distancia de propagación desde el punto de entrada propuesto para las especies exóticas. La aparición de las plantas auto-compatibles se demostró en ambas especies y este hecho podría explicar en parte la alta capacidad invasiva de las especies anuales Helianthus en Argentina.
Palabras clave: Flujo de genes; Sistema de apareamiento; Producción de semilla; Auto-compatibilidad; Auto-incompatibilidad esporofítica.
INTRODUCTION
Sunflower, Helianthus annuus L. (Asteraceae) is a self-incompatible species by nature and annual Helianthus spp. are mostly obligate allogamous (Heiser et al., 1969). Plant cross-pollination depends on insects, mainly bees. As in other members of the family, the self-incompatible mechanism is sporophytic, where the pollen grain phenotype is determined by the diploid genotype of the anther where they originate (Hiscock and McInnis, 2003). Self-incompatibility is based in protein interactions produced by a unique S-locus that comprises two genes, one expressed in the pistil (female determinant) and another in the anther or pollen grains (male determinant). These genes are closely linked and are considered as a single gene. Proteins produced by female and male determinants interact at the time of pollen grain germination on the stigmata and lead to the arrest of pollen tube growth. Fernandez Martinez and Knowles (1978) proposed a gene control based in a single locus with multiple alleles, with dominant expression in pollen and other mechanisms in style. Gandhi et al. (2005) confirmed a self-incompatible S-locus with multiple alleles in linkage group 17. S-alleles causing self-compatibility would originate a loss of function mutations (Hiscock and McInnis, 2003) where self-incompatibility is controlled by a dominant or semi-dominant allele and self-pollination is attributable to the recessive condition, ss.
In sunflower if there is no cross pollination, the hermaphrodite flower design allows self-pollination, although this mechanism is not efficient (Charlet et al., 1997). Values of up to 80-100% self-fertility have been achieved by breeding for self-compatibility. Self- compatible genotypes have higher yields than self-incompatible ones when bees are not abun dant and the trait is transmitted from elite lines to hybrids. Self-incompatibility and self-fertility degrees depend on three conditions: gene control, environment, and floral structures (Miller and Fick, 1997).
Natural selection during the invasion process of a species could originate self-compatible populations which derive from self-incompatible populations as ancestors. Therefore, invasive species with individuals which vary in their compatibility degree could originate self-compatible populations through mate system selection (Petanidou et al., 2012). A quantitative trait observed in self-incompatible mechanisms is pseudo-self-compatibility (PSC) in which different factors cause variation in the strength of the self-incompatible system, leading to some levels of self-fertilization; yet, seed set is typically lower than in completely compatible cross-pollinations. There are three types of PSC (Hiscock, 2000): a) environmental, induced by temperature and day length. In this regard, Stephens (2008) found that high temperatures induced PSC; b) artificial, generated by mechanical or chemical treatments; and c) genetic, caused by the modifying action of an independent locus different from the S-locus.
In absence of cross-pollination a PSC mechanism could operate, allowing a late acceptance of self-pollen under the control of a second G-modifier locus present in Asteraceae and Brassicaceae. This mechanism could have favored Senecio squalidus following its introduction and rapid spread in the UK (Brennan et al., 2011).
Gene flow from crops to wild and feral populations has been involved in the evolution of a number of weeds, including novel invasive species (Ellstrand, 2003). Helianthus petiolaris and H. annuus ssp. annuus were introduced in Argentina between 1940 and 1950 and, since then, both species quickly spread becoming invasive biotypes. Only one entry point for each species has been registered (Cantamutto et al., 2010). These species have long life cycles and produce many small heads, while domesticated sunflower has a shorter cycle and produce only one big head. Gene flow among both species and sunflower crop has been confirmed (Ureta et al., 2008; Gutierrez et al., 2010). The breakdown of self-incompatibility was studied in these species in their center of origin: heterospecific pollen can induce self-pollination in both species, i.e. H. petiolaris pollen on H. annuus stigmatas and vice-versa, an event known as mentor effect (Desrochers and Rieseberg, 1998).
Our observations during collection trips and in the experimental field indicate that some Helianthus plants from natural populations are likely able to self-pollinate, and that would explain in part their invasive ability. The aim of this work was to verify self-compatibility in different naturalized populations of these two annual Helianthus spp. and to relate this trait to the invasive process.
MATERIALS AND METHODS
In 2012, plants of five accessions of H. annuus ssp. annuus and five accessions of H. petiolaris representative of natural populations from different geographic locations of Argentina were established in the experimental field (Table 1). The populations differed in distance from the proposed entry point for each species (Cantamutto et al., 2010). A plot of commercial hybrid sunflower was sown at the same time. Previously, wild seeds were maintained at 4° C during one week to break dormancy. Seeds were sown in multicell plastic trays in the greenhouse and allowed to grow with proper watering until the seedlings had 4-6 leaves, stage at which they were transplanted to the experimental field belonging to Department of Agronomy, Universidad Nacional del Sur (S 38˚41'38'', W 62˚14'53'').
Table 1. Origin of wild accessions used in the study. Localities span over 988 km from East to West and 1000 km from North to South in Argentina

1 Entry point was Catriló (La Pampa) for H. petiolaris and Río Cuarto (Córdoba) for H. annuus annuus
Plots were established with 10 plants, each divided into rows 0.70 m apart with a distance of 0.20 m between plants. A drip irrigation system was used and weed control was manual. Five plants within each invasive population accession and 10 sunflower plants (cv. Nidera P104CL) were randomly sampled. At emergence of the ray flowers or in the previous reproductive stage R3, according to the scale by Schneiter and Miller (1981), three heads per plant of invasive Helianthus accessions were bagged to prevent the access of foreign pollen. Two of the three covered heads were manually selfed 2-3 times from the beginning to the end of the flowering period, with pollen from another flower of the same plant. To force self-fertilization two heads were rubbed together, or pollen was transferred with cotton brushes. The third head of each plant was kept bagged. Five sunflower heads were allowed to self-pollinate under bags placed at R4 crop stage. A fourth head of each Helianthus sp. plant and five crop sunflower heads, selected at the same stage and grown without cover, were used to assess open pollination.
Seed loss by shattering was prevented by bagging open pollination heads at R6 stage. The following data were collected: disc diameter, number of filled seeds (grains) per head, number of empty seeds per head, weight of filled grains. Based on these data, other parameters were calculated as follows: seed set, as the number of filled seeds / (filled seeds + empty seeds); grain biomass, as the weight of filled grains/grain number. Data were taken as proportions and the logit transformation, log (p/(100-p)), was applied to satisfy the normality assumption of ANOVA. Mean comparisons were made by the LSD Fisher test. Data were analyzed using the statistical package INFOSTAT (Di Rienzo et al., 2013).
Self-Compatibility Index (SCI, Lloyd and Schoen, 1992) was used to classify plants as self-compatible or self-incompatible. We calculated SCI as seed set after manual pollination/seed set after open pollination. SCI varies from 0 to ≥1, where 1 represents complete self-compatibility. A plant was considered self-compatible if SCI> 0.75. This index is useful to compare populations from different environments.
The progeny (N ≤12) of plants showing self-compatibility (SCI> 0.75) was tested the following season under the same experimental conditions. Seed production in the progeny by self-pollination (bagged and forced) and cross-pollination (open pollination) was measured. The SCI index was used to classify self-compatible or self-incompatible off-spring.
RESULTS
Comparisons were made between accessions of each wild species and for selfed and open-pollinated heads for six traits. No significant differences were found between accessions for most traits within both invasive species, but pollination type did show highly significant differences (Table 2). In H. petiolaris significant differences among accessions were found in filled seed, whereas in H. annuus highly significant differences were found in disc diameter and number of empty seed, and significant differences in seed set.
Table 2. ANOVA of reproductive traits of H. petiolaris and H. annuus accessions with two pollination types (selfing and open-pollination); ns not significant, * P <0.05, ** P<0.01


DISCUSSION
No significant differences for the interaction between accession and pollination type were found, except for number of empty seed and seed set in H. annuus.
As there was no accession x pollination type interaction, we performed a Kruskal-Wallis analysis to evaluate the effect of pollination type on six traits considering the accessions as replicates. No transformation of data was applied for this analysis, so means were readily compared (Table 3).
Table 3. Effect of pollination type on reproductive traits in Helianthus species. Different letters indicate significant differences in the Kruskal-Wallis test. BS: Bagged selfing, FS: forced selfing, OP: open

Most studied traits were influenced by the pollination type. Disc diameter was larger with open pollination (OP) in invasive biotypes, whereas it was not affected in cultivated sunflower. Open-pollination favoured seed production per head, seed set, and seed yield (in weight) in all accessions, but grain biomass was not affected by pollination type. The number of empty seed showed no differences between bagged and forced selfing in Helianthus spp., but highly significant differences were found between selfing and open pollination in the three biotypes. Grain yield was higher with forced selfing compared to natural selfing, and it was highest for open pollination in both wild species. Same values in seed set under open pollination were observed in the three biotypes.
SCI index showed that seed production within each accession was variable (Figure 1). Among Helianthus petiolaris accessions CAT, BAR, ROC and BUO, there were plants showing a certain degree of self-pollination. Some were considered to be self-compatible (SCI> 0.75), and others were only partially compatible (SCI 0.25-0.75). ALP was the only accession in which plants produced very scarce seed by selfing. ROC accession also showed high percentages of self-incompatible plants (SCI 0-0.25).

Figure 1. Variation in the mating system according to SCI index among plants in accessions of Helianthus petiolaris and H. annuus. SCI varies from 0 to ≥1. SCI> 0.75 considered self-compatible.
Code accessions in Table 1. CUL: cultivated sunflower.
Within H. annuus accessions, only three plants showed high seed production and could be considered self-compatible (SCI> 0.75), whereas most plants were self-incompatible (SCI 0-0.25), being the incompatibility 100% for BAR accession. The cultivated sunflower crop had the highest percentage of self-compatible plants.
Analyzed plants were plotted on a graph relating seed produced by natural selfing and by forced selfing (Figure 2). Most plants of both Helianthus species were self-incompatible and located in the low left corner of the graph. These plants produced very small amount of seed, if any. Among 50 analyzed wild plants, 10 had varying degrees of self-compatibility; of these, two individuals of H. petiolaris (BAR accession) and H. annuus (LMA accession) showed high self-compatibility.
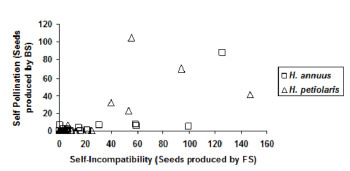
Figure 2. Seed production per plant with bagged selfing (BS) and forced selfing (FS) in invasive Helianthus accessions.
In general, self-compatibility of the progeny of plants subject to self-pollination was higher than in their parents (Figure 3). Some progenies of self-compatible plants of both species produced seeds and segregated for SCI index. In H. petiolaris, offpring of three out of nine analyzed

Figure 3. SCI index among progenies of self-fertilized plants of Helianthus petiolaris and H. annuus ssp. annuus. SCI varies from 0 to ≥1. SCI> 0.75 considered self-compatible. Code accessions in Table 1.
mother plants segregated for SCI index, whereas in H. annuus, offpring of nine out of 15 mother plants also showed segregation.
Regarding the entry point in both species, correlation between population distance and proportion of self-pollinating plants in the first generation was not significant: R2=0.31, p=0.32 for H. petiolaris and R2=0.02, p=0.82 for H. annuus annuus.
Seeds of invasive biotypes were collected at different localities to compare accessions in the experimental field. Selfed and open-pollinated plants of both H. petiolaris and H. annuus annuus showed similar values among accessions. We discarded mentor effect (Desrochers and Rieseberg, 1998) because selfed heads were bagged during all the experiment. The low number of filled seeds in H. petiolaris accessions CAT and BAR and the low total grain weight in CAT could be explained because in both places the species grows close to sunflower crop, H. annuus var. macrocarpus. Crop-wild gene flow has been confirmed (Ureta et al., 2008; Gutierrez et al., 2010) and presumably interspecific hybrids or their progenies could be partially sterile. Advanced generation hybrids or backcrosses to H. petiolaris are almost indistinguishable from the latter (Gutierrez et al., 2012). Among H. annuus accessions, RAN showed the lowest values for seed and grain parameters which was attributed to inbreeding depression, since it is a small population that has been isolated for more than ten years. BAR had the largest discs probably because of gene flow from sunflower crop, whereas DIA had the smallest ones, since this population is isolated from the crop.
As expected, open pollination favoured all traits in Helianthus spp. populations, which are naturally self-incompatible, as well as in the sunflower crop. The latter is self-compatible, but heads were covered and therefore were not accessible to bees for pollination. These species depend on pollinators, mainly bees and this explains the differences in the number of seeds and grain weight between bagged pollinated and manually pollinated heads.
Differences in disc diameter were attributed to the amount of seed set in each case. Higher seed production contributed to the development of the receptacle (disc) in Helianthus species. In H. annuus annuus the significant difference in total grain weight was probably due to flowers that were fertilized by crop pollen, since sunflower commercial hybrids were grown at the same time in the experimental field.
Seed set in open-pollinated invasive biotypes was not very high, 79% in Helianthus annuus and 77% in H. petiolaris, but this is not unusual. In a previous study in the same experimental field, the average seed set of four populations was 60% for H. annuus and 72% for H. petiolaris (Ureta et al., 2008). Seed set in cultivated sunflower was 79.3%, which was similar to that obtained in this work (80%).
Seed set differences between bagged (NS) and forced selfing (FS) in the wild biotypes were attributed to the effect of pollinators, known as geitonogamy, or pollination between different flowers of the same individual. In a commercial sunflower hybrid, the term applies to pollination among flowers of different plants, since all plants have the same genotype. This explains differences in seed production in the highly self-compatible cultivated biotype, although this varies according to genotypes (Astiz et al., 2011).
The degree of self-compatibility depends on environmental conditions and pollinating vectors visiting the flowers, and can vary between flowers and between plants (Luciano et al., 1965; Astiz et al., 2011). A plant is considered self-compatible if SCI> 0.75. This index is used to compare populations of different environments (Petanidou et al., 2012).
SCI index graphs for parental and progeny generations show an increase in plant frequency with higher SCI values in all the accessions; however, there were variations. For example, no SCI> 0.75 were observed among progeny of ROC plants and some low SCI plants appeared in ALP. This suggests that the self-compatibility trait is heritable and that there are factors affecting allelic interactions in the self-incompatibility system. Incompletely dominant or weak S alleles in wild H. annuus were suspected (Gandhi et al., 2005) but allelic relationships have not been studied in Helianthus, even though breeding has succeeded in converting sunflower into a self-compatible species (Luciano et al., 1965; Miller and Fick, 1997). Crosses are needed among different SCI indexed plants in order to unravel the allelic polymorphism in invasive Helianthus.
The two annual Helianthus species have spread widely with a striking success after their introduction in Argentina. They form extensive populations in the central part of the country and new patches can be found every year (Poverene et al., 2008). Entry occurred ca. 70 year ago; the way both species arrived is uncertain and they seem to have spread from a single entry point (Cantamutto et al., 2010). However, we were not able to find a correlation between self-pollination and spread distance from the entry point for both species.
According to Baker's Law, successful colonizing and invasive plants are usually self-compatible (Baker, 1967) but there are exceptions (Petanidou et al., 2012). Hiscock (2000) argued that during population bottlenecks self-incompatible mechanisms may break down, and pseudo-self-compatibility (PSC) facilitates the invasion by an otherwise strong self-incompatible species, as in Senecio squalidus (Asteracee). A modifying G-locus has been proposed in this species as a consequence of disturbances following introduction and rapid spread within a new environment (Brennan et al., 2011). In PSC seed set in self-pollinated plants is lower compared to open-pollinated plants. This was true for most plants producing seeds by self-pollination in Helianthus annuus and H. petiolaris accessions, though ratios of self-pollination to cross-pollination seeds were very variable. The ratio is environment - or age - dependent and also PSF is characterized by a continuous distribution of self-fertility in the progeny (Levin, 1996). This should be further investigated in Helianthus and we plan to make artificial crosses among plants showing different SCI indexes. Abbot et al. (2009) stressed the argument that hybridization has preceded invasion in S. squalidus, a feature that also holds for wild Helianthus species in Argentina. Introgression of crop genes could be the way to acquisition of null S-alleles that allow self-fertilization. Moreover, H. petiolaris and H. annuus differ in a number of structural chromosome changes, i.e. translocations and inversions, both involving linkage group 17 (Burke et al., 2004), where the S-locus is located (Gandhi et al., 2005). Hybridization between these taxa could disrupt and in some way alter gene expression in that locus, causing PSF and enhancing invasiveness. Even though the genetic mechanism deserves further research, it is clear that self-pollination occurs, and this trait is inherited by the next generations in both invasive Helianthus species.
ACKNOWLEDGEMENTS
This work was supported by grants ANPCYT PICT 2854 and PGI UNS 24A204.
REFERENCES
1. Abbott R.J., Brennan A.C., James J.K., Forbes D.G., Hegarty M.J., Hiscock S.J. (2009) Recent hybrid origin and invasion of the British Isles by a self-incompatible species, Oxford ragwort (Senecio squalidus L., Asteraceae). Biological Invasions 11:1145-1158. [ Links ]
2. Astiz V., Iriarte L.A., Flemmer A., Hernández L.F. (2011) Self-compatibility in modern hybrids of sunflower (Helianthus annuus L.). Fruit set in open and self-pollinated (bag isolated) plants grown in two different locations. Helia 54: 129-138. [ Links ]
3. Baker H.G. (1967) Support for Baker's Law - as a rule. Evolution 21: 853-856. [ Links ]
4. Brennan A.C., Tabah D.A., Harris S.A., Hiscock S.J. (2011) Sporophytic self-incompatibility in Senecio squalidus (Asteraceae): S allele dominance interactions and modifiers of cross-compatibility and selfing rates. Heredity 106: 113-123. [ Links ]
5. Burke J.M., Lai Z., Salmaso M., Nakazato T., Tang S., Heesacker A., Knapp S.J., Rieseberg L.H. (2004) Comparative mapping and rapid karyotypic evolution in the genus Helianthus. Genetics 167: 449-457. [ Links ]
6. Charlet L.D., Brewer G., Franzmann B.A. (1997) Sunflower insects. In: Schneiter A.A. (Ed.) Sunflower Technology and Production, Agronomy Monograph 35, ASA-CSSA-SSSA Press, Madison, Wisconsin, USA, pp. 183-261. [ Links ]
7. Cantamutto M.A., Torres L.I., Presotto A., Gutierrez A., Ureta S., Poverene M. (2010) Migration pattern suggested by terrestrial proximity as possible origin of wild annual Helianthus populations in central Argentina. Biological Invasions 12: 541-551. [ Links ]
8. Desrochers A., Rieseberg L.H. (1998) Mentor effects in wild species of Helianthus (Asteraceae). American Journal of Botany 85: 770-775. [ Links ]
9. Di Rienzo J.A., Casanoves F., Balzarini M.G., Gonzalez L., Tablada M., Robledo C.W. (2013) InfoStat versión 2013. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. URL http://www.infostat.com.ar [ Links ]
10. Ellstrand N.C. (2003) Dangerous liaisons? When cultivated plants mate with their wild relatives. The Johns Hopkins University Press, Baltimore and London. [ Links ]
11. Fernandez Martinez J., Knowles P.F. (1978) Inheritance of self incompatibility in wild sunflower. In: Proc 8th Int Sunflower Conf. Minneapolis, Int. Sunflower Assoc, Paris, pp. 484-489. [ Links ]
12. Gandhi S.D., Heesacker A.F., Freeman C.A., Argyris J., Bradford K., Knapp S.J. (2005) The self-incompatibility locus (S) and quantitative trait loci for self-pollination and seed dormancy in sunflower. Theoretical and Applied Genetics 111:619-629. [ Links ]
13. Gutierrez A., Carrera A., Basualdo J., Rodriguez R., Cantamutto M., Poverene M. (2010) Gene flow between cultivated sunflower and Helianthus petiolaris (Asteraceae). Euphytica 172: 67-76. [ Links ]
14. Gutierrez A., Cantamutto M., Poverene M. (2012) Introgression of cultivated sunflower into exotic Helianthus petiolaris populations. Journal of Basic and Applied Genetics 23: 25-36. [ Links ]
15. Heiser C.B., Smith D.M., Clevenger S.B., Martin W.C. (1969) The North American sunflowers (Helianthus). Memoirs of the Torrey Botanical Club 22: 1-218. [ Links ]
16. Hiscock S.J. (2000) Self-incompatibility in Senecio squalidus L. (Asteraceae). Annals of Botany 85 (Supplement A): 181-190. [ Links ]
17. Hiscock S.J., McInnis S.M. (2003) Pollen recognition and rejection during the sporophytic self-incompatibility response: Brassica and beyond. Trends in Plant Science 8: 606-613. [ Links ]
18. Levin D.A. (1996) The evolutionary significance of pseudo-self-fertility. American Naturalist 148: 321-332. [ Links ]
19. Lloyd D.G., Schoen D.J. (1992) Self-fertilization and cross-fertilization in plants. Functional dimensions. International Journal of Plant Sciences 153: 358-369. [ Links ]
20. Luciano A., Kinman M.L., Smith J.D. (1965) Heritability of self incompatibility in the sunflower (Helianthus annuus). Crop Science 5: 529-532. [ Links ]
21. Miller J.F., Fick G.N. (1997) The Genetics of Sunflower. In: Schneiter A.A. (Ed.) Sunflower Technology and Production, Agronomy Monograph 35, ASA-CSSA-SSSA Press, Madison, Wisconsin, USA, pp. 441-496. [ Links ]
22. Petanidou T., Godfreeb R., Songa D., Kantsaa A., Dupontd Y., Wasere N. (2012) Self-compatibility and plant invasiveness: Comparing species in native and invasive ranges. Perspectives in Plant Ecology, Evolution and Systematics 14: 3-12. [ Links ]
23. Poverene M., Cantamutto M., Seiler G.J. (2008) Ecological characterization of wild Helianthus annuus and H. petiolaris germplasm in Argentina. Plant Genetic Resources Characterization and Utilization 7: 42-49. [ Links ]
24. Schneiter A.A., Miller J.F. (1981) Description of sunflower growth stages. Crop Science 21: 901-903. [ Links ]
25. Stephens L.C. (2008) Self-incompatibility in Echinacea purpurea. HortScience 43: 1350-1354. [ Links ]
26. Ureta S., Cantamutto M., Carrera A., Delucchi C., Poverene M. (2008) Natural hybrids between cultivated and wild sunflowers in Argentina. Genetic Resources and Crop Evolution 55: 1267-1277. [ Links ]














